Clinical and biological correlates of the expression of select Polycomb complex genes in Brazilian children with acute promyelocytic leukaemia
De novo acute promyelocytic leukaemia (APL) is an aggressive subtype that accounts for 5–10% of all childhood acute myeloid leukaemia (AML). In some Latin American and European populations, APL is more frequent than in other geographic populations. Approximately 97% of patients with APL present t(15;17)(q22;q21.1)/promyelocytic leukaemia (PML)-retinoic acid receptor alpha (RARA) fusion protein and nearly all of the affected patients respond to therapy with all-trans retinoic acid (ATRA) combined with arsenic trioxide (ATO).1 Additionally, studies in transgenic mice revealed that this transcript is necessary, but not sufficient, for APL development, suggesting that additional genetic or epigenetic changes are also required for the APL establishment.2
The FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) has been frequently reported in patients with APL presenting a more aggressive disease course, with lower overall and disease-free survival rates. These mutations are known to co-operate with other initiating events to advance disease progression, but they do not initiate leukaemia independently.3 Regarding paediatric APL, genomic studies reported that APL cells have fewer genetic alterations than those from other AML subtypes.4 These data indicate that childhood APL development may require more than just genetic alterations to manifest the disease phenotype.
In this context, there are several reports on the epigenetic alterations implicated in AML development, and they are mainly presented in APL. For instance, patients with APL are characterised by a specific DNA methylation pattern, which may be due to relatively late events in APL leukaemogenesis, contributing to APL maintenance rather than leukaemia initiation.5
Besides, PML/RARA induces a multitude of alterations in chromatin architecture, including the recruitment of crucial epigenetic-modifying factors, such as histone deacetylase complexes and DNA methyltransferases. Moreover, pieces of evidence have revealed that the Polycomb repressor complex (PRC) could contribute to the alterations observed in the typical APL epigenetic landscape.6
Polycomb group (PcG) proteins are histone modifiers in two multiprotein complexes: Polycomb repressive complexes 1 and 2 (PRC1 and PRC2). Radulovic et al.6 highlighted the emerging implications of these genes in haematopoietic neoplasms, including myeloid neoplasia. Therefore, changes in the expression profile of individual PcG genes might yield novel information about APL pathogenesis.
We evaluated the PcG gene levels, namely, enhancer of zeste homologue 2 (EZH2), Yin and Yang 1 protein (YY1), BMI1 proto-oncogene, Polycomb ring finger (BMI1) and suppressor of zeste 12 protein homologue (SUZ12), in a cohort of 25 Brazilian children with APL, with and without a FLT3-ITD mutation. In addition, we compared them to those found in patients with other AML subtypes, with and without the FLT3 mutation, to verify whether this mutation status could be associated with the APL epigenetic landscape.
Amongst the 25 patients with APL, eight had additional chromosome abnormalities that mostly involved chromosomes 6, 8, 20 and 21. In the AML group, 39 patients had their karyotype evaluated and the abnormalities detected were as follows: 15 (38·5%) presented with lysine methyltransferase 2A (KMT2A) gene abnormalities; eight (20·5%) presented with non-recurrent chromosome abnormalities; four (10·3%) presented with RUNX1 translocation partner 1 (RUNX1)/RUNXT1 fusion genes; four (10·3%) presented with core binding factor subunit beta (CBFB)/myosin heavy chain 11 (MYH11) fusion genes; four (10·3%) presented with normal karyotypes; three (7·7%) presented with abnormalities in chromosomes 5 and 7; and one (2·4%) presented with no mitosis. In relation to FLT3 status, 21 (30·9%) patients had FLT3-ITD mutations, 13 (61·9%) were patients with APL and eight (38·1%) were patients with AML [AML-M5 (five patients), AML-M2 (two) and AML-M6 (one)] (Clinical data are in Table S1).
Gene expression analyses were carried out on bone marrow samples from healthy donors, and patients with AML and APL by quantitative real-time polymerase chain reaction (RT-qPCR) assay (Methodology shown in Data S1). The EZH2 expression levels were lower in both patients with AML (P = 0·002) and APL (P = 0·014) compared with the healthy donors (Fig 1A). EZH2 overexpression has been reported in acute lymphoblastic leukaemia and different lymphoma subtypes.6 The changes in this important PcG gene component altered the genomic expression programme through chromatin silencing and could present with either tumour-suppressing or oncogenic functions in various mouse models.7 Hence, the role of EZH2 in childhood APL needs to be further elucidated. Although the transcription levels of the SUZ12, YY1 and EZH2 genes were assessed in the APL and AML samples, no significant differences were detected between them (P > 0·05) (Fig 1C,D).
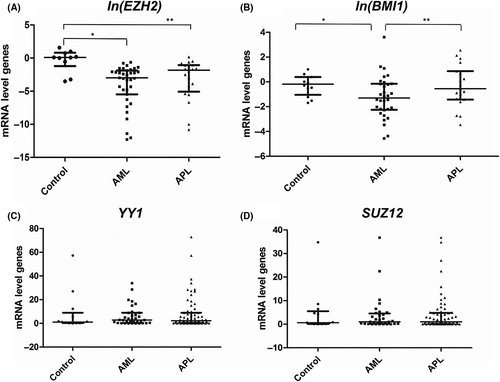
The same observation was noted for the BMI1 expression levels, which were significantly higher in the patients with APL than in those with AML (P = 0·002) (Fig 1B). Although BMI1 overexpression has been reported in paediatric acute lymphoblastic leukaemia, it has not been observed in paediatric AML. Darwish et al.8 reported BMI1 overexpression in different subtypes of adult AML and in two cell lines. However, concerning overexpression, they could not demonstrate a significant difference between adult patients with APL and those with AML subtypes. Thus, our present findings, in paediatric patients, are described for the first time in the literature and may suggest that childhood AML subtypes present different BMI1 expression profiles. Moreover, we corroborate the importance of epigenetic factors in the development of leukaemia, particularly their role in the expression of Polycomb complex genes.
In univariate analysis, we also found that the patients with APL were more highly associated with ages >8 years (P = 0·018), FLT3-ITD mutation status (P = 0·011) and BMI1 (P = 0·02) overexpression levels (Table I). In the multivariate analysis, only FLT3-ITD status (P = 0·03) and BMI1 overexpression (P = 0·003) were markedly correlated with APL occurrence (Table I). The final prediction model was internally validated using a bootstrap resampling procedure9 (Table S2).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95th CI | P | OR | 95th CI | P | |
| WBC <30 000 | 2·343 | 0·761–7·208 | 0·138 | |||
| Platelets <40 000 | 3·306 | 0·987–11·070 | 0·053 | |||
| FLT3-ITD (yes/no) | 5·400 | 1·478–19·729 | 0·011 | 4·258 | 1·122–16·163 | 0·033 |
| Age >8 | 4·052 | 1·266–12·970 | 0·018 | |||
| YY1 | 1·030 | 0·985–1·078 | 0·197 | |||
| EZH2 for each unit | 25·253 | 0·944–675·802 | 0·054 | |||
| ln (BMI1) for each unit | 1·586 | 1·073–2·344 | 0·021 | 1·875 | 1·234–2·850 | 0·003 |
| SUZ12 | 1·016 | 0·941–1·096 | 0·690 | |||
Although APL is a leukaemia that is mainly characterised by the presence of the PML/RARA gene fusion, the present study shows that clinical and laboratory data, such as older age and FLT3-ITD status, may be specifically associated with specific characteristics of paediatric APL. The results from our multivariate analysis, based on our molecular results, suggest that higher levels of the BMI1 gene transcripts and the FLT3-ITD mutation were significantly more highly correlated in paediatric patients with APL than those with other AML subtypes. In short, we present the potential biological significance of our predictive model, in which the BMI1 gene and FLT3 status were the main factors capable of predicting the APL phenotype. Concurrently, we believe that future epigenetics and functional studies will be necessary to allow major inferences about our findings in paediatric APL.
Acknowledgements
This work was supported by PROBRAL (DAAD no. 301/08), (DAAD no. 419/14), MINISTÉRIO DA SAÚDE DO BRASIL and INCT Para o Controle do Câncer (Conselho Nacional de Pesquisa- CNPq no.573806/2008-0).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Amanda F. de Figueiredo, Andre Mencalha, Renata Binato, Maria Luiza M. Silva, Marcelo G.P. Land and Eliana Abdelhay designed the research project. Marcelo G.P. Land performed the data analysis and statistical analysis; Amanda F. de Figueiredo, Roberto R. Capela de Matos and Maria Luiza M. Silva conducted the conventional cytogenetic analyses and performed the fluorescence in situ hybridisation. Thomas Liehr revised the molecular cytogenetic results; Amanda F. de Figueiredo, Gerson M. Ferreira, Renata Binato and Andre Mencalha performed the reverse transcriptase-polymerase chain reaction analysis; Amanda F. de Figueiredo and Andre Mencalha performed the FLT3-ITD screening; Amanda F. de Figueiredo, Roberto R. Capela de Matos, Renata Binato and Andre Mencalha participated in manuscript writing; Maria Luiza M. Silva, Marcelo G.P. Land, Thomas Liehr and Eliana Abdelhay revised the manuscript critically for important intellectual content.