Real world data of the impact of first cycle daratumumab on multiple myeloma and AL amyloidosis services
Daratumumab, a monoclonal antibody targeting CD38, is indicated for the treatment of patients with relapsed multiple myeloma (MM) either as monotherapy (Lonial et al, 2016) or in combination with either lenalidomide or bortezomib with dexamethasone (Dimopoulos et al, 2016; Palumbo et al, 2016). However, worldwide usage is currently limited by reimbursement. The most common adverse event was infusion-related reaction (IRR). In the phase 2 trial, the overall response rate was 29·2%. Grade 1–2 IRRs were reported in 42% (16 mg/kg) with first cycle median infusion times of: first infusion: 7·0 h (range 1·5–14·3) for the first infusion, 4·2 h (2·7–8·5) for the second infusion and 3·4 h (1·1–6·7) for subsequent infusions (Lonial et al, 2016). However as per summary of product characteristics recommendations, the fastest infusion should be 6·5 h (https://www.medicines.org.uk/emc/medicine/32088), longer than the fastest infusion time of 1·5 h reported. Consequently, real-world data may be different. With increased demands on chemotherapy infusion units due to immune-oncology agents, capacity is planned by chair time. We therefore report our initial experience with the first cycle of Daratumumab.
All patients with MM and AL amyloidosis treated with daratumumab at a single UK site (both National Health Service and private patients) were included. Infusion times and in-patient admission were recorded from nursing documentation and IRRs were graded by Common Terminology Criteria for Adverse Events v4.0 criteria (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Response rates were not collected as this study was restricted to the first cycle. Patients received Daratumumab weekly for the first cycle (MM: 16 mg/kg; AL amyloidosis: 8 mg/kg initially over 12 h, 16 mg/kg from second dose). All received pre-medications approximately one hour before the infusion, including paracetamol, anti-histamine, corticosteroid and, according to clinical discretion, montelukast (all with AL amyloidosis). Corticosteroids were given for the 2 days following infusions for the first cycle. Infusions were given on a day case basis where possible and completed as an in-patient. Amyloid patients had planned in-patient first dose infusions. First infusions were given in 1000 ml at 50 ml/h, increased in 50 ml/h increments to 200 ml/h as tolerated (except AL amyloid). Second infusions were 500 ml at 50 ml/h and increased to 200 ml/h. Subsequent 500 ml infusions were at 100 ml/h and increased to 200 ml/h.
Between November 2014 to February 2017, 28 patients received their first cycle of daratumumab (MM, 24; AL amyloidosis, 4), ranging from newly diagnosed to multiply relapsed patients and AL amyloidosis with renal and/or cardiac involvement (Table 1). Sixteen (57%) received daratumumab monotherapy, 10 (36%) with lenalidomide and dexamethasone and 2 (7%) with pomalidomide and dexamethasone. 46% reported baseline co-morbidities including 9 (23%) with cardiovascular (mainly hypertension) and 3 (7%) with respiratory disease (asthma, chronic airways disease).
| Demographic | N = 28 |
|---|---|
| Median age (range), years | 66·3 (49–77) |
| Gender | |
| Male | 12 (43%) |
| Female | 16 (57%) |
| International Staging System grade | |
| I | 11 (39%) |
| II | 6 (22%) |
| III | 11 (39%) |
| Isotype | |
| IgG Kappa; Lambda | 9 (32%); 3 (11%) |
| IgA Kappa; Lambda | 6 (21%); 2 (7%) |
| Light chain only | 4 (14%) |
| AL Amyloidosis | 4 (14%) |
| Renal | 2 |
| Cardiac | 4 (all Mayo stage III disease) |
| Liver | 1 |
| Co-morbidities | 13 (46%) |
| Cardiac | 9 (23%) |
| Respiratory | 3 (7%) |
| Asthma | 2 (5%) |
| Chronic obstructive pulmonary disease | 1 (3%) |
| Median time from diagnosis to first dose of daratumumab (range), months | 35 (0·7–150) |
| Median prior lines (range) | 2 (0–6) |
| Prior immunomodulatory drug | 23 (82%) |
| Prior proteasome inhibitor | 25 (89·2%) |
| Prior autologous stem cell transplantation | 15 (53·5%) |
| Daratumumab schedule (first cycle): | |
| Monotherapy | 16 (57%) |
| With lenalidomide and dexamethasone | 10 (36%) |
| With pomalidomide and dexamethasone | 2 (7%) |
The median infusion times (range) for MM patients were (Fig 1A): first dose 9·7 h (5·4–17·0), second dose 4·1 h (2·8–7·8), 3rd dose 3·6 h (2·8–6), 4th dose 3·5 h (3·2–6·0). Longer infusion times correlated with occurrence of IRRs. AL Amyloid patients were treated more cautiously due to organ involvement and overall frailty. Consequently, the median infusion times (range) were: first dose 12·3 h (12·0–12·5), second dose 8·2 h (6·0–12·0); third dose 6·0 h (6·0–7·1); fourth dose 6·0 h (3·2–7·6). IRRs were reported in 10 patients (36%), the majority (n = 9) occurred during the first dose with one reported during the second dose. All reactions were grade 1–2: nasal congestion (n = 3), sore throat (n = 2), dyspnoea (n = 2), tachycardia (n = 2), cough (n = 1), voice alteration (n = 1), paraesthesia (n = 1) and diarrhoea (n = 1). All were managed with interruption of the infusion and medical management (predominantly chlorpheniramine and paracetamol) then restarting at a slower rate. No patients discontinued treatment due to IRRs. All patients required in-patient admission to complete their first dose. Subsequent doses were planned as day cases unless the patient was in hospital due to other reasons (complications of MM) or had AL amyloidosis. Overall, infusions as a day case were administered in 19 (68%, second dose), 19 (68%, third dose) and 20 (71%, fourth dose) patients.
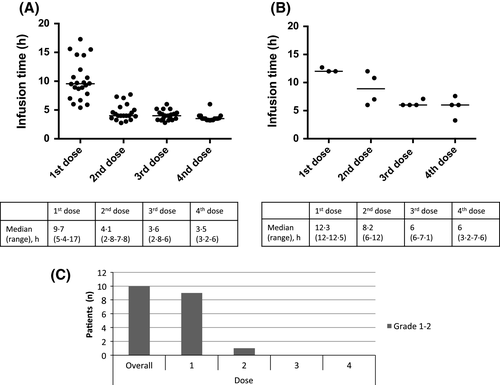
Median infusion times for MM were longer than reported (Lonial et al, 2016). Many were frail due to MM, leading to more cautious infusion rate escalations. There is very limited published data for daratumumab in patients with AL amyloidosis and infusion times have not been previously reported. Therefore, these patients were treated with deliberate caution as they had more advanced AL amyloidosis than the cases previously published (Sher et al, 2016).
In-patient admission was required for all first doses due to the length of the infusion time and day care opening times; however 68% received all subsequent doses as a day case. Those with a poor baseline performance status were more likely to be in-patients at the time of further doses. All patients with AL amyloidosis were planned to have the first dose as an in-patient.
The need for in-patient beds for all first doses would have a major impact; however optimisation of the treatment pathway may allow many first doses to be given as day cases. Once prepared, daratumumab is stable for 24 h (2–8°C) or 15 h (room temperature). Therefore, preparation the day before would allow the first infusion to commence early, potentially allowing the patient to go home. Montelukast, a leukotriene receptor antagonist has been reported to reduce IRRs and consequently quicken first infusion times to 6·7 h (median) (Chari et al, 2016).
Other CD38 monoclonal antibodies are under investigation for MM. Isatuximab has a reported first dose infusion time of 5·3 h and second dose of 4·4 h (Martin et al, 2015) and MOR202 has reported a 2-h infusion time (Raab et al, 2016). Mature phase 3 efficacy and safety data is still emerging, but both may have a future role in MM and AL amyloid. The sub-cutaneous formulation of daratumumab is currently under development (Usmani et al, 2016).
In conclusion, we report the infusion times for the first cycle of daratumumab for MM and AL amyloid patients in a real world setting. All patients required in-patient admission for the first dose; however with optimisation of the treatment pathway, such as preparing daratumumab the day before and the use of montelukast, infusion times are likely to improve such that most may be able to receive the first dose as a day case.
Acknowledgments
ED presented the abstract at the International Myeloma Workshop, New Delhi, 2017. RP and KY are supported by the UCL/UCLH NIHR Biomedical research centre.
Author Contributions
RP, ED, DP designed the study. ED, SA, DP collected and analysed the data. NR, CK, AW, SM, KY, RP contributed clinical data. RP, ED, AW wrote the manuscript, all authors reviewed and approved the final manuscript.




