Survival of very elderly patients with diffuse large B-cell lymphoma according to treatment intensity in the immunochemotherapy era: a Swedish Lymphoma Register study
Summary
Diffuse large B-cell lymphoma (DLBCL) incidence rises with increasing age. Rituximab-anthracycline-based regimens offer a potential cure but also risks of adverse events, especially in the elderly. Using Swedish registers, we conducted a nationwide, population-based study of DLBCL in the very elderly. We obtained information on clinical characteristics, residence, comorbidity, therapy and survival for the 1194 patients aged ≥80 years diagnosed in Sweden 2007–2014. To address selection bias, we also investigated treatment differences between Sweden’s Healthcare Regions and whether there were survival differences between the Regions. The 2-year overall and relative survivals were better in patients aged ≥80 years given treatment with curative intent (54%; 64%) than low-intensity (26%; 33%), or palliative treatment (6%; 7%). The fraction of patients treated with curative intent varied between the Healthcare Regions (45–76%). Survival was significantly inferior in Regions with few patients treated with curative intent (multivariable hazard ratio 1.3, 95% confidence interval 1.1–1.6). When treatment intensity and Regions competed, Regions were no longer independent, suggesting that Regional survival differences are due to therapeutic differences. Furthermore, we found that the age-adjusted International Prognostic Index was independently associated with survival. We conclude that patients aged ≥80 years with DLBCL appear to benefit from rituximab-anthracycline-based treatment given with curative intent.
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma and its incidence increases with age.1 The median age of diagnosis is 70 years, but with longer life-expectancy in the Western world, the proportion of very elderly patients aged ≥80 years will increase.2
The standard treatment for DLBCL is based on rituximab (R) combined with chemotherapy, particularly CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). However, very elderly patients (age ≥ 80 years) are rarely included in the randomised trials that provide the current evidence-base for the use of R-CHOP as standard treatment.3-5 The risk of severe adverse events in the very elderly has been reported to be high.6 On the other hand, recent data show that the life-expectancy for a person reaching the age of 80 years in 2013 was up to 10 years, which means that there is still a lot of time to gain if one survives DLBCL and patients aged ≥80 years who are alive 2 years after the diagnosis of DLBCL have almost the same life-expectancy as an age-matched general population.7 The prognosis in DLBCL worsens with increasing age, which reflects the fact that these patients have more comorbidity and do not tolerate full-dose chemotherapy.8-11 However, it might also partly result from under-treatment due to fears of severe side-effects or to different traditions regarding how to treat the very elderly. Optimal treatment regimens have not yet been defined for the very elderly because of the lack of randomised data.
In this report we present data from the Swedish Lymphoma Register (SLR) on the overall survival (OS) and relative survival according to treatment intensity in a large population-based cohort of patients aged ≥80 years with DLBCL treated in the rituximab era. To further validate our results and try to overcome selection bias by different comorbidities, we also investigated and compared overall treatment intensity and outcome in Sweden’s six Healthcare Regions.
Patients and methods
Study population
The study population includes all patients aged ≥80 years diagnosed with DLBCL from 1 January 2007 to 31 December 2014. The coverage of the SLR compared with the mandatory Swedish Cancer Registry has been 95–97%.12 Survival data were obtained from the Causes of Death Register. Calculation of the age-adjusted International Prognostic Index (aaIPI) risk group was performed as previously described.13 Patients were categorised as aaIPI 0–1 or 2–3. Patients who fulfilled the criteria for at least two risk factors included in the aaIPI-score but had missing data on the last parameter were coded as aaIPI 2–3, and those who had no risk factor but missing data on one parameter was coded as 0–1. Treatment was classified according to treatment intensity as curative intent, low-intensity treatment or palliative treatment. Curative treatment included (R-)CHOP variants, R-CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone), R-CEOP (cyclophosphamide, epirubicin, vincristine, prednisone), R-CVIP (cyclophosphamide, etoposide, idarubicine, prednisone) and VADRIAC (vincristine, adriamycin, cyclophosphamide). Low-intensive treatment included R-COP (cyclophosphamide, vincristine, prednisone) chlorambucil, R-bendamustine, radiotherapy, cyclophosphamide, and other less intensive chemotherapeutic agents. The palliative group received no treatment or only steroids. The cohort was linked to the National Patient Register to collect information on comorbidity during a period of 10 years prior to the diagnosis of DLBCL. This register contains diagnoses of hospital admissions nationwide since 1987 and outpatient care since 2001 with coverage of 85–95%.14 We categorised comorbidity according to the Charlson Comorbidity Index (CCI).15 The study was approved by the Ethics Committee, Stockholm, Sweden.
Treatment recommendations and healthcare regions
Rituximab was introduced as part of the standard treatment for all patients with CD20+ DLBCL in the national guidelines in Sweden in 2006. R-CHOP was recommended to patients aged >65 years during the studied period, but no specific recommendations were made for patients aged ≥80 years. Sweden is divided into six geographical Healthcare Regions, each with a population of between 0·9 and 2·3 million people and an academic referral centre. Local guidelines and traditions impact the choice of treatment in the very elderly, as well as clinical status and comorbidities of the patient. Patients are treated exclusively within the Healthcare Region of residence and in our dataset there were no significant differences between the regions concerning CCI. We therefore compared treatment intensity, OS and relative survival between the Regions. By doing this we aimed to estimate the impact of treatment intensity on survival also on a group level to reduce the effect of selection bias in treatment decisions. We first grouped the Regions by how intensively patients were treated, based on the fraction of patients treated with curative intent. Second, by comparing survival between Regions, we addressed patient selection bias. This method of analysis has been described by our group before.16
Statistical methods
Kaplan–Meier curves and the log-rank test were used to estimate OS. Factors that were significantly associated with survival in the univariable analysis competed in multivariable analysis, using forward stepwise Cox regression including age, aaIPI, gender, CCI, Region and treatment intensity. The proportional hazards assumption was checked with graphs based on Schoenfeld residuals. A significant correlation was detected between age and treatment intensity, as well as for age and aaIPI and the model was adjusted also for this by introducing correlating interaction variables. All P values are two-tailed and P < 0·05 was considered statistically significant. Patients were followed from the date of diagnosis to the date of death or last follow-up (10 October 2018). Relative survival was estimated as the ratio of the observed all-cause survival to the expected survival in an age-, sex-, and calendar-period-matched population (assumed lymphoma free). The Ederer II method was used to calculate expected survival in the matched general population using data obtained via the Human Mortality Database (www.mortality.org). Results are presented in relative survival graphs and as 2-year estimates with 95% confidence intervals (CIs). All statistical analyses were performed with STATA software, version 15 (StataCorp LLC 2015, College Station, TX, USA).
Results
Patient characteristics
In total, 1194 patients aged ≥80 years [median (range) 84 (80–105) years] were diagnosed with DLBCL during the study period. Baseline characteristics for included patients are shown in Table I. Most of the patients were aged 80–90 years (88%). More than one-third had a high risk aaIPI (2–3; 38%) and comorbidity was frequent with 42% of all patients having a CCI of ≥2.
| Baseline characteristic | Value |
|---|---|
| N (%): | |
| Curative treatment | 702 (59) |
| Low-intensity treatment | 205 (17) |
| Palliative treatment | 212 (18) |
| Missing | 75 (6) |
| Albumin <36 g/l | 560 (47) |
| Age, years, median (range) | 84 (80–105) |
| 80–84, n (%) | 644 (53) |
| 85–89, n (%) | 404 (34) |
| ≥90, n (%) | 146 (13) |
| N (%): | |
| Male | 566 (47) |
| aaIPI 0–1 | 492 (41) |
| aaIPI 2–3 | 456 (38) |
| Missing | 246 (21) |
| Bulky disease | 215 (18) |
| Elevated LDH | 610 (51) |
| WHO Performance Status 2–4 | 447 (37) |
| Stage I–II | 480 (40) |
| Stage III–IV | 559 (47) |
| Missing | 155 (13) |
| CCI 0 | 413 (35) |
| CCI 1 | 278 (23) |
| CCI ≥2 | 502 (42) |
| Region A | 291 (24) |
| Region B | 75 (31) |
| Region C | 528 (44) |
- Curative intent included R-CHOP variants, R-CHOEP, R-CEOP, R-CVIP and VADRIAC. Low-intensity treatment included R-COP, Chlorambucil, R-Bendamustine, radiotherapy, surgery, cyclophosphamide single, and other types of less intensive chemotherapeutic agents. The palliative group received no treatment or only steroids. Regions are grouped according to proportion of patients treated with curative intent and Region A is the most intensive one.
- aaIPI, age-adjusted international prognostic index; CCI, Charlson Comorbidity Index; LDH, lactate dehydrogenase; WHO, World Health Organization.
Treatment intensity and survival
The mean follow-up of survivors was 2·4 years. The OS and relative survival is shown in Fig 1. In the cohort, 702 patients were treated with curative chemotherapy, 205 with low-intensity chemotherapy, and 212 were given palliation. In patients treated with curative intent, 93% received CHOP or variants and 7% CHOEP, CEOP, CVIP or VADRIAC. Patients in the curative group were younger than in the low-intensity and palliative groups. Furthermore, they had a lower comorbidity burden according to the CCI (Table II).
| Characteristic | Curative treatment | Low-intensity treatment | Palliative treatment | P |
|---|---|---|---|---|
| N (%): | ||||
| Male | 356 (51) | 85 (41) | 85 (40) | 0·007 |
| aaIPI 0–1 | 340 (48) | 102 (50) | 38 (18) | <0·001 |
| aaIPI 2–3 | 289 (41) | 62 (30) | 77 (36) | |
| Missing | 72 (10) | 41 (20) | 98 (46) | |
| Age, years, median (range) | 83 (80–99) | 87 (80–96) | 86 (80–105) | <0·001 |
| 80–84, n (%) | 463 (66) | 59 (29) | 84 (39) | |
| 85–89, n (%) | 200 (28) | 100 (49) | 77 (36) | |
| ≥90, n (%) | 39 (6) | 46 (22) | 52 (24) | |
| CCI 0, n (%) | 284 (40) | 58 (28) | 52 (24) | <0·001 |
| CCI 1, n (%) | 162 (23) | 44 (21) | 54 (25) | |
| CCI ≥2, n (%) | 255 (36) | 102 (50) | 107 (50) |
- Chi-squared test for difference between the groups.
- aaIPI, age-adjusted international prognostic index; CCI, Charlson Comorbidity Index.
In multivariable analyses, curative treatment was significantly associated with superior outcome compared with low-intensity treatment [hazard ratio [HR] 2·3, 95% CI 1·8–2·9) and palliative treatment (HR 7·6, 95% CI 5·2–11·2). An aaIPI 2–3 correlated with worse outcome (HR 1·6, 95% CI 1·1–2·2), as did high CCI (HR 1·6, 95% CI 1·1–1·9 for CCI ≥2) and age (HR 2·2, 95% CI 1·6–2·7 for age 85–89 vs. 80–84 years; Table III).
| Model | Factor | HR (95% CI) | P |
|---|---|---|---|
| Treatment intensity | Curative treatment | Ref | <0·0005 |
| Low-intensity treatment | 2·3 (1·8–2·9) | ||
| Palliative treatment | 7·6 (5·2–11·2) | ||
| aaIPI 0–1 | Ref | 0·011 | |
| aaIPI 2–3 | 1·6 (1·1–2·2) | ||
| Age 80–84 years | Ref | <0·0005 | |
| Age 85–89 years | 2·1 (1·2–1·6) | ||
| Age ≥90 years | 4·2 (1·1–1·8) | ||
| Male | Ref | 0·007 | |
| Female | 0·8 (0·7–0·9) | ||
| CCI 0 | Ref | <0·0005 | |
| CCI 1 | 1·4 (1·2–1·7) | ||
| CCI ≥2 | 1·6 (1·3–1·9) | ||
| Regions | Region A | Ref | 0·009 |
| Region B | 1·1 (0·9–1·4) | ||
| Region C | 1·3 (1·1–1·6) | ||
| aaIPI 0–1 | Ref | <0·0005 | |
| aaIPI 2–3 | 2·2 (1·9–2·5) | ||
| Age 80–84 years | Ref | <0·0005 | |
| Age 85–89 years | 1·7 (1·5–2·0) | ||
| Age ≥90 years | 2·4 (1·9–3·0) | ||
| Male | Ref | 0·084 | |
| Female | 0·9 (0·8–1·0) | ||
| CCI 0 | Ref | <0·0005 | |
| CCI 1 | 1·4 (1·1–1·7) | ||
| CCI ≥2 | 1·7 (1·4–2·0) |
- aaIPI, age-adjusted international prognostic index; CCI, Charlson Comorbidity Index.
Of patients treated with curative intent, 16% did not receive rituximab, and the corresponding proportions in the low-intensity and palliative patients were 28% and 100%, respectively. The fraction of patients not receiving rituximab as part of their treatment with curative intent did not differ over time.
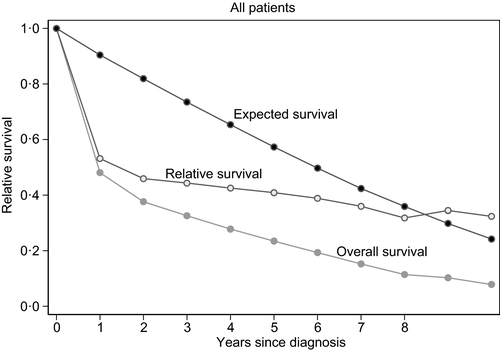
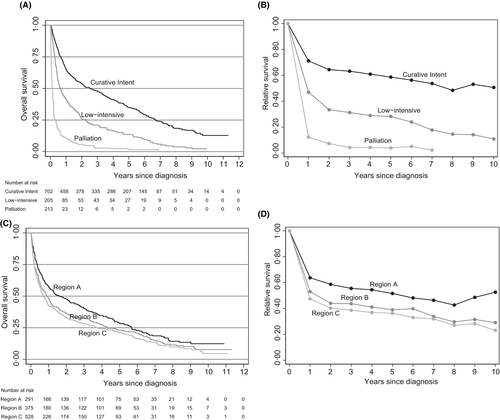
The 2-year OS for all patients was 38%. In patients receiving treatment with curative intent, 2-year OS was 54%, compared with 26% in the low-intensity group and 6% in the palliative group (Fig 2A). The 2-year relative survival in the curative group was 64%, 33% in the low-intensity group, and 7% in the palliative group (Fig 2B).
Regional differences in treatment intensity and survival
The proportion of patients treated with curative intent differed between the six Regions. Of all patients, 76% in Region 1 were treated with curative intent, 73% in Region 2, 64% in Region 3, 63% in Region 4, 49% in Region 5, and 45% in Region 6 (P < 0·001). This allowed us to coalesce the Regions into three larger pairs, according to treatment intensity: Region A (1 and 2) with >70% of patients treated with curative intent, Region B (3 and 4) with 50–70%, and Region C (5 and 6) with <50%.
Region A had a larger proportion of patients with aaIPI 0–1 than Regions B and C. However, the proportion of patients with aaIPI 2–3 was similar and missing data on aaIPI were more frequent in Region B and C, which could explain the difference in aaIPI 0–1 (Table IV). OS was superior in Region A, and inferior in Region C (Fig 2C).
| Characteristic | Region A | Region B | Region C | P |
|---|---|---|---|---|
| N (%): | ||||
| Curative intent | 216 (74) | 239 (63) | 247 (47) | <0·001 |
| of which included rituximab | 197 (91) | 168 (70) | 226 (91) | |
| missing data on treatment | 23 (8) | 21 (6) | 30 (6) | |
| Male | 137 (47) | 177 (47) | 252 (48) | 0·98 |
| aaIPI 0–1 | 147 (51) | 138 (37) | 207 (39) | <0·001 |
| aaIPI 2–3 | 109 (37) | 151 (40) | 196 (37) | |
| Missing | 25 (12) | 86 (23) | 125 (24) | |
| Age, years, median (range) | 84 (80–99) | 84 (80–105) | 84 (80–96) | 0·345 |
| 80–84, n (%) | 148 (51) | 213 (57) | 283 (54) | |
| 85–89, n (%) | 111 (38) | 119 (32) | 174 (33) | |
| ≥90, n (%) | 32 (11) | 43 (11) | 71 (13) | |
| CCI 0, n (%) | 109 (37) | 139 (37) | 166 (31) | 0·167 |
| CCI 1, n (%) | 73 (25) | 78 (21) | 127 (24) | |
| CCI ≥2, n (%) | 109 (37) | 157 (42) | 236 (45) |
- Chi-squared test for difference between the groups.
- aaIPI, age-adjusted international prognostic index. CCI, Charlson Comorbidity Index.
In multivariable analysis, there were significant differences in OS between the Regions A, B, and C (Table III), with inferior outcome in Region C compared with Region A (HR 1·3, 95% CI 1·1–1·6). The 2-year relative survival in Region A was 59%, in Region B 44%, and in Region C 40% (Fig 2D). When both treatment intensity and Regions were included in the multivariable model with age and aaIPI, Regions were no longer independent.
Discussion
With an ageing population and increasing life-expectancy also at the age of 80 years, we need to consider our very old patients with DLBCL as potentially curable, with several years left to live, and treat them accordingly.
In this large, population-based, nationwide study we show that very elderly patients with DLBCL benefit from R-CHOP-like regimens with curative intent despite their old age and comorbidity. The present study shows that, in this population, the 2-year relative survival for patients treated with curative intent is twice that of those given low-intensive chemotherapy and almost 10-times higher than in those receiving only palliative treatment, suggesting that the risk of dying of DLBCL is far more probable than dying of side-effects due to intensive treatment of the disease.
As the choice of treatment for patients aged ≥80 years is influenced by the estimation of risk of morbidity and mortality related to the treatment itself as well as the disease, it is complicated to adjust for all known and unknown clinical factors in retrospective material. To look at the data from a different angle, we decided to use the system of Healthcare Regions in Sweden to see if different local traditions in treatment intensity of the very elderly had an impact on OS and relative survival. In this way we could use the geographical area for each patient to compare outcome based on region of residence instead of administered treatment, to see if more intensive treatment traditions translate into better survival also in the elderly population with complicating comorbidity.
We found that the regions treating >70% of patients with R-CHOP or other intensive regimens had significantly better OS and relative survival than the less intensive regions. Adverse risk factors and CCI did not differ significantly between the regions and could therefore not explain the survival differences nor did socioeconomic differences. Regional differences in median income, tertiary education, and life-expectancy were not significant (data not shown) and of the two healthcare regions comprising the most intensive pair, Region A, one is the most rural and the other is the most urban region. When both treatment intensity and Regions were included in the multivariable model, Regions were no longer independent risk factors for survival indicating that the difference between Regions in terms of OS is mediated primarily through the regional variances in use of intensive treatment.
Previous studies have shown that anthracycline-based therapy is correlated with favourable outcome in very elderly patients with DLBCL, although in smaller cohorts or single centres.17, 18 Boslooper et al.9 demonstrated, in a retrospective study of 103 patients with DLBCL aged >75 years, that those who received full-dose R-CHOP had significantly superior 2-year OS (70%) than those administered reduced regimens (2-year OS 28%) or palliative care (2-year OS 21%). The French Lymphoma Study Association (LYSA) group presented data on R-mini-CHOP as an alternative for patients aged >80 years in a multicentre, single-arm phase II study in 2011 with a 2-year OS of 59% with acceptable toxicity.19 The same group performed a similar study on of atumumab combined with mini-CHOP in 120 patients with DLBCL aged >80 years; the 2-year OS was 64·7%.20 Prospective clinical trials on very elderly patients always face the risk of selection bias, as patients with severe comorbidity might not be included.4 Our present study, based on consecutive patients treated in daily clinical practice in all of Sweden; show a 2-year OS of 54% when treated with curative intent, comparable to the data from the LYSA study on R-mini-CHOP, which further strengthens the assumption that R-CHOP-like regimens are correlated with superior outcome also in the very elderly population.
Another important finding of the present study is that the prognostic index aaIPI, originally developed for patients aged ≤60 years, is a useful prognostic tool also in patients aged ≥80 years. This was also noted by Peyrade et al.20, but has not been consistently shown before in this age group.
As in all retrospective studies, there is a risk of selection bias when it comes to choosing patients for more intensive treatment. We have made an effort to analyse our present data from different angles, using the geographical Healthcare Regions, to try to minimise this bias. By looking at treatment intensity by region, we could validate our findings that treatment with curative intent is associated with better survival. In the present data, some patients were not given rituximab, despite curative intent and CHOP-based regimens. This could represent a group of undertreated patients amongst the elderly. However, the superior survival in patients treated with curative intent remained significant also when excluding patients treated without rituximab from the analysis, both concerning treatment intensity and regions.
It is reasonable to assume that a proportion of the patients in the present study received dose reductions, but as has been shown before pre-planned dose reductions can be associated with good response and survival in the very elderly.21, 22 In the LYSA study reduced R-CHOP in form of R-mini-CHOP proved to be an acceptable alternative for patients aged >80 years.20 The strength of our present study is the population-based large dataset of very elderly patients treated for DLBCL in the immunochemotherapy era. The ability to perform regional analysis on survival and intensity of treatment further validate our present findings.
We conclude that patients with DLBCL, aged ≥80 years, appear to benefit from rituximab-anthracycline-based treatment given with curative intent despite advanced age and comorbidity.
Acknowledgments
This work was supported by grants from Cancerfonden, Svenska Sällskapet för Medicinsk Forskning (SSMF), Svenska läkaresällskapet and Stockholm County Council.
Conflicts of interest
The authors have no conflict of interest.




