British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018
Abstract
Linked Editorial: Wang and Bagot. Br J Dermatol 2019; 180:443–444.
1 Purpose and scope
The overall objective of the guideline is to provide up-to-date, evidence-based recommendations on the management of primary cutaneous lymphoma in the U.K. The document aims to: (i) offer an appraisal of all relevant literature up to February 2018 focusing on any key developments; (ii) address important, practical clinical questions relating to the primary guideline objective; (iii) provide guideline recommendations with, where appropriate, some health economic implications; and (iv) discuss potential developments and future directions.
The guideline is presented as a detailed review with highlighted recommendations for practical use in the clinic (see section 12). A patient information leaflet on mycosis fungoides is available on the British Association of Dermatologists (BAD) website: www.bad.org.uk/for-the-public/patient-information-leaflets. Further information can also be found at www.lymphomas.org.uk/about-lymphoma.
1.1 Exclusions
These guidelines do not cover patients presenting with or found to have skin involvement as part of a systemic lymphoma.
2 Methodology
This set of guidelines has been developed using the BAD's recommended methodology1 and with reference to the Appraisal of Guidelines Research and Evaluation (AGREE II) instrument (www.agreetrust.org).2 The recommendations were developed for implementation in the National Health Service using a process of considered judgement based on the evidence (Appendix S1; see Supporting Information). Targeted literature searches were carried out in the PubMed, MEDLINE and Embase databases and the Cochrane Library, for meta-analyses, randomized and nonrandomized controlled clinical trials, case series, case reports, open studies and cohort studies on primary cutaneous lymphoma and specified treatments to February 2018. The search terms and strategies are detailed in Appendix S2 (see Supporting Information). Additional relevant references were also retrieved from citations in the reviewed literature.
All identified English-language titles were screened and those relevant for first-round inclusion were selected for further scrutiny. The abstracts for the shortlisted references were then reviewed and the full papers of relevant material were obtained; disagreements in the final selections were resolved by discussion with the entire Guideline Development Group (GDG). The structure of the 2003 guidelines was then discussed and re-evaluated, with headings and subheadings revised where appropriate; different coauthors were allocated separate subsections. Each coauthor then performed a detailed appraisal of the selected literature with discussions within the GDG to resolve any issues, for example the quality of evidence and making the appropriate recommendations. All subsections were subsequently collated, circulated within the GDG and edited to produce the final guideline.
3 Introduction
3.1 Incidence and epidemiology
Primary cutaneous lymphomas represent a heterogeneous group of extranodal non-Hodgkin lymphomas, consisting of cutaneous B-cell lymphoma (CBCL) and cutaneous T-cell lymphoma (CTCL). CTCL represents around 70% and CBCL about 30% of primary cutaneous lymphomas. Primary cutaneous lymphomas have been defined by the European Organisation for Research and Treatment of Cancer (EORTC)–World Health Organization (WHO) classification3, 4 and incorporated into the most recent WHO classification (Table 1).5
Cutaneous B-cell lymphoma
|
Cutaneous T-cell lymphoma
|
- aClassified as extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the 2016 version. bChanges from the 2008 version.
These guidelines are broadly consistent with U.S. National Comprehensive Cancer Network guidelines (www.nccn.org), and the EORTC and European Society for Medical Oncology clinical practice guidelines.4, 6, 7
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common clinicopathological subtypes of CTCL.3 A recent U.K. National Cancer Information Network audit of newly diagnosed cases of CTCL from 2009 to 2013 suggests an annual incidence of 0·7 per 100 000 U.K. population, with a significantly higher male incidence (1·6 : 1·0) and a peak age between 50 and 74 years. Completion of staging data was poor (7% in 2009, 20% in 2013).8 While the peak incidence of MF/SS occurs in the 50–74-year age group, approximately 20% of patients are diagnosed with CTCL aged 25–49 years, and MF may rarely present in childhood. Increasing age is a poor prognostic factor and the vast majority of younger patients present with early-stage disease where the outlook is excellent. However, management of younger adults with intermediate or advanced stages of CTCL may require a more aggressive approach than usual.
MF and SS are closely related both clinically and pathogenetically but are distinct from other relatively rare types of primary CTCL. Of these rarer CTCLs, the primary cutaneous CD30+ lymphoproliferative disorders have an excellent prognosis, while some of the other CTCL variants have poor prognosis and are more closely related to peripheral T-cell lymphomas (PTCLs).9
CBCL is classified into three main types: the more common, low-grade primary cutaneous follicle centre lymphoma (PCFCL) and primary cutaneous marginal zone lymphoma (PCMZL), which can occur at any age, and the rare, primary cutaneous, diffuse, large B-cell lymphoma (PCLBCL) leg type, which occurs typically in elderly female patients.3
A list of the abbreviations used in this guideline is available in Appendix S3 (see Supporting Information).
4 Diagnosis, staging, prognosis and end points
4.1 Diagnosis
The diagnosis of different cutaneous lymphoma variants is based on an assessment of the clinical and pathological features, which forms the basis for the National Cancer Information Network minimum dataset for primary cutaneous lymphomas. MF is characterized by a distinct clinical morphology consisting of polymorphic patches or plaques, with the more advanced cutaneous stage associated with tumours and erythroderma; peripheral adenopathy may or may not be present. SS is much less common (5% of CTCL) and is defined according to the WHO criteria by the presence of erythroderma, peripheral lymphadenopathy and Sézary cells comprising > 1000 cells μL−1, or with a CD4 : CD8 ratio > 10 or loss of one or more T-cell antigens on flow cytometry and a T-cell clone (stage B2). Human T-lymphotropic virus-1 serology should be checked in all patients to exclude adult T-cell leukaemia/lymphoma, which can mimic CTCL. As for all new patients with lymphoma, HIV status should also be checked, in line with the Department of Health recommendations.10
Repeated biopsies may be required to establish the diagnosis, especially in MF. MF has a characteristic CD4+ epidermotropic infiltrate of T cells, although rare CD8+ phenotypic variants occur. T-cell receptor gene analysis is an important diagnostic and staging technique especially in patients with MF/SS, where analysis of peripheral blood provides critical prognostic information. It is essential that results are always interpreted in the context of the clinicopathological features.
Patients with other CTCL variants do not have the characteristic polymorphic patches and plaques seen in MF and may present with cutaneous tumours, nodules or subcutaneous infiltration with distinctive pathology and immunophenotypic findings.3 Lymphomatoid papulosis (LyP) represents part of a spectrum of primary cutaneous CD30+ lymphoproliferative disorders and is defined by a history of recurrent, self-healing papules and nodules, and characteristic pathology.
CBCL presents with nodules, plaques and tumours with a characteristic pathology and immunophenotype, and site localization can be an important distinguishing feature. A definitive diagnosis can only be made on a representative biopsy, preferably an excision or elliptical biopsy. Borrelia burgdorferi infection has been reported in association with PCMZL, and serology is indicated in all patients.11, 12 Clonal IgH gene rearrangements can aid diagnosis.13
4.2 Staging
Staging investigations should include a computed tomography (CT) scan of the neck, chest, abdomen and pelvis in all patients with CTCL and CBCL, with the exception of those with early stages of MF (IA/IB) and LyP unless there is palpable lymphadenopathy.14, 15 Positron emission tomography (PET) CT should be considered for patients with PCLBCL leg type in keeping with local policies for PET CT in aggressive B-cell lymphomas.16 The value of PET scans in MF/SS and other CTCL variants remains unclear at present, although this may indicate which nodal basin to sample.17, 18 In MF and SS, morphological assessment of peripheral blood for Sézary cells is required (defined as lymphocytes with hyperconvoluted nuclei). This should be carried out by flow cytometry to quantify the total number of Sézary cells and T-cell-receptor gene analysis.
In SS, an identical clonal T-cell-receptor gene rearrangement is detected in skin and peripheral blood in combination with (i) a total Sézary cell count > 1000 cells μL−1, (ii) an expanded CD4+ T-cell population with a CD4 : CD8 ratio ≥ 10 or (iii) an expanded CD4+ T-cell population with abnormal immunophenotype including CD4+ CD7− (> 30%) or CD4+ CD26− (> 40%). Bone marrow aspirate and trephine biopsies are rarely indicated in MF, SS and LyP, unless there is an unexplained haematological abnormality, but they are more commonly required for other CTCL variants and for high-grade PCLBCL leg type. Bone marrow biopsy and aspirate is considered optional for low-grade PCMZL but should be considered for PCFCL, as systemic follicle centre lymphoma with secondary cutaneous involvement may occur.19, 20 Serum lactate dehydrogenase has prognostic significance and should be checked at diagnosis.21
Existing staging systems for nodal lymphomas are not applicable for primary cutaneous lymphomas. The original TNM (primary tumour, regional nodes, metastasis) and clinical staging system for MF and SS22 has now been replaced by an International Society for Cutaneous Lymphomas (ISCL)–EORTC revised staging system,23 which distinguishes patches and plaques, incorporates a molecular assessment of lymph node and peripheral blood, and provides a quantitative method for assessing peripheral blood disease in SS (Tables S1–S3; see Supporting Information).24 These revised staging systems have been adopted by the American Joint Committee on Cancer.25
4.3 Prognosis
Recent studies have defined prognostic risks with CTCL and CBCL, providing rates for overall survival (OS), disease-specific survival and progression-free survival (PFS) (Tables 2 and 3).21, 26-30
| Stage | Overall survival | Disease-free survival | Progression-free survival | ||||
|---|---|---|---|---|---|---|---|
| 5 year | 10 year | 5 year | 10 year | 5 year | 10 year | ||
| IA | T1a | 97% | 91% | 100% | 96% | 95% | 91% |
| T1b | 91% | 80% | 96% | 92% | 88% | 82% | |
| IB | T2a | 85% | 75% | 90% | 82% | 85% | 72% |
| T2b | 81% | 64% | 86% | 72% | 75% | 56% | |
| IIA | 78% | 52% | 89% | 67% | 83% | 67% | |
| IIB | 40–65% | 34% | 50–80% | 42% | 52% | 42% | |
| IIIA | 47% | 37% | 54% | 45% | 47% | 38% | |
| IIIB | 40% | 25% | 48% | 45% | 18% | 27% | |
| IVA1 | 37% | 18% | 41% | 20% | 38% | 17% | |
| IVA2 | 18% | 15% | 23% | 20% | 23% | 20% | |
| IVB | 18% | – | 18–20% | – | 18% | – | |
| 5-Year survival | Overall | Disease specific |
|---|---|---|
| pcCD30+ anaplastic large cell lymphoma | 90% | NA |
| pcCD30+ lymphomatoid papulosis | 100% | NA |
| pc marginal zone lymphoma MZL | > 95% | 100% |
| pc follicle centre lymphoma | > 95% | > 95% |
| pc diffuse large B-cell lymphoma | 36–45% | 61% |
- pc, primary cutaneous; NA, not available.
Unlike CTCL, prognosis for CBCL is determined by the type of primary CBCL, not its stage (Table 3).3, 11, 31-33 Most patients with early stages of MF, primary cutaneous CD30+ lymphoproliferative disorders, and marginal zone and follicle centre lymphomas have a good prognosis, but disease progression and disease-specific mortality may occur. The 5- and 10-year OS rates for MF are 80% and 57%, respectively, with disease-specific survival rates of 89% and 75% at 5 and 10 years, respectively.27 Approximately 25% of early-stage patients develop disease progression.30 In contrast, patients with SS have an 11% 5-year survival, with a median survival of 32 months from diagnosis. Patients with low-grade PCMZL and PCFCL have 5-year OS rates approaching 100%, but for PCLBCL leg type the prognosis is poor, with 5-year OS rates of between 36% and 45%.
Several multivariate analyses of large cohorts of patients with MF/SS have identified potential independent prognostic factors at diagnosis. A study of 1502 U.K. patients identified separate prognostic models for early and advanced MF.30, 34 Specifically for early-stage disease, male sex, age (≥ 60 years), presence of plaques (T1b/T2b), histological evidence of folliculotropic disease and palpable or histologically confirmed dermatopathic peripheral nodes (N1/Nx) were adverse factors for progression and survival. For late-stage disease, nodal involvement (N2/3) and blood and visceral disease are additional important prognostic factors. Patients with stage IIB disease have a poor prognosis, but published data show marked variation in survival, perhaps reflecting the heterogeneous nature of tumour-stage disease in terms of both tumour burden and biology.26, 27 The probability of survival for patients with stage III erythrodermic MF, without evidence of lymph node or peripheral blood involvement, is broadly similar to that for stage IIB MF.
A Cutaneous Lymphoma International Consortium study of prognostic factors in 1275 patients with advanced MF/SS from 29 international sites confirmed similar survival probability for patients with stage IIB and III disease, with significantly worse survival in stage IV.21 Four independent prognostic factors were identified, namely age > 60 years, large cell transformation in skin, raised lactate dehydrogenase and stage IV disease. A prognostic index model combining these four factors identified three risk groups with significantly different 5-year survival rates: low risk (68%), intermediate risk (44%) and high risk (28%).21 This prognostic index is in the process of being prospectively validated.
4.4 Disease response and end points
Historically, most trials in cutaneous lymphomas have been retrospective reports of case series or phase II studies, with no standardized definitions of end points or response criteria. Consequently, the detail and quality of response assessments have been variable, and most studies have reported responses based on assessment of skin disease alone. This has led to the publication of ISCL–EORTC consensus criteria for disease end points, and assessment tools for MF/SS, which are based on utilization of the modified Severity Weighted Assessment Tool technique (Table S4; see Supporting Information)35 and include a global scoring system based on responses of cutaneous and noncutaneous disease (Table S5; see Supporting Information).35
In addition, a similar consensus proposal is in preparation for other primary non-MF/non-SS cutaneous lymphomas. These standards for disease assessment should now be routinely used for monitoring clinical outcomes in all patients with primary cutaneous lymphomas in conjunction with quality-of-life measurements.
5 National Institute for Health and Care Excellence Improving Outcomes Guidance for primary cutaneous lymphomas
5.1 Specialist multidisciplinary teams
Primary cutaneous lymphomas are rare and heterogeneous malignancies that can present considerable diagnostic difficulties. In addition, effective standard treatments have yet to be defined for many variants, particularly those with a poor prognosis. As such, it is critical that patients have effective pathways of care that allow rapid access to centres with broad experience of primary cutaneous lymphomas. Such pathways have been defined in the National Institute for Health and Care Excellence (NICE) Improving Outcomes Guidance (IOG) for skin cancers including melanoma (Table 4).36, 37 Patients outside the groups where review by the supranetwork skin lymphoma multidisciplinary team (MDT) is mandated may benefit from review by this team, especially where there is difficulty in establishing a diagnosis or the patient's management is proving challenging, depending on the expertise of the specialist skin MDT.
| Category | Specialist MDT | Supranetwork MDT |
|---|---|---|
| Stage IA–IIA MF | Required | a |
| Stage IA–IIA MF refractory to skin-directed therapy | Required | Essential |
| Stage III–IVA1 erythrodermic MF/SS | Required | Essential |
| Stage IIB–IV MF/SS | Required | Essential |
| pcCD30+ lymphoproliferative disorders | Required | a |
| CTCL variants | Required | Essential |
| CBCL | Required | a |
- MF, mycosis fungoides; SS, Sézary syndrome; pc, primary cutaneous; CTCL, cutaneous T-cell lymphoma; CBCL, cutaneous B-cell lymphoma; MDT, multidisciplinary team. aThese patients may benefit from review by the supranetwork skin lymphoma MDT. Table adapted from the National Institute for Health and Care Excellence guidance.36
All patients with suspected or proven primary cutaneous lymphomas should be reviewed at regional specialist skin cancer MDT meetings. There must be a close working relationship between the regional specialist skin cancer and haemato-oncology MDTs, especially for patients who develop systemic disease.
5.2 Supranetwork multidisciplinary teams
The NICE IOG also defines which patients with primary cutaneous lymphomas should be referred to supranetwork MDTs across England (Table 5), at which dermatologists, clinical oncologists, haemato-oncologists and both dermatopathologists and haematopathologists review the patient's diagnosis and management plan. The IOG recommends that all patients with stage IIB or higher MF, and all with SS and rare CTCL variants should be reviewed by the supranetwork MDT. These supranetwork MDTs also provide the appropriate pathway to review cases where diagnostic or classification difficulties arise. Patients should be reviewed at supranetwork centres to provide access to clinical trials, which are often the standard of care, and to agree on use of newly approved therapies.
| TSEB | ECP | |
|---|---|---|
| Supranetwork MDTs | ||
| Birmingham (Queen Elizabeth Hospital) | Yes (Coventry and Warwickshire University Hospital) | Yes |
| Leeds (St James's University Hospital) | Yes | No, referred to Rotherham Hospital |
| Liverpool (Royal Liverpool Hospital) | Yes (Clatterbridge Hospital, Wirral) | Yes |
| London (Guy's and St Thomas' Hospital) | Yes | Yes |
| Manchester (The Christie Hospital) | Yes | Yes |
| Newcastle (Freeman Hospital) | Yes | Yes |
| Nottingham (City Hospital) | Yes | Yes |
| Other network skin lymphoma services | ||
| Belfast | Yes | No, would be referred |
| Cardiff | No, referred to Guy's and St Thomas’ | No, referred to Bristol University Hospitals |
| Glasgow | No, referred to Newcastle | Yes |
- TSEB, total skin electron beam; ECP, extracorporeal electrophoresis.
The supranetwork MDT is also responsible for reviewing patients who require specialist treatment options, such as total skin electron beam therapy (TSEB) and extracorporeal photopheresis (ECP). Although not stipulated in the IOG, patients with stage IB disease that is refractory to skin-directed therapy (SDT) should be referred to the supranetwork MDT to ensure that they have appropriate access to clinical trials and TSEB. All patients who are being considered for stem cell transplantation should be reviewed by a supranetwork MDT.
6 Treatment of mycosis fungoides/Sézary syndrome
Therapeutic options range from SDT consisting of topical agents, phototherapy and radiotherapy, to systemic treatment with biologics, chemotherapy and stem cell transplantation. Selection of appropriate treatment is based on the stage of disease. Following treatment, patients with advanced disease often develop recurrent, low-grade disease that can be responsive to SDT. There have been very few randomized controlled studies. Recently Quaglino et al. published a retrospective analysis of 853 patients with advanced MF/SS from 21 centres.38 This large, multicentre retrospective study showed a substantial heterogeneity of treatment approaches in advanced MF/SS between U.S. and non-U.S. centres, with up to 24 different drugs, modalities or combinations used as first-line treatment, but these differences did not influence survival outcome. However, it was observed that chemotherapy, when used as first-line treatment, is associated with a higher risk of death and/or change of therapy. For the purposes of this guideline, separate recommendations for MF/SS have been made according to stage.
6.1 Skin-directed treatment
6.1.1 Topical therapies
6.1.1.1 Stage IA–IIA mycosis fungoides
There have been few randomized controlled trials (RCTs) of topical therapies for MF. The lack of well-controlled studies limits the quality of evidence, although all of the topical therapies listed have some clinical efficacy for patches and thin plaques (Table 6). However, accurate data on end points such as duration of response and freedom from relapse are lacking. Many of the topical therapies reviewed remain unlicensed for use in MF.
| Therapy | Design | Level of evidencea | Stage | Response rate |
|---|---|---|---|---|
| Mechlorethamine | RCT40 | 1+ | IA–IIA | 59% gel vs. 48% ointment (noninferiority design by CAILS) |
| Retrospective case series41-46 | 3 | IA–IB | 51–80% (IA) 26–68% (IB) | |
| Mechlorethamine + betamethasone | Prospective open-label phase II47 | 2+ | IA–IB | 58% complete response (duration 7·7 months) |
| Bexarotene gel | Phase II50 | 2+ | IA–IB | 63% |
| Phase III51 | IA–IIA | 44% | ||
| Carmustine (BCNU) | Case series48, 49 | 3 | IA–IIA | 76% |
| Corticosteroids | Case series39 | 3 | IA–IB | 94% (IA), 82% (IB) |
- RCT, randomized controlled trial; CAILS, Composite Assessment of Index Lesion Severity. aSee Appendix S1 in the Supporting Information.
6.1.1.1.1 Topical corticosteroids
Topical corticosteroids, especially class 1 (very potent) compounds, are effective for patches and plaques in some patients with early-stage IA/IB MF, but responses are rarely complete or durable.39
6.1.1.1.2 Topical mechlorethamine (nitrogen mustard)
The single largest RCT randomized 260 patients to a standard nitrogen mustard 0·02% ointment or a novel nitrogen mustard 0·02% gel preparation, used once daily for up to 12 months; no adjunctive therapy was allowed. The gel preparation was statistically noninferior to the ointment, with response rates of 58·5% (gel) vs. 47·7% (ointment) as measured by the Composite Assessment of Index Lesion Severity (CAILS).40 An extension study of 98 patients who failed to achieve complete response used a nitrogen mustard 0·04% gel, with a CAILS response rate of 26% (6% complete response). European Medicines Agency (EMA) approval of this new gel preparation (Ledaga®) provides a more practical formulation than the historical, in-house compounded ointment and aqueous solutions.
These trials confirm that mechlorethamine is an effective topical therapy for early-stage MF. Previous studies have been predominantly retrospective and confounded by the use of other therapeutic modalities.41-46 A prospective, nonrandomized study of 64 patients with early-stage MF treated with a 0·02% aqueous solution of mechlorethamine followed by betamethasone cream twice weekly for 6 months reported 58% complete responses.47
The duration of response to mechlorethamine varies, and the efficacy of maintenance therapy and whole-body application remains unclear, but rare patients with stage IA disease may be cured. Irritant contact dermatitis is the most common adverse effect, occurring in 10–40% of patients, depending on the preparation used. A recent 30-year population-based cohort study showed no increased risk of secondary cancers (including nonmelanoma skin cancer or melanoma) in patients treated with mechlorethamine. Mortality and cause-specific mortality were not influenced by mechlorethamine.46
6.1.1.1.3 Topical carmustine (BCNU)
Limited data suggest that topical carmustine has similar efficacy to mechlorethamine.48, 49 However, it is more extensively absorbed, increasing the risk of bone marrow suppression, although the incidence of irritant contact dermatitis is lower (10%).
6.1.1.1.4 Other topical therapies
Topical bexarotene (1% gel) has been shown to be effective in prospective phase I/II open studies50 and phase III trials for refractory or persistent, early-stage MF.51 Although approved by the U.S. Food and Drug Administration (FDA), topical bexarotene is not licensed in Europe. Irritant contact dermatitis is common and was reported in 12% of patients.
Limited data from case reports and series suggest that imiquimod 5% cream,52-59 5-fluorouracil cream,60 topical retinoid preparations (tazarotene 0·1%, tretinoin 0·1%)61 and tacrolimus 0·1% ointment62 may have efficacy in early stages of MF. The value of simple emollient therapy in MF was highlighted by a 24% placebo response rate to ointment base alone in an RCT of topical peldesine (28% response rate, difference not statistically significant).63
6.1.1.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 MF/Sézary syndrome (SS); stage IVA2–B MF/SS
There is no evidence to suggest that topical therapies have a significant impact on the course of the disease for advanced stages of MF/SS, although SDT can alleviate skin symptoms such as pain and pruritus, and most patients will require intermittent topical treatments, especially topical steroids.
6.1.2 Phototherapy
6.1.2.1 Stage IA–IIA mycosis fungoides
Phototherapy (Table 7) is the standard of care for patients with early stages of MF whose disease is not controlled by topical therapy, producing high complete remission rates.64-91 However, the duration of response is often limited. Repeated courses may be considered but the increased risk of skin cancer (including melanoma) limits the number of phototherapy courses in a lifetime.
| Therapy | Design (all nonrandomized) | Level of evidencea | Stage | Response rate | Response duration (months) |
|---|---|---|---|---|---|
| UVB | Retrospective66 | 2− | IA–IB | 75% | 51 |
| UVB | Retrospective65 | 2− | IA–IB | 71% | 22 |
| NB UVB | Retrospective68 | 2− | IA–IB | 84% | 12·5 weeks |
| NB UVB | Retrospective69 | 2− | IA–IB | 84% (IA), 78% (IB) | |
| NB UVB | Retrospective91 | 2− | IA–IB | 100% | > 60 (80% CR and 60% no recurrence) |
| PUVA | Retrospective74 | 2− | IA–IB | 95% | 43 |
| PUVA | Prospective79 | 2+ | IA–IB | 100% | 20 (IA), 17 (IB) |
| PUVA | Retrospective80 | 2− | IA–II | 63% (CR) | 39 |
- Regimens: psoralen–ultraviolet A (PUVA), twice weekly for 12–14 weeks; Narrowband ultraviolet B (NB UVB), two to three times weekly for 12–14 weeks. CR, complete response. aSee Appendix S1 in the Supporting Information.
There are few comparative studies or RCTs involving psoralen–ultraviolet A (PUVA), and most case series lack a validated scoring system to assess tumour burden. The critical questions as to whether PUVA affects time to progression and disease-specific survival remain unanswered.
6.1.2.1.1 Ultraviolet B phototherapy
Both narrowband ultraviolet (UV)B (TL-01: 311–313 nm) and broadband UVB (290–320 nm) phototherapy can produce high complete response rates.64-69 Responses are more likely in patients who have only patches. There have been no prospective RCTs of narrowband UVB, but a retrospective case series showed it to be as effective as PUVA for treatment of early-stage disease, with no difference in time to relapse.70 In a paediatric case series, high response rates were seen (> 80%), including a number of complete responses in children with the hypopigmented variant of MF.71
6.1.2.1.2 Ultraviolet A phototherapy
High-dose UVA1 phototherapy (340–400 nm), which penetrates more deeply than both UVB and UVA, has shown clinical efficacy in a small case series.72
6.1.2.1.3 Psoralen–ultraviolet A photochemotherapy
Extensive, nonrandomized and retrospective case studies have reported high complete response rates for PUVA in early stages.73-76 Data from an RCT showed that a minority (25–30% freedom from relapse at 5 years) of patients achieving a complete response77 may have a prolonged duration of response. Many patients receive maintenance therapy but the benefits are uncertain.78, 79 A retrospective study of long-term outcomes following complete remission from PUVA monotherapy at a single institution reported that 30–50% of patients had a durable remission (10-year disease-free survival), but maintenance PUVA was given to almost all responding patients.80 A total of 30% of patients showed chronic photodamage and secondary skin cancers. This study showed no significant difference in the OS rate for the nonrelapsing and relapsing patient cohorts. Recent data from a prospective cohort study suggest that maintenance therapy does not prevent future relapse.81, 82
For PUVA, an increased risk has been identified for patients receiving more than 250 treatments and/or > 2000 J cm−2.83, 84 Based on the data available, the cumulative lifetime PUVA exposure should be limited (1200 J cm−2 and/or 250 sessions).85 For maintenance PUVA the risks may outweigh the benefits, and generally this should be avoided for those patients who achieve excellent clinical responses, although there are some exceptional refractory patients for whom maintenance treatment may provide important symptomatic relief.
6.1.2.1.4 Photodynamic therapy
Photodynamic therapy has been reported as a treatment for solitary plaques that are resistant to topical treatment. Small case series have shown durable responses in 70–75% of patients.86, 87 Photodynamic therapy is safe and well tolerated and may be appropriate for certain sites, but a specific therapeutic role in MF has yet to be defined.
6.1.2.1.5 Excimer laser
Several small case series have demonstrated the efficacy of the 308-nm excimer laser in early-stage skin disease. Treatment with an excimer laser appears to be safe, effective and well tolerated for patches, but its precise therapeutic role remains to be established.88-90
6.1.2.2 Stage IIB mycosis fungoides
Patients with tumours invariably have coexisting patches and plaques and may benefit from phototherapy if individual tumours respond to other options such as localized radiotherapy.
6.1.2.3 Stage III/IVA1 erythrodermic mycosis fungoides/Sézary syndrome
Patients with erythrodermic MF/SS are often intolerant of phototherapy due to aggravation of pruritus, but they can rarely respond.
6.1.2.4 Stage IVA2–B mycosis fungoides/Sézary syndrome
PUVA can often be used as a salvage therapy, as patients with advanced disease often relapse or have persistent low-grade skin disease characterized by patches and plaques following systemic treatments.
6.1.3 Combination psoralen–ultraviolet A regimens
PUVA combined with interferon-alpha or bexarotene does not improve overall response but may improve duration of response and can the reduce cumulative UVA dose (Table 8).
| Therapy | Design | Level of evidencea | Stage | ORR (CR), % | Number of patients | Duration (months) | 5-year RFS, % |
|---|---|---|---|---|---|---|---|
| PUVA + interferon | Case studies94 | 2− | IA–IV | 89 | 9 | ||
| PUVA + interferon | Case series97 | 2− | IA–IV | 68 (45) | 22 | 20–75 | |
| PUVA + interferon | Prospective phase II96 | 2+ | IB–IIA | 98 (84) | 89 | 14 | |
| PUVA + interferon | Case studies101 | 2− | 100 (100) | 5 | 74 | ||
| PUVA + interferon | Case studies93 | 2− | IB–IVB | 90 (100) | 39 | ||
| PUVA + interferon | Prospective phase II95 | 2+ | IA–IVA | 81 (75) | 63 | 32 | 75 |
| PUVA + bexarotene | RCT EORTC-2101177 | 1+ | IB–IIA | 77 (31) | 87 | 5·8 | 25 |
| PUVA + bexarotene | Case series100 | 2− | I–III | 67 (29) | 14 | 2–10 | |
| PUVA + bexarotene | Case series102 | 2− | IA–IIB | 100 (63) | 8 |
- ORR, overall response rate; RFS, relapse-free survival; RCT, randomized controlled trial; EORTC, European Organisation for Research and Treatment of Cancer. aSee Appendix S1 in the Supporting Information.
6.1.3.1 Stage IA–IIA mycosis fungoides
6.1.3.1.1 Psoralen–ultraviolet A and interferon-alpha
RCTs have established that combined PUVA and interferon-alpha are associated with a reduced cumulative UVA dose to best response and an improved duration of response compared with both PUVA alone and interferon-alpha combined with acitretin.92-94 However, overall complete response rates for PUVA and interferon-alpha appear to be similar to those with PUVA alone.93, 95-102
6.1.3.1.2 Psoralen–ultraviolet A and retinoids
The combination of PUVA and retinoids (acitretin) is associated with a reduction in cumulative UVA dose to best response, but with no difference in response rates compared with PUVA alone.99 PUVA and bexarotene have also been combined safely, with similar response rates to PUVA alone.100 Weaknesses of these studies include the lack of a validated scoring system to assess tumour burden, and data regarding the duration of response and disease-free survival/OS. A recent RCT (EORTC-21011) comparing PUVA with combined PUVA and bexarotene showed no significant difference in response rates or duration of response, but a trend for lower cumulative UVA dose to achieve complete response in the combination arm was noted.77
6.1.3.2 Stage IIB mycosis fungoides (MF); stage III erythrodermic MF/Sézary syndrome (SS); stage IVA2–B MF/SS
Although there is a lack of data, combination regimens involving PUVA have been utilized as first-line treatment for patients with erythrodermic MF, but patients with SS tolerate PUVA poorly. In contrast, combination PUVA regimens are rarely indicated as first-line therapy for tumour or nodal disease, but are often used as an adjuvant or salvage therapy for those patients with persistent cutaneous disease following debulking treatment for cutaneous tumours or nodal/visceral disease, despite a lack of supportive evidence.
6.1.4 Radiotherapy (localized radiotherapy)
6.1.4.1 Stage IA–IIA mycosis fungoides
MF is a highly radiosensitive malignancy, and localized radiotherapy remains an effective treatment for patients with all stages of disease.103-105 Palliative radiotherapy is very effective for plaques and small or large tumours, which respond to relatively low doses of radiotherapy, such as 8 Gy in two fractions.106 The dose-fractionation regimen should take into account the size of the treatment area, the treatment site and potential risk of acute and late damage to adjacent organs. MF is a multifocal disease, and while local control with radiotherapy is readily achievable, this is usually a palliative approach. Therefore, it is appropriate to use the minimum dose of radiotherapy to obtain local control. Using such a low-dose approach also facilitates, if required, repeat treatment or treatment to an adjacent area where fields may overlap with a previous treatment field. This is especially important for sites with relatively poor skin tolerance to radiotherapy, such as the lower legs. Single fractions of 7–8 Gy have been shown to be effective and convenient for the patient, but allow less re-treatment and may not be suitable for areas of skin with poor tolerance.107 The use of higher doses of radiotherapy is seldom justified. Localized radiotherapy can be used at the same time as other SDT, including phototherapy.
For treatment of large areas of skin such as the trunk, limbs or whole scalp, smaller doses per fraction should be considered, for example 20 Gy in 10 fractions. Regions such as the eyelids may also benefit from smaller doses per fraction to limit acute and long-term toxicity; 4 Gy in two fractions may produce symptomatic benefit in this situation
To define the radiotherapy clinical target volume (CTV) a margin of ≥ 1 cm should be allowed around the disease plaque or tumour (gross tumour volume), and larger margins may be required for some techniques. Further margins need to be added according to the local protocol and radiotherapy mode to determine the planning target volume and field size required to treat the area. To ensure that the full dermis (3–5 mm thick) and a 5-mm-deep margin are being treated, the treatment dose should be delivered to a depth of 8–10 mm below the normal skin surface.
Rarely, MF can present as a solitary patch or plaque, and in this setting local radiotherapy may be used with curative intent (doses of 20–30 Gy in 2-Gy fractions).108-111
Modern radiotherapy techniques such as complex matched electrons, intensity-modulated radiotherapy, helical Hi-ART therapy (TomoTherapy®) and high-dose-rate brachytherapy mould techniques are increasingly being used to treat large areas of disease on curved surfaces of the skin such as the scalp, trunk and limbs, and can provide excellent durable disease control for many patients.
6.1.4.2 Stage IIB mycosis fungoides
Low-dose palliative radiotherapy (e.g. 12 Gy in three fractions) is very effective for MF tumours. Tumours involving large areas require smaller doses per fraction, with schedules of 20–30 Gy in 10–15 fractions. Radiotherapy field margins on the skin should be determined as outlined above. The treatment depth required is determined by the thickness of the tumour and its depth of invasion, which in turn determines the radiotherapy mode required, for example orthovoltage X-rays, electrons or photons. The depth of invasion may need to be assessed using ultrasound, CT or magnetic resonance imaging.
6.1.4.3 Stage III–IVA1 erythrodermic mycosis fungoides/Sézary syndrome
Local radiotherapy may be used for patients who develop isolated, symptomatic fissured lesions or tumours on a background of erythrodermic disease. It can also be useful for treating extensive involvement of the hands and feet.
6.1.4.4 Stage IVA2–B mycosis fungoides/Sézary syndrome
Localized, peripheral nodal disease can be treated with local external beam radiotherapy, as per standard protocols for non-Hodgkin lymphoma. Visceral metastases can also be treated with external beam radiotherapy using the same dosing schedules. Relatively low doses or single fractions can provide effective palliation and should be considered.112 Central nervous system disease in MF has a very poor prognosis. Palliative low-dose, whole-brain radiotherapy can be used in patients who are fit for treatment with a good performance status.113, 114
6.1.5 Radiotherapy: total skin electron beam therapy
TSEB is a highly effective treatment for MF with excellent complete response rates for all stages.115-134 EORTC consensus guidelines for the use of TSEB in CTCL have been published.115 Critical issues to be addressed include maintenance of response to TSEB.
6.1.5.1 Stage IA–IIA mycosis fungoides
A systematic review and meta-analysis of mostly nonrandomized studies has established that the rate of complete response in patients with early-stage disease treated with TSEB is dependent on the stage of disease, skin-surface dose and energy delivered, with very high complete response rates observed.116 Greater skin-surface dose (32–36 Gy) and higher energy (4–6-MeV electrons) are associated with a higher rate of complete response and reasonable 5-year relapse-free survival (Table 9).116-118 The conventional Stanford schedule of TSEB is 36 Gy delivered with a six dual-field technique to the whole skin, delivering 2-Gy fractions per 2-day cycle over 9 weeks, with local boost treatments to tumours and thick plaques, and supplemental radiotherapy to a median dose of 20 Gy to ‘shadowed’ or self-shielding areas, such as the perineum, soles of feet, scalp and inframammary folds.119 The standard U.K. modified Stanford protocol involved 30 Gy to the whole skin delivering 1·5-Gy fractions per 1-day cycle over 5 weeks.117 TSEB delivering 30–36 Gy is associated with significant toxicity in some patients. Fatigue has been reported in 38% of patients, erythema and desquamation in 47–76%, alopecia in 95%, lower-leg oedema in 26%, blisters in 19–52% and skin infection in 31–32%.117, 120
| Therapy | No. of patients | Dose (Gy) | Level of evidencea | Stage | ORR, % | CR, % | Median RFS/PFS (months) |
|---|---|---|---|---|---|---|---|
| High dose127 | 114 | 30–36 | 2+ | IA | 100 | 97 | 50 |
| High dose117, 119 | 103, 17 | 30–36 | 2+ | IB | 100 | 59–75 | 18–29 |
| High dose117, 119 | 77, 19 | 30–36 | 2+ | IIB | 95–100 | 47 | 9 |
| High dose117, 131 | 3, 45 | 30–36 | 2+ | III | 100 | 33–60 | 6–9 |
| Low dose122, 134 | 21 | 10 | 2+ | IB–IV | 95 | 57 VGPR | 5·8 |
| Low dose124 | 33 | 12 | 3 | IB–III | 88 | 27 | MDOCB 16–17 |
| Low dose121 | 33 | 5 to < 10 | 2+ | IB–III | 85–100 | 0–25 | 12 |
| Low dose121 | 51 | 10 to < 20 | 2+ | IB–III | 96–100 | 7–52 | 25·7 |
| Low dose121 | 32 | 20 to < 30 | 2+ | IB–III | 83–100 | 29–37 | 29·3 |
| Low dose125 | 103 | 12 | 2+ | IB–IV | 87 | 18 | 13·2 |
| 54 | IB | 94 | 20 | 26·5 | |||
| 33 | IIB | 97 | 21 | 8·7 | |||
| 12 | III | 50 | 8 | 10·6 | |||
| 4 | IV | 25 | 0 | – |
- ORR, overall response rate; CR, complete response; RFS, relapse-free survival; PFS, progression-free survival; VGPR, very good partial remission with modified Severity Weighted Assessment Tool score < 1; MDOCB, mean duration of clinical benefit (date of TSEB to date of next whole-skin-equivalent treatment). aSee Appendix S1 in the Supporting Information.
A recent review has shown similar response rates and duration of response with lower doses of TSEB (10–30 Gy),121 but a lower median response duration was reported following a total dose of 10 Gy.122 Very low doses of TSEB (4 Gy) have been tested, with reported inadequate responses.123 The results of low-dose TSEB using 12 Gy have been reported from a pooled analysis from three phase II trials. They showed an overall response rate (ORR) of 88%, with a median duration of clinical benefit of 70·7 weeks.124 A series of low-dose TSEB (12 Gy in eight fractions over 2 weeks) in 103 patients has shown a similar very high ORR, with a median duration of response of 18 months in patients with stage IB MF and 10 months in patients with stage IIB MF, with significantly less toxicity than standard-dose TSEB.125 Studies of low-dose TSEB in combination with newer agents are currently under way.
One RCT comparing TSEB with topical mechlorethamine showed similar rates of complete response and duration of responses in early stages (IA–IB) of disease.126
The use of adjuvant PUVA and topical mechlorethamine after TSEB has been reported, but the data are based on limited retrospective studies of small cohorts.127, 128 It is not possible to recommend this as standard treatment, although PUVA can be used as an effective early salvage therapy following TSEB. Furthermore, a recent report has shown no clinical advantage to the use of topical mechlorethamine as adjuvant treatment after TSEB.119
TSEB should be considered as second-line treatment for stage IB MF that has relapsed or is refractory to other SDT. It can also be considered as first-line treatment in patients with extensive cutaneous disease (stage T2b).
6.1.5.2 Stage IIB-IVB mycosis fungoides
Patients with tumour stage IIB MF can achieve high rates of durable complete responses to TSEB.121 One RCT included 103 patients with MF/SS (all stages) and compared TSEB and multiagent chemotherapy (CAVE; cyclophosphamide, doxorubicin, vincristine and etoposide) with sequential topical therapy including superficial radiotherapy and phototherapy. It revealed a higher complete response rate in the TSEB–chemotherapy group (38% vs. 18%), but without a significant difference in disease-free survival and OS.129 Nevertheless, a combination of chemotherapy and TSEB can be invaluable for patients with severe skin disease and late stages of disease (stage IVA2–B). In addition, TSEB can be utilized as debulking treatment prior to allogeneic stem cell transplantation.130
6.1.5.3 Stage III-IVA1 erythrodermic mycosis fungoides/Sézary syndrome
A retrospective study of TSEB as monotherapy for erythrodermic CTCL noted a higher complete response rate and lower rate of disease progression in patients without peripheral blood involvement (stage III disease) and in those receiving a more intense regimen (32–40 Gy).131 These retrospective data suggest that TSEB can be considered as second-line, and even first-line therapy for patients with erythrodermic MF, although patients with erythrodermic disease can be very sensitive to radiotherapy and have severe acute radiation reactions. A radiotherapy test dose to a limited area of skin should be used before treatment, and careful patient selection is advised.
A retrospective study comparing TSEB alone with TSEB followed by ECP in erythrodermic MF/SS suggests that the combination of TSEB and ECP is associated significantly with improved disease-free and cause-specific survival when corrected for peripheral blood and nodal involvement, but these retrospective data may be open to selection bias.132 There may be potential for TSEB to be used alone or in combination for both skin and blood debulking in patients with SS, but this needs further investigation in clinical trials.133
6.2 Systemic biological therapies
6.2.1 Interferon-alpha
Interferon-alpha is licensed in the European Union for the treatment of MF/SS (Table 10). There have been numerous, relatively small open nonrandomized studies of interferon-alpha in all stages of pretreated MF/SS, with variable dose schedules (3–9 megaunits, three to seven times weekly). ORRs > 50% and complete response rates > 20% have been reported. Response rates are higher in early stages of disease and with higher doses of interferon.135-137 A small, randomized study comparing PEGylated interferon-alpha-2b plus PUVA with standard interferon-alpha-2b plus PUVA showed a higher ORR with less constitutional side-effects for patients treated with PEGylated interferon-alpha-2b plus PUVA.94 However, the patients in the PEGylated interferon arm had a higher rate of myelosuppression and liver toxicity than those treated with standard interferon.
| Therapy | Design | Level of evidenceb | Stage | ORR, % | CR, % | Duration |
|---|---|---|---|---|---|---|
| Interferon-α | Case series135 | 2− | IA–IVB | 45–74 | 10–27 | NA |
| Interferon-α | Case series136 | 2− | IA–IIA | 88 | ? | NA |
| Interferon-α | Case series138 | 2− | IIB–IVB | 29–63 | ? | NA |
| Bexarotene | Phase II145 | 2+ | IA–IIA | 54 | 0 | 516 days |
| Bexarotene | Phase II148 | 2+ | IIB–IVB | 45–51 | 0 | 299 days |
| Brentuximab vedotin | Phase II160 | 2+ | IB–IVB | 73 | 35 | 32 weeks (range 3–93) |
| Brentuximab vedotin | Phase II161 | 2+ | IB–IVB | 70 | 3 | 54% of responders progression free at 12 months |
| Brentuximab vedotin | RCT Phase III162 | 1+ | 1A–IVB | 56 | 16 | 16·7 months |
| Denileukin diftitoxa | Phase II163 | 2 + + | IB–IVA | 30 | 10 | 6·9 months |
| Denileukin diftitoxa | RCT phase III164 | 1+ | IA–III | 44 | 10 | > 2 years (PFS) |
| Alemtuzumab high dose | Case series151, 152 | 2− | IIB–IV | 37–100 | 25–47 | 6–9 months |
| Alemtuzumab low dose | Case series154, 155 | 2− | IIB–IV | 85 | 21 | TTF 12 months |
| Vorinostata | Phase II165 | 2+ | IB–IVA | 24–29·7 | 0 | 106–185 days |
| Romidepsina | Phase II166 | 2+ | IB–IVA | 34 | 6 | 13·7–14·9 months |
| Bortezomib | Case series167 | 2− | III–IV | 70 | 10 | 7–14 months |
- ORR, overall response rate; CR, complete response; RCT, randomized controlled trial; NA, not available; PFS, progression-free survival; TTF, time to treatment failure. aApproved by the U.S. Food and Drug Administration but not the European Medicines Agency. bSee Appendix S1 in the Supporting Information.
6.2.1.1 Stage IA–IIA mycosis fungoides
Although higher response rates have been reported for interferon in patients with early-stage MF,135-137 interferon-alpha should not be used in patients with early-stage MF who are responsive to SDT, as these patients have a good prognosis and there is no evidence that interferon affects long-term outcome.
However, for patients with early-stage MF who are refractory to SDT, interferon and PUVA can be used in combination, and evidence suggests that this may reduce the cumulative UV dose required to achieve a complete response.99 One RCT comparing PUVA and interferon with interferon plus acitretin in stage I and II patients demonstrated that PUVA and interferon were superior, with a 70% complete response rate compared with 38% for the drug combination.92
6.2.1.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IVA2–B MF/SS
Most studies of interferon have involved patients with refractory, advanced-stage disease but have not distinguished between patients with erythroderma and either tumour-stage disease or nodal involvement. Responses are dose dependent but complete response is rare.135, 136, 138 Responses have been reported with combined interferon-alpha and acitretin in patients with MF and SS resistant to interferon alone.139 Aviles et al.140 reported good response rates (80%) at 1 year with combinations of interferon (5 megaunits three times a week) and methotrexate or interferon and trans-retinoic acid. Data on combined interferon-alpha and bexarotene are currently limited to case series.141 Patients with erythrodermic MF treated with immunosuppressive therapy such as ciclosporin for suspected inflammatory disorders may have a more aggressive disease course and worse outcome.142
6.2.2 Retinoids and rexinoids
6.2.2.1 Stage IA–IIA mycosis fungoides
Retinoic acid receptor retinoids (acitretin) have modest effects in early stages of MF as monotherapy, but in combination with PUVA they may reduce the cumulative UVA dose and time to response, and improve duration of response,143, 144 although the only RCT92 suggested no benefit.
Prospective studies of bexarotene, a retinoid X receptor rexinoid, have shown significant efficacy and good duration of response with low rates of disease progression in early-stage disease.145 Bexarotene has been used in combination with PUVA;102, 146 however, the EORTC-21011 RCT in low-risk MF comparing PUVA alone with PUVA plus bexarotene showed no difference in response rates.77
6.2.2.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IVA2–B MF/SS
Bexarotene is licensed in the European Union for the treatment of late-stage MF/SS refractory to at least one systemic agent, and U.K. consensus guidelines for bexarotene prescribing and management have been published.147 Maximum responses may take 6 months, and bexarotene should be continued until loss of response.
Prospective studies of bexarotene in refractory, late-stage disease have shown encouraging ORRs and response durations at doses of 300 mg m−2 daily,148 which have been confirmed in subsequent case series.146, 149 These results suggest that bexarotene has a useful role, particularly for erythrodermic disease, but monotherapy is unlikely to be effective for tumours or nodal disease. Whether adjuvant bexarotene has a role in maintaining remission or partial responses in those patients with pretreated advanced disease is currently unknown.
6.2.3 Antibody therapies
6.2.3.1 Stage IA–IIA mycosis fungoides
At present, there is no evidence that antibody therapies should be used for patients with early-stage disease.
6.2.4 Alemtuzumab (CAMPATH-IH)
6.2.4.1 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IIVA2–B MF/SS
Phase II trials of alemtuzumab (a humanized anti-CD52 antibody) in small cohorts of patients with advanced MF/SS have shown encouraging ORRs.150-156 Reported overall response and complete remission rates are high, but typically responses are short lived, with only a minority of patients achieving responses for longer than 12 months. Clinical responses are best in SS, where symptomatic benefit may be dramatic. In contrast, recent evidence suggests that alemtuzumab is ineffective in patients with MF with tumours and large cell transformation.157
Reports of possible cardiac toxicity158 have not been confirmed and the safety profile has been acceptable even in elderly patients, although infection and specifically cytomegalovirus reactivation remains an issue and patients need irradiated blood products.155 In the majority of studies, alemtuzumab has been administered intravenously at the standard dose of 30 mg three times per week. However, one study examined the use of a lower-dosage subcutaneous schedule at 10 mg three times per week, with similar efficacy but reduction in toxicity.154 Alemtuzumab remains an important second-line therapeutic option for patients with advanced disease, particularly erythrodermic stage III–IVA1 MF/SS.
6.2.5 Brentuximab vedotin
Brentuximab vedotin is an antibody–drug conjugate comprised of an anti-CD30 monoclonal antibody attached by an enzyme-cleavable linker to the antimicrotubule agent, monomethyl auristatin E, which is released upon internalization into CD30-expressing tumour cells. It is administered intravenously at 1·8 mg kg−1 over 30 min every 21 days, for a maximum of 16 cycles.159
Phase II trials demonstrated encouragingly high response rates,160, 161 where the proportion of CD30 tumour cell expression appeared to correlate with tumour responses. Those with < 5% CD30 expression had a lower likelihood of global response than did those with CD30 expression ≥ 5%.161
Further to these phase II trials, a randomized, open-label phase III trial (ALCANZA) was designed to evaluate single-agent brentuximab vedotin vs. a control arm of the investigator's choice of standard therapies, namely methotrexate or bexarotene, in patients with CD30-expressing CTCL, including those with primary cutaneous anaplastic large cell lymphoma (pcALCL) or MF. The primary end point was objective response lasting at least 4 months (ORR4) as assessed by Global Response Score. Brentuximab vedotin resulted in a statistically significant improvement in the rate of ORR4 (56·3% vs. 12·5% in the control arm). The median PFS was 16·7 months vs. 3·5 months, a significant difference favouring brentuximab (P < 0·001).
In November 2016, the FDA granted brentuximab vedotin Breakthrough Therapy designation for the treatment of patients with CD30-expressing MF and pcALCL who require systemic therapy and have received one prior systemic therapy. The phase III data from this trial have also led to EMA approval in patients with CD30+ CTCL.162 Peripheral neuropathy is a frequent side-effect, occurring in over 60% of patients, but resolution or improvement of symptoms on cessation of treatment is reported.
6.3 Chemotherapy
Systemic chemotherapy (Table 11) is usually reserved for patients with advanced disease, or disease refractory to SDT or immunobiological therapy, and is palliative rather than curative.168-189 Although good responses are reported with both single-agent and combination regimens, overall the results are disappointing when compared with other lymphomas. A particular difficulty in interpreting data in this area is the variable and largely suboptimal reporting of response assessment and the heterogeneity of risk factors within the spectrum of the patients with MF and SS who are treated. In the absence of randomized, phase III trials, comparison of response rates across phase II studies is fraught with difficulties of interpretation, and therefore recommendations are subject to inherent investigator and reporting bias.
| Therapy | Design | Level of evidencea | ORR, % | CR, % | Median RFS or PFS (months) |
|---|---|---|---|---|---|
| Pentostatin | Case series174-177, 181 | 2− | 35–71 | 6–32 | 9 |
| Cladribine | Case series178 | 2− | 28–41 | 14–19 | 4·5 |
| Fludarabine | Case series179, 188 | 2− | 30–51 | 11·4 | 5·9 |
| Liposomal doxorubicin | Case series183 | 2− | 80–88 | 45–66 | 15 |
| Liposomal doxorubicin | EORTC-21012184 | 2+ | 40·8 | 6·1 | 6 |
| Gemcitabine | Case series180-182 | 2− | 68–75 | 8–22 | 10–15 |
| Methotrexate | Case series170 | 2− | 58 | 41 | 31 |
| Trimetrexate | Case series171 | 2− | 45 | Not recorded | |
| Pralatrexate | Phase II189 | 2+ | 45 | Not recorded | |
| Combination chemotherapy | Systematic review168 | 2+ | 81 | 38 | 5–41 |
| Chlorambucil | Case series169 | 2− | 89 | 36 | Not recorded |
| EPOCH | Phase II187 | 2+ | 80 | 27 | 8 |
- ORR, overall response rate; CR, complete response; RFS, relapse-free survival; PFS, progression-free survival; EORTC, European Organisation for Research and Treatment of Cancer; EPOCH, etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin. aSee Appendix S1 in the Supporting Information.
6.3.1 Stage IA–IIA mycosis fungoides
Chemotherapy is contraindicated for early stages of disease, as low-grade disease is relatively resistant to chemotherapy and response duration is short.
6.3.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IVA2–B MF/SS
Quaglino et al. found treatment heterogeneity between U.S. and non-U.S. centres but no difference in survival.38 However, a constant geographical feature was the finding that first-line chemotherapy was associated with a higher risk of death.
One RCT comparing combination chemotherapy and TSEB against conservative SDT showed a better overall response in the combination arm but greater morbidity and no improvement in OS.129 A systematic review of uncontrolled open studies of single-agent regimens in 526 patients with CTCL treated between 1988 and 1994 revealed OR rates of 62%, with complete response rates of 33% and median response durations of 3–22 months.168 Single-agent therapies showing clinical efficacy in MF/SS include alkylating agents (chlorambucil and cyclophosphamide),169 antimetabolites (methotrexate and trimetrexate),170, 171 purine analogues (pentostatin, 2-chlorodeoxyadenosine and fludarabine),172-179 the pyrimidine antimetabolite gemcitabine180-182 and liposomal doxorubicin.183, 184 A recent U.K. prospective phase II study assessing a combination regimen incorporating gemcitabine and bexarotene in advanced MF/SS (GEMBEX) showed a low response rate following chemotherapy at 12 weeks (31%, all with partial response), reducing to a 14% ORR at 24 weeks despite bexarotene maintenance. In addition, control of hyperlipidaemia was challenging with this combination.185
A systematic review of different combination chemotherapy regimens in patients with MF and SS stages IIB–IVB treated between 1988 and 1994 showed an OR rate of 81% in 331 patients and a complete response rate of 38%, with response duration ranging from 5 to 41 months.168 The most commonly reported regimen used in MF/SS is CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). Prospective, nonrandomized studies of other multiagent chemotherapy regimens – VICOP-B (etoposide, idarubicin, cyclophosphamide, vincristine, prednisolone, bleomycin) and EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin) – have revealed similar OR rates and durations of response to those for CHOP.186, 187 Overall survival rates remain unchanged for these more intensive regimens and toxicity may be considerable; therefore, these regimens have little clinical benefit over less intensive regimens.
The patient's quality of life should always be considered before embarking on chemotherapy regimens with limited efficacy. Patients with CTCL are at high risk of neutropenic sepsis, and therapy-related mortality with combination chemotherapy is a significant risk. Single-agent regimens appear to have similar efficacy to combination regimens but with lower toxicity, and therefore are preferable as palliative therapy in late stages of MF and SS. Durable responses with either single- or multiagent regimens are rare.
The limited data suggest that methotrexate and pentostatin are most appropriate for erythrodermic SS, while gemcitabine and liposomal doxorubicin are more appropriate for advanced stages of MF. Maintenance or adjuvant treatment with bioimmunotherapy, SDT or TSEB for patients achieving a response with chemotherapy may be considered. Patients frequently relapse, with early stages of disease manifested by patches and plaques, although there is no trial evidence suggesting that any such approach improves outcomes, and further well-designed studies are required.
While outcomes are invariably poor for patients with advanced disease, with median survival of < 12 months (stage IV), for those stage IVA2 patients with a good performance status, the aim is to achieve a durable complete or good partial remission and to proceed to a reduced-intensity allogeneic transplant.
6.4 Extracorporeal photopheresis
6.4.1 Stage IA–IIA mycosis fungoides
Although responses to ECP have been reported in early-stage MF, this is based mostly on a small number of patients in poorly controlled single-centre studies, including some in which patients were treated with adjuvant therapies (Table 12).190-192 The only randomized study looking at patients with early-stage MF treated with either PUVA or ECP showed that PUVA was more effective over a 6-month treatment period.193 Therefore, there is no evidence at present to suggest that ECP should be used for patients with early-stage disease.
| Therapy | Design | Level of evidencea | Stage | ORR, % | CR, % | OS (months) |
|---|---|---|---|---|---|---|
| ECP | Systematic review | 2+ | IB–IV | 63 | 20 | |
| ECP | Systematic review | 2+ | III | 35–71 | 14–26 | 39–100 |
| ECP | Multiple case series | 2− | III | 31–86 | 0–33 |
- ORR, overall response rate; CR, complete response; OS, overall survival. aSee Appendix S1 in the Supporting Information.
6.4.2 Stage IIB mycosis fungoides
6.4.3 Stage III–IVA1 erythrodermic mycosis fungoides/Sézary syndrome
A systematic review of nonrandomized and mostly retrospective studies of ECP in 650 patients from 30 published studies showed an ORR of 63% and a complete response rate of 20%.196 Response rates are highest in those with erythrodermic stage III–IVA1 MF/SS.190, 197-200 Treatment is given on two consecutive days and repeated every 4 weeks, but it may be given more frequently (e.g. every 2 weeks) in those with a high blood-tumour burden.194, 196, 201 Responses may take up to 6 months to develop and therapy should continue until loss of response. The U.K. consensus guidelines for ECP in CTCL recommend ECP as first-line systemic treatment in patients with erythrodermic CTCL with evidence of blood involvement, treating with one to two cycles per month. The guidelines were recently updated in 2017 with the recommendation that ECP treatment should be continued until loss of response.196, 202
A recent U.K. audit report of 97 patients with CTCL treated since 2010 found that 45% had received ECP as the first-line systemic agent.203 The response rate at 6 months was 61% and the median length of treatment was 9 months. There have been numerous small case series of erythrodermic patients treated with combinations of ECP, interferon-alpha and/or retinoids, as well as bexarotene, which suggest that combination therapy can be highly effective, as both clinical and molecular remissions have been documented.196, 204-213
Although these studies were uncontrolled, the evidence suggests that ECP can be an effective and well-tolerated therapy for patients with erythrodermic stage III–IVA1 MF/SS and that such patients may benefit from combination regimens combining ECP with other systemic therapies. ECP is an FDA-approved treatment for MF/SS. In contrast, there is a lack of evidence for use of ECP in stage IVA2–B MF/SS.
6.5 Systemic therapies (Food and Drug Administration but not European Medicines Agency approved)
6.5.1 Toxin therapies (denileukin diftitox: diphtheria interleukin-2 fusion toxin; Ontak)
Denileukin diftitox is a fusion protein targeting the interleukin-2 receptor (CD25). Large RCTs have shown significant efficacy and improved PFS in all stages of disease (Table 9). Denileukin diftitox is FDA but not EMA approved for MF/SS, but it is currently not available in the U.S.A. because of a manufacturing change, and results of an ongoing trial are awaited. Toxicity includes hypersensitivity reactions and vascular leak.
6.5.1.1 Stage IA–IIA mycosis fungoides
Toxin therapies should not be used for early-stage disease in view of potential adverse effects, but denileukin diftitox can be effective for early-stage patients who are resistant to SDT.
6.5.1.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IVA2–B) MF/SS
Denileukin diftitox (18 μg kg−1 per day for 5 days, as a 21-day cycle) is effective in heavily pretreated patients with late stages of disease,163, 164, 214 and efficacy may be improved by combining with bexarotene.215
6.5.2 Histone deacetylase inhibitors
Histone deacetylase (HDAC) inhibitors are an emerging class of drugs that increase acetylation of histones and nonhistone proteins affecting gene transcription, which results in cell-cycle arrest and apoptosis. Several phase II studies have established that HDAC inhibitors have clinical efficacy in all stages of MF/SS, with an acceptable safety profile.165, 166, 216, 217 These studies led to FDA approval for vorinostat (suberoylanilide hydroxamic acid) and romidepsin for the treatment of refractory MF/SS. Neither HDAC inhibitor is licensed for use in Europe.
6.5.2.1 Stage IA–IIA mycosis fungoides
HDAC inhibitors should not be used for early-stage MF but can be effective for patients resistant or refractory to SDT.
6.5.2.2 Stage IIB mycosis fungoides (MF); stage III–IVA1 erythrodermic MF/Sézary syndrome (SS); stage IVA2–B MF/SS
Phase II studies of vorinostat and romidepsin have shown similar ORRs in advanced disease including stage III patients.165, 166, 216, 217 Evaluation of patients on vorinostat for over 2 years provides evidence of long-term safety and tolerability.218 One study also showed a significant improvement in pruritus in 43% of patients, including those who had no objective clinical response.166
6.5.3 Other systemic chemotherapies
Pralatrexate belongs to a class of folate analogues, known as the 10-deazaaminopterins, and is emerging as a promising new agent in the treatment of T-cell lymphomas, including CTCL. Phase II trials have demonstrated good response rates, although adverse effects, including mucositis, are a concern.219 A further study, using a lower dose of pralatrexate, reported better tolerance but similar response rates.189
6.6 Systemic therapies (not Food and Drug Administration or European Medicines Agency approved)
Bortezomib, a proteasome inhibitor, has shown clinical efficacy in a small series of patients with advanced stages of CTCL,167 but its clinical role remains to be established. Temozolomide has also been reported to be effective in a minority of patients in a small multicentre study of patients with advanced MF/SS220 and those with central nervous system progression.114
6.7 Autologous or allogeneic peripheral blood or bone marrow stem cell transplant
Autologous haemopoietic stem cell transplant (HSCT) appears to be associated with only short-term remission and is therefore difficult to justify.221-223 In contrast, allogeneic HSCT is a complex form of immunotherapy aimed at consolidating treatment of advanced MF/SS to produce a durable complete remission.224 A retrospective study from the European Group for Blood and Marrow Transplant (EBMT) included a heterogeneous group of patients who received both myeloablative and reduced-intensity conditioning (RIC) regimens. The study showed an estimated 3-year survival rate of 54%.225 However, the treatment-related mortality of 25% at 1 year for CTCL was a concern. The EBMT data suggested that alemtuzumab should not be used as part of the pretransplant conditioning regimen because of a higher rate of early relapse and increased treatment-related mortality.225
A number of other case reports and small retrospective series have also described clinical responses and durable remissions of more than 3 years following myeloablative allo-HSCT226-229 and reduced-intensity conditioning allo-HSCT.229-234 A single-centre report described outcomes in 19 recipients of allo-HSCT using an RIC regimen that included TSEB irradiation prior to transplant. Eleven patients achieved sustained complete remissions, some of which were following donor lymphocyte infusion, immunosuppression withdrawal and/or chemotherapy.130 An update of this study utilizing pretransplant TSEB in the majority of patients showed that 60% achieved complete skin remission as a result.235
A more recent analysis of the EBMT cohort (60 patients with MF/SS) in 2014 reported an OS of 46% and PFS of 32% at 5 years.236 Relapse or progression occurred in 45% at a median of 3·8 months following HSCT, but many patients were rescued with donor lymphocyte infusions. Nonrelapse mortality was 22%, with the last nonrelapse death occurring 14 months following HSCT. Multivariate analysis confirmed worse outcomes for myeloablative conditioning regimens and transplants from unrelated donors.236 A meta-analysis of allogeneic or autologous SCT in MF and SS supports the view that allogeneic transplantation provides a better survival and disease-free outcome than autologous transplantation.237
There is increasing enthusiasm for RIC allograft approaches for a number of reasons. CTCL affects older patients, many of whom have chronically infected skin tumours, and the risk of life-threatening sepsis is lower with reduced-intensity allografts. Allogeneic transplant is successful in part because of the graft-versus-lymphoma effect of the donor graft, independent of the conditioning regimen, but this has to be balanced against the morbidity associated with chronic graft-versus-host disease.225 In addition, and relevant to the above, a major risk following allogeneic transplant is disease relapse. Clearly, some patients can be salvaged with donor lymphocyte infusion, but this in turn may lead to significant graft-versus-host disease.
Maximal benefit from RIC-HSCT is observed when it is performed before patients develop highly refractory disease and when there is low disease bulk at the time of transplantation.225 Appropriate data registration is required to identify the optimal conditioning regimen for allo-HSCT and to define clinical outcomes. Considering the numerous biological agents that are becoming available and the potential efficacy of allo-HSCT, younger patients with stage IIB–IV disease and poor prognostic factors or aggressive disease should be reviewed at supranetwork MDTs and, if appropriate, referred early to a specialist transplant centre.
6.8 Future perspectives
FDA-approved therapies include topical bexarotene gel, mechlorethamine gel, oral bexarotene, denileukin diftitox, photopheresis and the HDAC inhibitors vorinostat and romidepsin.
In contrast, only topical mechlorethamine ointment for all stages of MF, and interferon-alpha, brentuximab and oral bexarotene for late-stage refractory MF/SS, are EMA approved. Currently, there is an unmet need for improved treatments in CTCL and an urgent need for well-designed RCTs with appropriate clinical end points.
Recommendations
- The diagnosis of MF and SS is based on a combination of clinical and pathological criteria and requires close MDT collaboration between different specialities. (Strength of recommendation D (GPP))
- All patients with early-stage MF refractory to SDT and late-stage MF/SS should be reviewed by supranetwork MDTs to agree a management plan and provide the opportunity for consideration in appropriate clinical trials. (Strength of recommendation D (GPP))
- The aim of treatment is to control the patient's disease and symptoms with the minimum of intervention. Management may range from an expectant policy through to nonmyeloablative allogeneic stem cell transplantation. (Strength of recommendation D (GPP))
- SDTs, including phototherapy and local radiotherapy, are the standard of care for patients with early-stage IA–IIA MF. (Strength of recommendation C)
- Patients with stage IA–IIA MF who are refractory to SDT often require TSEB and systemic biological therapies. There is no evidence to support the use of maintenance phototherapy. (Strength of recommendation C)
- Patients with stage IIB MF can have an unpredictable clinical course: some patients develop only small and infrequent skin tumours and often obtain durable responses to localized radiotherapy and other SDT options for persistent patches and plaques; other patients develop extensive bulky skin tumours and rapidly progressive disease requiring TSEB and systemic chemotherapy. (Strength of recommendation C)
- Following treatment, patients with advanced MF may develop recurrent, low-grade disease where SDT is a treatment option. (Strength of recommendation D (GPP))
- Patients with erythrodermic MF (stage III) and SS (stage IVA1) often require single or combination systemic therapies such as methotrexate, photopheresis, bexarotene and interferon-alpha as first-line treatment. (Strength of recommendation C)
- Options for SS (stage IVA1–2) also include clinical trials and alemtuzumab. (Strength of recommendation C)
- For stage IVA2–B MF/SS, radiotherapy (including TSEB for selected stage IV patients) and single-agent chemotherapy regimens are the preferred option, but response duration is often short. (Strength of recommendation C)
- Brentuximab offers an effective option for refractory stage IB disease and advanced stages of MF/SS with tumour CD30 expression. (Strength of recommendation B)
- All patients with early-stage MF refractory to SDT and stage IIB–IV MF/SS should be offered the opportunity to participate in well-designed clinical trials. (Strength of recommendation D (GPP))
- Autologous HSCT should not be considered for advanced stages of MF/SS. (Strength of recommendation A)
- Reduced-intensity allogeneic HSCT should be considered for selected groups of patients with advanced MF/SS to consolidate treatment responses. (Strength of recommendation B)
- The conditioning regimens and outcomes of RIC allo-HSCT should be collected through data registries such as the EBMT registry. (Strength of recommendation D (GPP))
7 Treatment of non-mycosis fungoides/Sézary syndrome primary cutaneous T-cell lymphomas
7.1 Primary cutaneous CD30+ lymphoproliferative disorders
Primary cutaneous CD30+ lymphoproliferative disorders represent a spectrum of disorders, and the diagnosis is dependent on careful assessment of both clinical and pathological features. Specifically, MF must be excluded by a clinical evaluation that fails to identify pre-existing or coexistent polymorphic patches and plaques in a characteristic limb-girdle distribution. There is only one RCT involving this condition, and the evidence is based on the EORTC, ISCL and United States Cutaneous Lymphoma Consortium consensus statement,15 which is the appropriate source for references supporting all the treatment recommendations below.
7.1.1 Primary cutaneous CD30+ anaplastic large cell lymphoma
pcALCL is characterized by solitary tumours, grouped tumours or thick plaques of disease. Rarely, patients present with generalized multifocal lesions. Partial regression may occur but it is rarely complete, and this clinical feature helps to distinguish pcALCL from LyP. The tumour phenotype is CD30+ ALK−, and this CTCL variant is biologically distinct from its nodal counterpart. The prognosis is excellent, with 5-year survival rates of 96%.31 Staging is based on the non-MF/SS staging recommendations,24 and CT scans and bone marrow trephine biopsies are required to confirm that the skin disease is not a manifestation of a systemic lymphoma. Primary cutaneous CD30+ ALK+ ALCL has been rarely described but there is no evidence that such patients have a worse prognosis.
7.1.1.1 Surgery
Surgical excision can be an effective treatment for solitary tumours. If the lesion has been completely excised with clear histological margins, it may be appropriate to manage the patient with adjuvant radiotherapy or active surveillance.
7.1.1.2 Radiotherapy
Radiotherapy is an effective treatment modality for pcALCL, with very high complete remission rates reported. Cutaneous recurrences have been reported in 41–64% of patients.15 International consensus recommends a dose of 24–30 Gy.238
7.1.1.3 Chemotherapy
Doxorubicin-based, multiagent chemotherapy regimens are effective with high complete response rates, but response duration is limited with relapses in > 60% of patients.15 Vinblastine has been effective as a single agent in pcALCL.239 There are only limited reports of single-agent chemotherapy agents such as methotrexate, etoposide and gemcitabine.
7.1.1.4 Antibodies
The fusion toxin brentuximab vedotin (SGN-35) in a large phase III RCT in pcALCL has shown significant benefit in terms of durable response and improved PFS compared with methotrexate or bexarotene.162
7.1.2 Lymphomatoid papulosis
LyP is characterized by recurrent, papulonodular eruptions that resolve spontaneously to leave varioliform scars. The pathology shows variable proportions of CD30+ large cells (type A–D).
7.1.2.1 Expectant policy
For patients with limited disease and infrequent eruptions, an active monitoring policy is acceptable.
7.1.2.2 Phototherapy
Phototherapy is effective in LyP but there is a lack of data on dosage, and relapse is common when treatment is withdrawn.
7.1.2.3 Chemotherapy
Weekly low-dose oral methotrexate is highly effective in LyP, but only a minority of patients experience a durable remission following treatment withdrawal.15
7.1.2.4 Radiotherapy
Radiotherapy can be very effective especially for patients with regional LyP, and responses can be durable.15
7.1.2.5 Immunobiological therapy
There are very limited data on retinoids, interferon-alpha and bexarotene, but responses have been reported with all of these agents in LyP. Brentuximab is effective.160
7.2 Rare cutaneous T-cell lymphoma variants
There are various rare and aggressive CTCL variants including γδ CTCL and PTCL (not otherwise specified, NOS), which can primarily involve cutaneous tissues. The diagnosis in such cases requires careful evaluation of the clinical features, staging investigations and pathology. Human T-lymphotropic virus-1 virology should be considered to exclude a cutaneous presentation of adult T-cell leukaemias/lymphoma; there are no controlled trial data. Treatment options are the same as those recommended in the British Committee for Standards in Haematology 2013 guidelines.240
Rare CTCL variants such as subcutaneous panniculitis-like T-cell lymphoma (SPTCL), CD4+ small/medium T-cell lymphoproliferative disorder, acral CD8+ T-cell lymphoma and aggressive epidermotropic cytotoxic CD8+ CTCL are characterized by distinct clinical and/or pathological features and, with the exception of the latter, have an excellent prognosis.
7.2.1 Subcutaneous panniculitis-like T-cell lymphoma
SPTCL is a rare CTCL variant characterized by subcutaneous plaques and tumours and cytologically atypical CD8+ T cells rimming fat lobules.241 Localized disease may respond to radiotherapy. Patients may be steroid responsive but are likely to relapse, although immunosuppressive drug therapy has been more effective than chemotherapy for some patients.242 Patients with haemophagocytic syndrome and steroid-resistant disease respond to doxorubicin-based chemotherapy regimens (CHOP), with durable remissions reported.241 Durable responses have also been reported following high-dose chemotherapy and stem cell transplantation.243 Ciclosporin may be effective in patients failing cytotoxic chemotherapy.244
7.2.2 CD4+ small/medium pleomorphic T-cell lymphoproliferative disorder and primary cutaneous CD8+ acral T-cell lymphoma
Primary cutaneous small/medium CD4+ lymphoproliferative disorder has an excellent prognosis, although it can be difficult to distinguish from reactive lymphoid proliferations. Surgical excision and local radiotherapy are acceptable treatment options.245 Rare CD8+ acral CTCL variants also have an excellent prognosis and can also be treated with surgical excision or radiotherapy.246 However, the disease may follow a more aggressive path in patients who present with large rapidly progressive tumours or multifocal disease; this requires treatment as for PTCLs.247
7.2.3 CD8+ aggressive epidermotropic cytotoxic cutaneous T-cell lymphoma and primary cutaneous γδ T-cell lymphoma
Recommendations
- Primary cutaneous CD30+ lymphoproliferative disorders are diagnosed on the basis of careful clinicopathological correlation. Staging investigations are not appropriate for LyP but are essential for ALCL or borderline cases. (Strength of recommendation D (GPP))
- Treatment for LyP ranges from an expectant policy for patients with limited disease to phototherapy, radiotherapy, low-dose methotrexate or interferon-alpha for patients with extensive disease. (Strength of recommendation C)
- Treatment for CD30+ pcALCL consists of surgical excision and/or radiotherapy for localized disease. (Strength of recommendation B)
- Combination chemotherapy or brentuximab may be appropriate for patients with CD30+ pcALCL with extensive cutaneous disease or those with systemic progression. (Strength of recommendation B)
- Treatment of rare CTCL variants depends on the subtype, as some (SPTCL, CD4+ small/medium pleomorphic, CD8+ acral T-cell lymphoma) are indolent with an excellent prognosis, while others are aggressive and may require chemotherapy and consideration for stem cell transplant in line with recommendations for systemic PTCL (NOS). (Strength of recommendation C)
- Primary cutaneous PTCLs (NOS) are rare and heterogeneous in behaviour. Some present with limited skin disease that can be managed with skin-directed options such as radiotherapy, while others present with extensive or bulky skin disease and will require treatment options as for systemic PTCL (NOS). (Strength of recommendation C)
8 Treatment of primary cutaneous B-cell lymphomas
For the purpose of this guideline, separate recommendations have been made for low-grade lymphomas (PCMZL and PCFCL), high-grade PCLBCL and the rare PCLBCL (NOS). PCMZL and PCFCL have an excellent prognosis, and although cutaneous relapses are frequent, systemic spread is rare. PCLBCLs are aggressive lymphomas with considerable mortality and require systemic chemotherapy in most instances. There are no RCTs but there are published EORTC consensus guidelines.11
8.1 Primary cutaneous marginal zone and follicle centre lymphoma
8.1.1 Excision
Complete surgical excision may be considered for small lesions for both diagnosis and therapy, but there is no literature to guide margins of resection. In patients with PCMZL and PCFCL treated with complete excision, over 40% are reported to relapse.11 Where the lesion has been excised completely, a policy of monitoring or radiotherapy to the scar with a 1·5-cm margin to CTV around the scar238 can be considered to reduce the risk of local recurrence. In larger lesions where complete excision would result in a significant cosmetic scar or functional defect, a diagnostic biopsy should be performed and followed by radiotherapy.
8.1.2 Antibiotics
In patients with PCMZL and positive B. burgdorferi serology there are reports of complete response following antibiotic treatment.11 The U.K. Health Protection Agency guidance on treatment for Borrelia with oral antibiotics should be followed before considering other therapies.
8.1.3 Radiotherapy
Radiotherapy is the standard treatment for both PCMZL and PCFCL. The EORTC consensus recommendations11 reported data on 132 patients with PCMZL and 460 with PCFCL treated with radiotherapy. Of the patients with PCMZL, 99% achieved complete remission and only 46% of patients showed one or more relapses. Extracutaneous progression was reported in only three of 132 patients, one of whom died of lymphoma. The radiotherapy doses reported varied from 10 Gy to 50 Gy, and most studies used a treatment margin around the tumour of 1–5 cm.
Of the patients with PCFCL, almost all achieved complete response with radiotherapy. Three major studies in PCFCL combining 147 patients reported a relapse rate of 30% when margins > 2 cm were used.248-250 This differs from the relapse rate of 67% in a small study that used margins of 0·5–1 cm, where frequent in-field and marginal recurrences were reported.251 On the basis of this evidence, it is recommended that the radiotherapy CTV should be the tumour plus a 1·5-cm margin of normal skin around the tumour.238
In the U.K., the standard radiotherapy dose for indolent non-Hodgkin lymphoma is 24 Gy in 12 fractions, based on the results of a multicentre, prospective RCT comparing 40 Gy vs. 24 Gy in 2-Gy fractions for indolent follicular and marginal zone lymphoma.252 Lower doses or shorter fractionation schedules (e.g. 8 Gy in two fractions for multiple lesions; 15 Gy in five fractions for solitary lesions) have been used in many centres with good results.253 The Leiden group have reported complete response rates of 72% and re-treatment rates of 29% with 4 Gy in two fractions, and complete response achieved in all patients retreated with 20 Gy in eight fractions.106 A U.K. RCT has shown that 4 Gy in two fractions can be effective in the palliative setting, with an ORR of 74·1% (complete response rate 44·3%). However, this was significantly inferior to 24 Gy in 12 fractions, with an ORR of 81% and complete response rate of 60·3%.254 The OS rate was the same with both fractionations but there were fewer patients with PCMZL or PCFCL in this study.
8.1.4 Intralesional interferon
There are limited data on the use of interferon-alpha. Vandersee et al. reported a 67% response rate in 15 patients, with 90% of responders relapsing. The median time to relapse was 15·5 months.255
8.1.5 Rituximab
The limited data on rituximab are based on case reports and small case series. Treatment is either as systemic therapy 375 mg m−2 intravenously weekly for 4–8 weeks, or as intralesional therapy 5–30 mg one to three times per week. For intralesional therapy, the complete response rates for both PCMZL and PCFCL are reported to be 70–90%, with relapse rates of 40–60%.256 Systemic therapy is preferred for more widespread disease. For systemic therapy, the complete response rates for PCMZL and PCFCL are similar, ranging from 67% to 87·5%, but again the relapse rates are relatively high, with a median time to relapse of 25 months.257, 258
8.1.6 Chemotherapy
There are relatively little data published on the results of chemotherapy for PCMZL and PCFCL. For PCMZL, chlorambucil for multifocal skin lesions is associated with a complete response rate of 64% and relapse rate of 33%, based on reports of 14 patients. CHOP is associated with a complete response rate of 85% and relapse rate of 57%.11
For PCFCL, cumulative data on 104 patients from eight studies treated with CHOP or CHOP-like regimens for widespread skin lesions or high skin tumour burden give a complete response rate of 85% with a relapse rate of 48%.11 Only five patients received chemotherapy without an anthracycline (COP or CVP; see section 6·3·2). There is a lack of data on the efficacy of rituximab–CVP and rituximab–bendamustine, which are the most commonly used regimens in nodal or systemic indolent lymphomas, in disease confined to the skin.
Recommendations
- Solitary lesions should be treated with the intention of cure. (Strength of recommendation B)
- Small individual skin lesions can be completely excised and monitored, but treatment with radiotherapy (tumour or scar with a 2-cm margin of skin to define CTV) is preferred to reduce the risk of local relapse. (Strength of recommendation B)
- Following a diagnostic biopsy, the first-line treatment for individual skin lesions should be radiotherapy. The radiotherapy CTV should be the tumour plus a 1·5-cm margin of normal skin around the tumour. (Strength of recommendation B)
- Active monitoring is an option for patients with asymptomatic disease. (Strength of recommendation D (GPP))
- Systemic chemotherapy should be reserved for patients with widespread extensive lesions or high tumour burden (stage T2c–T3), or patients with nodal or visceral progression. Treatment is palliative, and for disease confined to the skin consideration should be given to single-agent rituximab, or chlorambucil, as this is well tolerated and effective. Further choice of chemotherapy should be in line with local guidelines for the management of indolent lymphomas. (Strength of recommendation C)
- Patients who relapse with nodal or visceral lymphoma should be treated according to local and national management guidelines for systemic lymphoma. (Strength of recommendation B)
8.2 Primary cutaneous diffuse large B-cell lymphoma
8.2.1 Primary cutaneous diffuse large B-cell lymphoma leg type
The number of reported cases of PCLBCL is small. The response rate to radiotherapy alone is high, but relapse rates are ≥ 58% with a significant risk of extracutaneous recurrences.11 Data on CHOP-like chemotherapy, R-CHOP and R-CHOP plus local radiotherapy are limited to small numbers of patients and short follow-up periods. In a review of 32 patients from eight separate studies treated with CHOP-like chemotherapy, 81% achieved complete response but 56% relapsed.11 Rituximab as a single treatment has an ORR of 75% in eight reported cases but all patients relapsed, with a median disease-free survival of 5·25 months.259 Current international consensus and guidelines recommend that PCLBCL leg type should be treated like a systemic diffuse large B-cell lymphoma (DLBCL), with three to six cycles of R-CHOP and local radiotherapy of 30–36 Gy.6
8.2.2 Primary cutaneous diffuse large B-cell lymphoma (not otherwise specified)
Recommendations
- PCLBCL leg type should be treated with R-CHOP chemotherapy followed by local radiotherapy as per local guidelines for systemic DLBCL. Current protocols recommend three to six cycles of R-CHOP chemotherapy followed by 30–36 Gy in 2-Gy fractions of local radiotherapy. (Strength of recommendation B)
- Palliative radiotherapy alone can be considered for patients unfit for chemotherapy. (Strength of recommendation D)
- PCLBCL (NOS) should be treated as per local guidelines for systemic DLBCL. (Strength of recommendation B)
Future research recommendation
- Future trials should concentrate on improving clinical outcomes for patients with PCLBCL. (Strength of recommendation D (GPP))
9 Economic and practical considerations
- Primary cutaneous lymphomas are rare and consequently the economic costs of treatment are modest, especially for early stages of CTCL and primary cutaneous B-cell lymphomas. In contrast, advanced cutaneous T-cell lymphomas require complex and expensive interventions, often with only palliative intent.
- There has been a growing difference between the U.S.A. and European Union for approval of novel drugs. However, the emergence of several pharmaceutically sponsored phase III RCTs in the last few years suggests that this issue is likely to be resolved, allowing U.K. patients to have access to these new treatments.
- Because of disease rarity, few large RCTs have been concluded. This situation is now improving as both the FDA and EMA have adopted the same approach for orphan drug indications, but for some interventions such as stem cell transplantation the evidence base will develop only through consistent use of data registries.
10 Future directions
- Low-risk, early-stage IA–IIA MF: future trials should address the need to improve duration of response without increasing toxicity following PUVA and TSEB.
- Early-stage IA–IIA MF refractory to SDT: future trials should address the need to improve specific clinical end points such as time to relapse or progression, PFS and OS.
- High-risk advanced-stage IIB–IV MF/SS: future trials are required to address an urgent need to improve PFS and OS.
- The conditioning regimens and outcomes of RIC allo-HSCT for selected groups of patients with advanced disease should be collected through data registries such as the EBMT registry, and large cohort studies should be published with adequate follow-up.
- There is an urgent need for the identification of novel targets in CTCL based on improved understanding of disease pathogenesis.
11 Recommended audit points
In the last 20 consecutive patients seen with primary cutaneous T-cell lymphoma:
- Was the WHO 2016 classification used in recording the following?
- a Cutaneous lymphoma subtype.
- b Tumour phenotype.
- c Folliculotropism in MF.
- d Large cell transformation in MF.
- Were patients staged appropriately?
- Is there a record of performance status?
- Is there a record of Sézary cell count, CD4 : CD8 ratio or abnormal T-cell phenotype on flow cytometry at diagnosis and clinical evolution?
- Is there an annual log of a cumulative treatment summary?
The audit recommendation of 20 cases per department is to reduce variation in the results due to a single patient, and to allow benchmarking between different units. However, departments unable to achieve this recommendation may choose to audit all cases seen in the preceding 12 months.
12 Summary
Figures 1-4 show treatment guidelines for MF, SS, other CTCLs and CBCL, respectively. See the manuscript for details of the evidence.
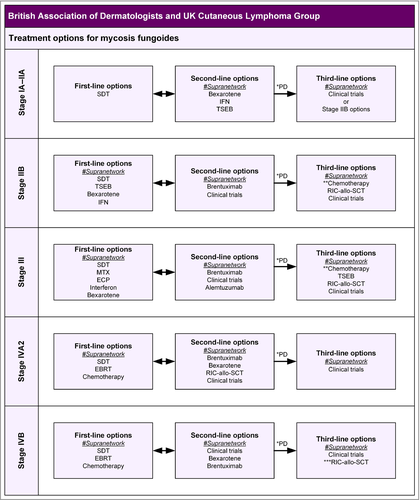
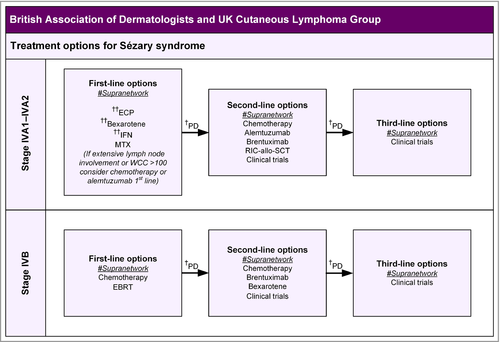
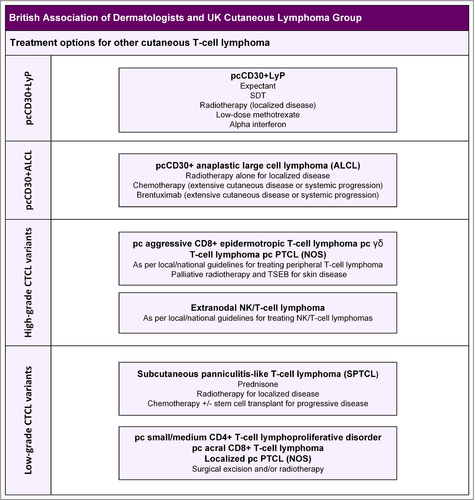
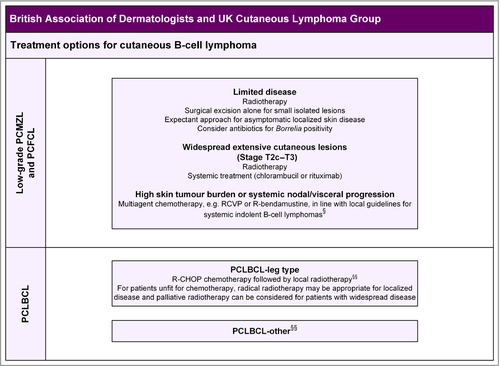
13 Stakeholder involvement and peer review
The GDG consisted of consultant clinical dermatologists, haematologists and oncologists. The draft document was circulated to the BAD membership and the British Society for Haematology, Royal College of Pathologists, British Dermatological Nursing Group, Primary Care Dermatological Society, Royal College of Radiologists, British Lymphoma Pathology Group, British Society for Dermatopathology, U.K. Lymphoma Association and Guy's & St Thomas’ NHS Foundation Trust Skin Lymphoma Discussion Group for comments, and was peer reviewed by the Clinical Standards Unit of the BAD (made up of the Therapy & Guidelines Subcommittee) prior to publication.
14 Limitations of the guidelines
This document has been prepared on behalf of the BAD and is based on the best data available when the document was prepared. Please check the BAD website for any updates (http://www.bad.org.uk/healthcare-professionals/clinical-standards/clinical-guidelines).260 It is recognized that under certain conditions it may be necessary to deviate from the guidelines and that the results of future studies may require some of the recommendations herein to be changed. Failure to adhere to these guidelines should not necessarily be considered negligent, nor should adherence to these recommendations constitute a defence against a claim of negligence. Limiting the review to English-language references was a pragmatic decision but the authors recognize this may exclude some important information published in other languages.
15 Plans for guideline revision
The proposed revision date for this set of recommendations is scheduled for 2023; where necessary, important interim changes will be updated on the BAD website: http://www.bad.org.uk/healthcare-professionals/clinical-standards/clinical-guidelines.
16 Mogamulizumab update
On 20 September 2018, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Poteligeo, intended for the treatment of mycosis fungoides (MF) or Sézary syndrome (SS) having received at least one prior systemic therapy. The active substance of Poteligeo is mogamulizumab, a monoclonal antibody (ATC code: L01XC25) that selectively binds to CC chemokine receptor-4 (CCR4) expressed on the surface of some cancer cells including T-cells of T-cell malignancies, such as MF and SS, resulting in depletion of the target cells. The benefits with Poteligeo are its ability to improve progression-free survival in patients. The approval by FDA and EMA is supported by the MAVORIC (Mogamulizumab anti-CCR4 Antibody Versus ComparatOR In CTCL) study, a Phase 3 open-label, multi-center, randomized study of mogamulizumab versus vorinostat in patients with MF and SS who have failed at least one prior systemic treatment.261 The results showed that mogamulizumab demonstrated significantly superior PFS at a median of 7·6 months [95% CI: 5·6, 10·2] compared to 3·1 months with vorinostat [95% CI: 2·8, 4·0], [hazard ratio 0·53: 95% CI: 0·41, 0·69; P < 0·001]. The confirmed overall response rate for mogamulizumab and vorinostat was 28% and 5%, respectively (P < 0·001). These findings suggest that Mogamulizumab will have a key role in the treatment algorithm for intermediate/advanced stages of MF/SS who are refractory to at least one systemic treatment.
Acknowledgments
We are very grateful to Dr Jennie Wimperis (British Society for Haematology) for her input and our U.K. CLG colleagues Eve Gallop Evans, Eleanor James and Richard Cowan, as well as everyone including the patient organizations who commented on the draft during the consultation period.




