Sudden blastic transformation in treatment-free remission chronic myeloid leukaemia
Chronic myeloid leukaemia (CML) progresses through three distinct phases, referred to as chronic, accelerated and blast. With the emergence of tyrosine kinase inhibitors (TKIs), and more recently the success of TKI discontinuation (Ross et al, 2013; Hehlmann et al, 2014; Cortes et al, 2016; Etienne et al, 2017; Saussele et al, 2018), a “fourth phase”, treatment-free remission (TFR-CML), has now emerged. This phase starts after discontinuation of TKI therapy, and is characterized by continued remission with undetectable – or stable low detectable – disease by the most sensitive method of testing, in the absence of CML-directed treatment. Unlike chronic phase, this phase is characterized by lack of further molecular, cytogenetic and haematological progression. Long-term follow-up and rates of transformation to accelerated or blast phase are not yet well defined. Herein, we describe a case of sudden lymphoid blastic transformation in a patient who had attempted treatment discontinuation.
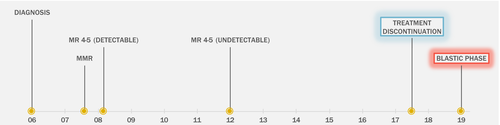
A 54-year-old woman presented in 2006 with one-week history of anorexia, flank pain and 2·7 kg weight loss over the previous month. Work-up revealed a white blood cell (WBC) count of 174 × 109/l. She had 3% blasts and 7% basophils. The spleen was 20 cm below the costal margin. Bone marrow biopsy confirmed chronic phase CML. She was started on hydroxycarbamide and, upon presentation to our institution, she was enrolled in clinical trial evaluating BMS-354825 (dasatinib) in chronic phase CML (NCT00254423). Her treatment was complicated by pleural effusion and the dose was reduced. By 2007 she achieved major molecular response [MMR; ≤0·1% International Standard (IS)] and by 2008 she achieved MR4·5 (≤0·0032% IS), however with persistently detectable transcript, which became undetectable by polymerase chain reaction (PCR) in 2012 (Figure 1). While she remained in sustained MR4·5 with undetectable transcripts, due to recurrence of pleural effusion attributed to dasatinib despite further reduction in dasatinib dose, the patient was offered treatment discontinuation, and she stopped dasatinib in September 2017. The patient sustained MR3·5 after discontinuation with PCR transcripts of 0·03% IS at its highest measured value (Figure S1). The last PCR check was in October 2018, 6 months prior to her presentation with blastic phase transformation. Importantly, the patient had regular follow-up with the primary care provider (PCP) and had a normal full blood count (FBC) with differential in January 2019 (Figure S1). In March 2019, the patient presented to her PCP with abdominal pain, night sweats, bruising and fatigue. FBC revealed a WBC count 53 x109/l with 82% blasts. The patient was transferred to our facility where bone marrow biopsy revealed 90% blasts by morphology in 90% cellular marrow. Flow cytometry was positive for CD10, CD19, CD22(partial), CD34, CD38, cytoplasmic CD79a, HLA-DR and TdT, consistent with precursor-B cell immunophenotype. Fluorescence in situ hybridisation (FISH) analysis was positive for BCR-ABL1 rearrangement [180/200 in interphases]. No analysable metaphases were recovered for karyotype. Using quantitative real-time RT-PCR, the percentage of BCR-ABL1 to ABL1 transcripts was greater than 100% with co-expression of b3a2 and b2a2 BCR-ABL1. There were no mutations detected in the coding sequence of ABL1 kinase domain. An 81-gene mutation panel (Table S1) was negative. Intrathecal chemotherapy was given and no blasts were detected in the CSF. The patient was enrolled on clinical trial of blinatumomab plus ponatinib (NCT03263572), and day14 bone marrow aspiration showed 2% blasts by morphology in a cellular (50-60%) bone marrow. FISH analysis was negative for BCR-ABL1 rearrangement. Flow cytometry was negative for measurable residual disease (MRD), and RT-PCR for BCR-ABL1 transcript was positive at 0·046% [IS]. Day-21 bone marrow showed a hypercellular marrow with FISH analysis negative for BCR-ABL1 rearrangement. Flow cytometry was negative for MRD, and RT-PCR for BCR-ABL1 transcript was undetectable (MR4·5).
Longer exposure to TKIs have been linked to deeper molecular remission (MR4·0 and beyond) in both first- and second-generation TKIs (Hehlmann et al, 2014; Cortes et al, 2016; Saussele et al, 2018). However, due to serious adverse events, such as pleural effusion and cardiovascular events, particularly with second-generation TKIs, as well as other adverse events, such as musculoskeletal pain, sleep disturbance and fatigue (Nazha et al, 2011; Cortes et al, 2017), dose reductions of TKI and full treatment discontinuation have been a desirable option for selected patients (Ross et al, 2013). Moreover, the financial burden on patients as well as on the health care system as the prevalence of CML rises, has led to growing interest in treatment discontinuation (Experts in Chronic Myeloid Leukemia, 2013). Criteria for discontinuation and current recommendations for proper monitoring are discussed in more detail elsewhere (Cortes et al, 2019). However, once treatment discontinuation starts, proper monitoring is crucial as the risk of an unrecognized relapse can be devastating. In older studies, monitoring was done monthly for at least the first 6–12 months, and every 2 months for 6–12 months, and every 3–6 months afterwards. In more recent trials, monitoring was done every 4 weeks for the first 6 months, then every 3 months afterwards (Saußele et al, 2016). National Comprehensive Cancer Network guidelines recommend monthly monitoring for the first 12 months, then every 6 weeks through months 13-24, and every 12 weeks afterwards (Pallera et al, 2016).
In this case, the patient had been on TKI treatment for 11 years and in sustained MMR and MR4·5 with undetectable transcripts for 10 and 5·5 years, respectively, before treatment discontinuation. The sudden transformation to blastic phase CML was not preceded with progression to chronic phase, as the patient had FBC and RT-PCR for BCR-ABL1 checked 2 and 6 months prior to transformation, respectively. Sudden blastic transformation has been reported in less than 1% of patients undergoing treatment with imatinib (Jabbour et al, 2006). It is unclear as to what sustains remission in the absence of CML-directed treatment in TFR-CML, and it is not clear why sudden transformation in this patient occurred. One hypothesis suggests that the cellular immunity, in the form of natural killer cells and cytotoxic T lymphocytes (Jo et al, 2018), may play a role in TFR-CML. It could be the failure of such immune surveillance or breakthrough of the disease, that results in this sudden blastic transformation. Contrary to what is mostly seen in the blastic transformation phase of CML, our case is notable for the rapid and deep response to TKI re-initiation, the lack of ABL1 kinase domain mutations, and the absence of any other molecular mutations associated with progressive disease. It also seems that re-initiation of TKI, in combination with other treatment modalities, in selected cases of blastic phase-CML after TFR-CML can be successful in achieving rapid and deep molecular response, however further reports will be needed.
While this is the first reported case in the literature of sudden blastic transformation in TFR-CML, the frequency of such transformation is likely to be more evident as the practice of treatment discontinuation is gaining momentum. It is important to be informed about the possibility of blastic transformation upon treatment discontinuation, and further studies evaluating this rare event are warranted.
Funding
This study was supported in part by the MD Anderson Cancer Centre Support Grant (CCSG) CA016672, the MD Anderson Cancer Center Leukemia SPORE CA100632, the Charif Souki Cancer Research Fund and generous philanthropic contributions to the MD Anderson Moon Shots Program.
Author contributions
M.A. and G.R.C compiled and summarized the data, M.A., E.J. PV, K.N, K.S, J.C. and N.P. designed treatment strategy and treated the patient. All authors contributed to the writing and reviewing of this letter.




