Increased fracture rates in people with haemophilia: a 10-year single institution retrospective analysis
People with haemophilia are at higher risk for reduced bone mineral density (BMD) compared to the general population (Barnes et al, 2004; Wallny et al, 2007; Gerstner et al, 2009; Iorio et al, 2010). It is currently unclear how this predilection for decreased BMD translates into fracture rates. We carried out a decade-long retrospective analysis in a large cohort of haemophilia subjects and found that the rate of fractures was significantly greater than in the general population. We also investigated the impact of haemophilia type, severity and presence of inhibitor and determined that people with severe factor deficiency had an increased risk of fractures. This is the first study in the developed world comparing fracture rates in people with haemophilia to the general population.
We performed a retrospective analysis of all male patients with haemophilia A and B that were seen at The Hemophilia Center at Oregon Health & Science University (OHSU) from 1 January 2003 to 31 December 2012. The OHSU institutional review board approved the use of the clinical database for this project. Our retrospective cohort included a total of 382 patients (316 with haemophilia A and 66 with haemophilia B). Pertinent baseline characteristics are outlined in Table 1. Data was gathered from a total of 2053 patient years of observation. Incidence rates (IR) and relative risk (RR) of bone fractures were calculated. Our results were compared to those from a large prospective cohort study in the United States that measured gender- and age-specific fracture rates in the general population (Brinker & O'Connor, 2004).
| Haemophilia A (N = 316) | Haemophilia B (N = 66) | |
|---|---|---|
| Age, years; mean (SD) | 20 (18) | 26 (21) |
| Severity; n (%) | ||
| Mild | 108 (34) | 14 (21) |
| Moderate | 42 (13) | 28 (42) |
| Severe | 165 (52) | 24 (36) |
| HIV; n (%) | ||
| Positive | 35 (11) | 3 (5) |
| Hepatitis C; n (%) | ||
| Positive | 78 (25) | 22 (33) |
| Negative | 131 (42) | 21 (32) |
| Unknown | 106 (34) | 23 (35) |
| Inhibitor; n (%) | 27 (8·6) | 1 (1·5) |
- SD, standard deviation; HIV, human immunodeficiency virus.
The fracture incidence in haemophilia subjects was considerably higher, at 24·8 fractures per 1000 patient-years, than in the control population, at 9·6 fractures per 1000 patient-years (Fig 1A). This correlated to a significantly greater relative risk of fracture in our haemophilia population compared with the control population [P < 0·0001, RR 10·7, 95% confidence interval (CI): 8·2–14·1]. Additionally, subjects with mild to moderate haemophilia had a significantly decreased risk of fracture compared to those with severe disease (P = 0·038, RR 0·56, 95% CI: 0·32–0·97, Fig 1B).
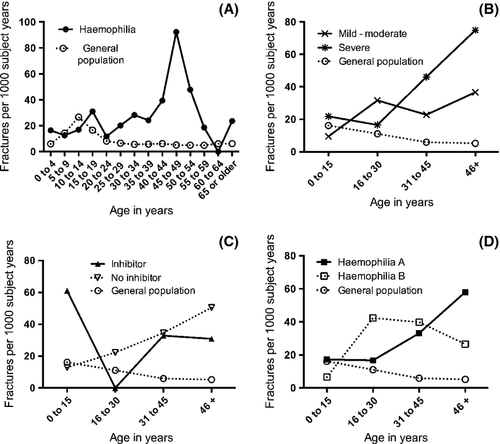
There was a trend toward higher fracture incidence in haemophilia subjects with an inhibitor compared to those with no inhibitor, at 35·9 vs. 21·7 fractures per 1000 patient-years, but no statistically significant difference in fracture risk was found (P = 0·12, RR = 1·84, 95% CI: 0·86–3·97, Fig 1C). The incidence of bone fractures was similar between patients with haemophilia A and B with 22·9 and 23·0 fractures per 1000 patient-years respectively (Fig 1D). Fracture risk was also not significantly different between the haemophilia A and B cohorts (P = 0·95, RR 0·98, 95% CI: 0·48–2·00).
In our cohort, fracture risk increased with age. People older than 31 years were more than twice as likely to suffer a fracture than those younger than 31 years of age (P = 0·0047, RR 2·15, 95% CI: 1·26–3·65). Age also had a dose effect, with risk of fracture increasing about 1·3% per year of age (P = 0·037, 95% CI: 0·1–2·5%).
Hepatitis C and human immunodeficiency virus (HIV) infection were not associated with fracture risk in this study. In an age-matched analysis, we found no significant differences in fracture risk between haemophilia patients with and without hepatitis C (P = 0·45, 95% CI: 0·59–3·25) or HIV (P = 0·48, 95% CI: 0·61–2·85).
The magnitude of fracture risk in people with haemophilia and how this compares to the general population was previously unknown. We found a markedly increased rate of bone fractures in our cohort of haemophilia subjects with a RR of 10·7 compared with the general population. Fracture risk was elevated across all severities of haemophilia (Fig 1A,B). This is the first study of its kind in the developed world. The only prior investigation was a small (N = 50) case–controlled study from western India, which showed increased fracture rates among young people with haemophilia (Nair et al, 2007).
Bone disease in haemophilia has been attributed to bleeding sequelae from chronic inflammation and decreased weight-bearing activity from painful and debilitating haemarthroses (Ghosh & Shrimati, 2012). Chronic hepatitis C and HIV infections are associated with poor bone health and have a high incidence in the haemophilia population secondary to past exposure to contaminated blood products (Lo et al, 2012). However, within our haemophilia population, people with and without HIV and hepatitis C infections had no significant differences in fracture risk. Thus, the increased fracture rates we observed are not due to HIV and/or hepatitis C infection alone.
Recent studies out of our laboratory have shown that factor VIII-knockout mice without evidence of bleeding had decreased BMD and biomechanical strength compared with controls suggesting that factor deficiency has a direct impact on skeletal health (Liel et al, 2012; Recht et al, 2013). In the present study, severe haemophilia subjects were 44% more likely to have a fracture than those with mild to moderate disease, suggesting that the factor levels correlate with bone strength.
Despite a known propensity for decreased BMD in people with haemophilia, there is a paucity of data describing how this translates into fracture risk. Our cohort study demonstrates that fracture rates are elevated significantly in haemophilia subjects and represents an important co-morbidity to be considered when caring for this population. More studies are needed to address how clinical bone health interventions (including calcium and vitamin D supplementation, weight-bearing exercise and bisphosphonate therapy) and factor replacement strategies may affect fracture rates. Our findings of increased fracture risk with increasing severity of haemophilia coupled with the evidence for bleeding-independent mechanisms of decreased skeletal health suggest factor replacement may directly impact bone health and fracture risk in people with haemophilia.
Authors contributions
S.C.L., M.S.L., M.R., and J.A.T. designed the initial research project. S.C.L., M.S.L., N.D.G., P.S., and J.A.T. performed retrospective data analysis. N.D.G., S.C.L., P.S., M.R. and J.A.T. wrote the paper.
Conflict of interest
Jason A. Taylor receives haemophilia research funding from Novo Nordisk, Kedrion Biopharma, and Baxter BioScience. Michael Recht, Meghan S. Liel, Nathan D. Gay, Sarah C. Lee these following authors have no competing financial interests.




