Prognostic impact of monocyte count at presentation in mantle cell lymphoma
We read with interest the recent report describing that peripheral blood absolute monocyte count (AMC) at the time of diagnosis of mantle cell lymphoma (MCL) is prognostic for long term outcome (Aprile von Hohenstaufen et al, 2013). The authors hypothesized that interaction between the MCL cells and host monocyte populations are reflective of tumour biology and therefore the rate of tumour progression and sensitivity to chemotherapy when treatment is indicated.
The impact of microenvironment and AMC as its surrogate marker on survival outcomes is increasingly recognized as an important prognostic factor in many tumour types. (Porrata et al, 2013). Monocyte subpopulations including circulating type II suppressive monocytes have been repeatedly identified as having prognostic impact in many types of lymphoma (Lin et al, 2011). However, the prognostic impact of AMC may be abrogated by the introduction of rituximab-containing chemotherapy in the context of follicular lymphoma (Watanabe et al, 2013)
In MCL, the MCL International Prognostic Index (MIPI; Hoster et al, 2008) and biological MIPI scores (Geisler et al, 2012) have incorporated host and tumour-related factors in risk stratifying patients with MCL. Additional novel biomarkers are likely to add to the stratification sensitivity and allow refinement of treatment decision-making.
Rituximab, in combination with hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone), has shown excellent complete response rates of >85% in previously untreated patients with MCL. Despite this, a steady pattern of ongoing disease recurrence without an evident plateau in the progression-free survival (PFS) curve is seen (Romaguera et al, 2010) Several prospective trials have reported favourable outcomes with upfront auto-haematopoietic stem cell transplantation consolidation in MCL cases receiving rituximab-containing induction regimens (Geisler et al, 2008; Damon et al, 2009)
Given that the findings of Aprile von Hohenstaufen et al, (2013) were derived from a heterogeneous population of patients with MCL, who received a wide range of therapeutic interventions of varied intensity, including five who received no treatment, we wished to explore whether the peripheral blood AMC at diagnosis retained its prognostic power in an uniformly treated cohort of patients with MCL.
At our institutions, 37 sequential patients diagnosed with MCL have received a standardized approach using either HyperCVAD+rituximab (n = 35) or if co-morbidity precluded this regimen, intensified CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)+rituximab (n = 2) followed by autologous stem cell transplantation (ASCT) with busulphan-melphalan (BuMel) conditioning in chemo-responsive patients (Ritchie et al, 2006). The median age was 54 years (range 29–69) and 30 (81%) patients were males.
Of these patients, 78% had stage four disease, the median β2-microglobulin level was 2·3 mg/l (normal range 0·8–3·0) and 33% of 36 evaluable patients had an intermediate/high risk MIPI score. The median AMC at diagnosis in our cohort was 0·4 × 109/l (range 0·1–1·46). With initial therapy, 94% achieved complete remission before ASCT. One patient died during transplant and 78% (n = 29) were alive at last follow-up with a median follow up of 55 (range 5·9–137) months. The median PFS of the cohort is 64 months and, overall, 13 (35·1%) patients have relapsed but with no relapses seen beyond 64 months despite nine patients remaining at risk. The estimated overall survival (OS) at 5 years was 84·6% (Fig 1).
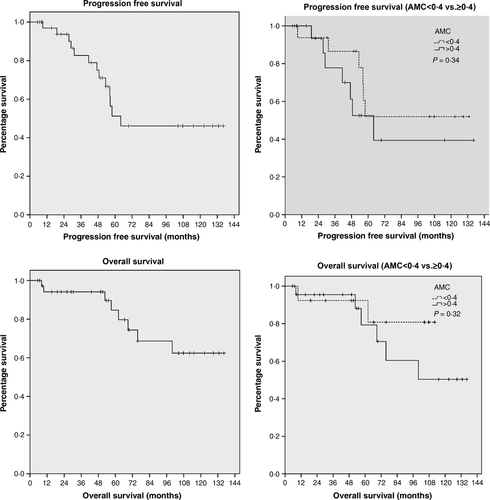
In our cohort, we observed that, in keeping with previously published data, the MIPI (low risk versus intermediate/high risk) defined groups with significantly different PFS and OS. However, in contrast to the findings by Aprile von Hohenstaufen et al, (2013), we found that there was no impact on either the PFS or OS in this cohort when analysed by a peripheral blood AMC at diagnosis dichotomized at the median of 0·4 × 109/l (Fig 1) or at 0·5 × 109/l, as described in the article.
Clearly there are substantial differences in the treatment schedule between the cohort reported by Aprile von Hohenstaufen et al, (2013) and ours. Among the 97 patients reported in the previous study (Aprile von Hohenstaufen et al, 2013), five did not receive any treatment and of those treated with chemotherapy, 65% did not receive rituximab as a part of their treatment whereas only 47% were treated with an anthracycline-containing regimen, and only 6% had an ASCT in first response.
Our data suggest that the prognostic power of AMC and hence the influence of microenvironment on survival outcomes may be overcome by intensive rituximab-containing chemotherapy and ASCT. Furthermore, the excellent PFS and OS in our cohort, with some patients in ongoing remission beyond 10 years suggest that cure may be feasible in some patients with this dose intensive regimen.




