Lenalidomide as a second-line therapy after failure of hypomethylating agents in patients with myelodysplastic syndrome
Article summary
- Lenalidomide is a drug of choice for del(5q) myelodysplastic syndrome (MDS) but also can be effective in non-del(5q) MDS.
- Lenalidomide showed good responses and survival in hypomethylating agent failure MDS patients without excess toxicities.
A study of lenalidomide for non-5q deletion myelodysplastic syndrome (MDS) patients showed that the transfusion independency rate was 26%, which was relatively acceptable and suggested that lenalidomide could be used for non-5q deletion MDS patients (List et al, 2005; Raza et al, 2008). We conducted a prospective phase II study (VIOLET) of second-line lenalidomide therapy after first-line hypomethylating agent (HMA) failure in MDS.
Patients with MDS according to the World Health Organization classification 2008 (Arber et al, 2016) and treatment failure after HMAs (azacitidine or decitabine), defined as intolerant to hypomethylating agents or progressive disease after HMA, were enrolled and treated with a cyclic dosing regimen of oral lenalidomide (10 mg) once daily on days 1–21 of a 28-day cycle. The general principle for dose modification was treatment interruption and dose modification for grade 3 or greater adverse events. Initial treatment was continued for a minimum of 16 weeks (4 cycles; Figure S1). Treatment could be maintained after 4 cycles in subjects who demonstrated a haematological response or clinical benefit to lenalidomide therapy until intolerance or disease progression at the discretion of the attending physician. Patients could proceed to allogeneic haematopoietic cell transplantation (alloHCT) at any time after the completion of at least 4 cycles of lenalidomide. Of the total 38 patients included in this analysis (Figure S2), 25 (65·8%) patients were male (Table SI) and one patient did not receive any study drug. Among the 19 patients who completed 4 cycles, 11 (57·9%) had their lenalidomide dose reduced to 5 mg; 14 (36·8%) patients had their dose reduced and 16 (42·1%) patients stopped lenalidomide before 4 cycles.
The responses by 4 cycles were: complete response (CR) in 4, stable disease (SD) in 14, and failure/not evaluable in 20 patients (Table SII). Overall response rate (ORR) was 4/38 (10·5%) with clinical benefit (18/38; 47·4%, inclusive of SD) by 4 cycles. The maximal responses were CR in 7, marrow CR (mCR) in 1, partial response (PR) in 3, haematological improvement (HI) in 3, SD in 10 and failure in 14. Maximal ORR was 14/38 (36·8%) patients, with clinical benefit (24/38; 63·2%, inclusive of SD). Median time to response was 4·3 (3·7–5·5) months and the median cycles of lenalidomide was 3 (0–21+). Among 19 patients who completed 4 cycles, the responses were CR in 4 (21·1%), SD in 9 (47·4%) and failure/not evaluable in 6 (31·6%) patients. The maximal responses of these 19 patients were CR in 7, mCR in 1, PR in 3, HI in 1, SD in 6 and failure in 1. Dose reduction of lenalidomide was not associated with maximal ORR (P = 0·859) or clinical benefit (P = 0·618). Among 3 patients with 5q deletion, 1 patient achieved CR but 2 patients failed. Of the 10 patients with a JAK2 mutation, the responses were CR in 2, SD in 3 and failure in 5 patients. Thirteen patients suffered disease progression at a median 3·5 (0·5–18·8) months and progression was unevaluable in 15 patients. Two patients underwent alloHCT after lenalidomide failure. All patients (except for 1 patient who has continued lenalidomide) received supportive care after lenalidomide failure.
Prognostic factors for predicting maximal ORR were evaluated (Table 1). Univariate analysis identified Eastern Cooperative Oncology Group performance status (ECOG PS) = 0 (P = 0·152), International Prognostic Scoring System (IPSS) lower risk at diagnosis (P = 0·001), revised IPSS (IPSS-R) lower risk at diagnosis (P = 0·089), IPSS lower risk at enrolment (P = 0·027), IPSS chromosome good (P = 0·111), IPSS-R chromosome very good/good (P = 0·044) and bone marrow (BM) blasts <5% (P < 0·001) as significant prognostic factors for ORR. However, only BM blasts <5% was a significant prognostic factor for maximal ORR [Hazard ratio (HR) 10·5, 95% confidence interval (CI) 1·514–72·811; P-value 0·017] in multivariate analysis. ORR by 4 cycles and best ORR in patients with BM blasts <5% was 22·2% and 50·0%, respectively. The toxicity profile was tolerable except for haematological adverse events including neutropenia and thrombocytopenia (Table SIII). Febrile neutropenia occurred in 7 (14·9%); asthenia/fatigue in 7 (14·9%); dyspnoea in 6 (12·8%); pruritus in 5 (10·6%) and rash in 5 (10·6%). There was no reported secondary malignancies.
| Maximal overall response | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Univariate | Multivariate | Univariate | Multivariate | ||||
| P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value | |
| Male gender | 0·728 | – | – | – | 0·434 | – | – | – |
| Age <65 years | 0·557 | – | – | – | 0·224 | – | – | – |
| RA/RARS/RCMD | 0·420 | – | – | – | 0·092 | 1·056 | 0·259–4·305 | 0·939 |
| Prior HMA | ||||||||
| CR, mCR, PR | 0·501 | – | – | – | 0·567 | – | – | – |
| HMA duration >8 months | 0·804 | – | – | – | 0·414 | – | – | – |
| Azacitidine | 0·728 | – | – | – | 0·312 | – | – | – |
| JAK2 V617F mutation | 0·268 | 0·566 | – | – | – | |||
| ECOG PS = 0 | 0·152 | 4·117 | 0·411–41·222 | 0·229 | 0·162 | 1·287 | 0·378–4·386 | 0·686 |
| Prognostic score at diagnosis | ||||||||
| IPSS lower risk | 0·001 | 1·0E+9 | 0·0 | 0·998 | 0·116 | 1·620 | 0·409–6·421 | 0·492 |
| IPSS-R lower risk | 0·089 | 3·098 | 0·206–46·591 | 0·414 | 0·108 | 1·272 | 0·446–3·622 | 0·653 |
| Prognostic score at enrolment | ||||||||
| IPSS lower risk | 0·027 | 2·4E+7 | 0·0 | 0·998 | 0·090 | 1·324 | 0·357–4·911 | 0·674 |
| IPSS-R lower risk | 0·331 | – | – | – | 0·053 | 1·206 | 0·385–3·779 | 0·748 |
| RBC transfusion <20 units | 0·712 | – | – | – | 0·321 | – | – | – |
| Laboratory tests at enrolment | ||||||||
| WBC >3 × 106/l | 0·240 | – | – | – | 0·255 | – | – | – |
| Haemoglobin >90 g/l | 0·296 | – | – | – | 0·936 | – | – | – |
| Platelet >100 × 109/l | 0·240 | – | – | – | 0·078 | 1·184 | 0·441–3·181 | 0·737 |
| BM blast <5% | <0·001 | 10·5 | 1·514–72·811 | 0·017 | 0·001 | 4·540 | 1·569–13·135 | 0·005 |
| IPSS chromosome good | 0·111 | 9·965 | 0·162–611·899 | 0·274 | 0·024 | 2·583 | 0·397–16·801 | 0·321 |
| IPSS-R chromosome very good/good | 0·044 | 2·2E+8 | 0·0 | 0·998 | 0·050 | 1·261 | 0·138–11·504 | 0·837 |
| Time from HMA stop to lenalidomide <2·5 months | 1·000 | – | – | – | 0·268 | – | – | – |
- 95% CI, 95% confidence interval; alloHCT, allogeneic haematopoietic cell transplantation; BM, bone marrow; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HMA, hypomethylating agent; HR, hazard ratio; IPSS, International Prognostic Scoring System; IPSS-R, revised International Prognostic Scoring System; mCR, marrow complete response; PR, partial response; RA, refractory anaemia; RARS, refractory anaemia with ringed sideroblasts; RBC, red blood cell; RCMD, refractory cytopenia with multilineage dysplasia; WBC, white blood cell.
Median overall survival (OS) was 15·4 (95% CI 5·9–24·9; Fig 1A–D) months. Causes of deaths were infection in 11 (64·7%), haemorrhage in 2 (11·8%), acute transfusion reaction 1 (5·9%) and unknown in 3 (17·6%). OS of patients who achieved at least HI with lenalidomide (CR, mCR, PR, HI) was significantly longer than that of patients who did not achieve HI (median 24·9 vs. 13·8 months; P = 0·032). Patients with at least SD after lenalidomide showed longer OS than patients who had failed lenalidomide (median 16·0 vs. 4·3 months; P = 0·005). Prognostic factors for predicting maximal OS were evaluated (Table 1). Refractory anaemia (RA)/RA with ring sideroblasts/refractory cytopenia with multilineage dysplasia (P = 0·092), ECOG PS = 0 (P = 0·162), IPSS lower risk at diagnosis (P = 0·116), IPSS-R lower risk at diagnosis (P = 0·108), IPSS lower risk at enrolment (P = 0·090), IPSS-R lower risk at enrolment (P = 0·053), platelet count >100 × 109/l (P = 0·078), BM blasts <5% (P = 0·001), IPSS chromosome good (P = 0·024), IPSS-R chromosome very good/good (P = 0·050) were identified as significant prognostic factors for OS in univariate analysis. Only BM blast <5% remained a significant prognostic factor for maximal OS (HR 4·540, 95% CI 1·569–13·135; P-value 0·005) in multivariate analysis.
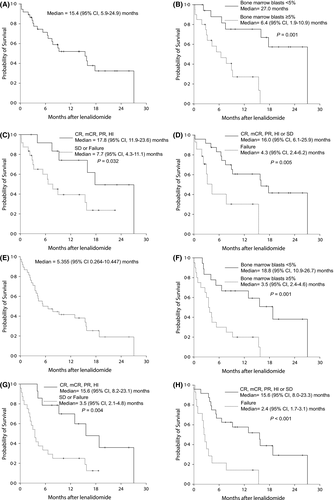
Median progression-free survival (PFS) was 5·355 months (95% CI 0·3–10·4; Fig 1D–H). PFS of patients who achieved at least HI with lenalidomide (CR, mCR, PR, HI) was significantly longer than that of patients who did not achieve HI (median 15·6 vs. 3·5 months; P = 0·004). PFS in patients with at least SD after lenalidomide was longer when compared with patients who had failed lenalidomide (median 15·6 vs. 2·4 months; P < 0·001). Also, BM blasts <5% was of statistical significance in terms of PFS (median 18·8 vs. 3·5 months; P = 0·001). Survival after progression was very short, at a median 2·7 (range, 0·0–10·7) months.
Our study showed a reasonable activity of lenalidomide in MDS patients who were refractory/intolerant to HMA. The dosing schedule was tolerable but required longer therapy for achieving better response. Patients with lower BM blasts could be suitable candidates for lenalidomide. In conclusion, lenalidomide for HMA-failed non-del(5q) MDS was feasible and effective, especially for patients who had low BM blasts.
Acknowledgement
Lenalidomide and study funding were provided by Celgene.
Author contributions
HK designed the study and wrote the manuscript. YJK supervised the study. HK, JHL, WSL, IK, JHM, CWC, HSL, JP, YC, HJS, SHC, KHK, SYK and YJK performed research. HK and JHM performed data analysis.
Conflict of Interest
All authors have no conflict of interest to disclose.




