Continuous usage of a hair dye product containing 2-methoxymethyl-para-phenylenediamine by hair-dye-allergic individuals
Funding sources:
None.
Conflicts of interest:
C.G. and M.K. are employees of the Procter & Gamble Company. The hair dye ingredient studied in this article is currently used in commercial products marketed by the Procter & Gamble Company. B.B. and P.J.C. participated as experts in skin sensitization.
Summary
Background
Despite a positive patch test reaction to para-phenylenediamine (PPD) and/or toluene-2,5-diamine (PTD), many people attempt to continue dyeing their hair with products containing PPD or its derivatives.
Objectives
Investigation of elicitation reactions among PPD/PTD-allergic individuals to hair dye products containing the less sensitizing PPD derivative 2-methoxymethyl (ME)-PPD.
Methods
Elicitation reactions were studied in 43 PPD/PTD-allergic individuals by a 45-min pretest with an ME-PPD-containing hair dye on their forearm. Upon a negative result this was followed by exposure to subsequent hair colour treatment(s).
Results
Overall, 38 of 43 PPD/PTD-allergic individuals did not develop an elicitation reaction during the pretest with ME-PPD-containing hair dye products, and were eligible for subsequent hair colour treatments. Of these 38 PPD/PTD-allergic individuals, 29 tolerated subsequent hair dyeing with ME-PPD-containing hair dye products, while seven showed mild and two showed moderate/marked allergic reactions upon the first hair colour treatment.
Conclusions
Hair dye products with the less sensitizing ME-PPD were tolerated by 29 of 43 (67%) PPD/PTD-allergic individuals throughout continued hair dyeing with an average of nine treatments per year. Five individuals reacted upon pretesting, while only mild-to-moderate/marked skin reactions occurred upon hair dyeing in nine individuals who were not identified by the pretest. To our knowledge this is the first study among PPD/PTD-allergic individuals indicating that a negative 45-min pretest with a hair dye product helps to avoid severe allergic reactions.
Allergic contact dermatitis related to the use of hair dye products is an important health concern for a certain subgroup of consumers. This is related mainly to the use of the primary intermediates para-phenylenediamine (PPD) and toluene-2,5-diamine (para-toluenediamine, PTD), which provide excellent hair colouring performance while being considered as major contact allergens among hair dye precursors. Less frequently, other aromatic amine hair dyes are also involved.1
Following the idea of primary prevention of skin sensitization and the onset of contact dermatitis,2 the recently developed precursor 2-methoxymethyl (ME)-PPD3 provides excellent hair colouring performance4, 5 with significantly lower skin-sensitizing properties compared with PPD or the structurally related compound PTD (for the chemical structures see Fig. 1).3 Consequently, based on the available preclinical data, the risk of allergic induction through ME-PPD is considered very low for hair dye consumers because the maximal on-head exposure is more than 100-fold lower than the allergy induction threshold (8·8 vs. 1075 μg cm2). For PPD and PTD the values are much closer to the threshold (16·1 vs. 27·5 and 22·7 vs. 41·3 μg cm2, respectively).3

In contrast to sensitization induction, there is no standard predictive approach to the identification of safe exposure levels for elicitation in hair-dye-allergic individuals,6 nor can this be predicted from knowledge of the relative potency for the induction of skin sensitization.7 For oxidative hair dyes, cross-reactions between PPD, PTD and other para-substituted benzene components are frequently reported.1, 6, 8, 9 Consequently, dermatologists generally recommend discontinuing the use of oxidative hair colours for individuals allergic to PPD or PTD, to avoid cross-reaction with other para-substituted benzene compounds. Nevertheless, many hair-dye-product consumers consider hair dyeing an important personal care need,10 in particular when related to grey hair coverage, and continue to use hair dyes even when they have been advised to discontinue using the respective hair colorants.6, 11
In a recent study in the Netherlands, our group investigated whether PPD-allergic individuals (with a documented history of hair-dye-related allergy) develop cross-reactions to ME-PPD-containing hair dye test products when exposing their forearm, simulating typical oxidative hair dye usage conditions (i.e. application of the hair colour tint mixed with hydrogen peroxide for approximately 30–45 min followed by rinsing). Cross-elicitation to the ME-PPD-containing hair dye test product occurred in nine of 30 individuals (30%), while 70% did not react.12
In the present study in Germany, we analysed whether hair-dye-allergic individuals who did not respond to a 45-min exposure on their forearm (simulating hair dyeing conditions) would tolerate repeated and continued full hair colour treatments at their hairdresser. We report the results of the (cross-)elicitation pretest, as well as the experience with continuous hair colour treatments over an observation period of 1 year.
Materials and methods
Cases
The participants (43 women) were recruited in Germany. They had contacted our consumer service because they were interested in the PPD/PTD alternatives due to their history of allergic contact dermatitis related to hair dyeing. Inclusion criteria were intolerance of hair dye usage and a PPD- and/or PTD-positive diagnostic patch test(s). For all 43 individuals a relevant contact allergy to PPD and/or PTD was documented by a dermatologist in their individual allergy card. For 17 of the 43 individuals the allergy card contained information on the strength of the diagnostic patch test reaction to PPD and/or PTD, and for the other 26 individuals the strength of the patch test response was not specified.
Pretesting with 2-methoxymethyl-para-phenylenediamine-containing hair dye products
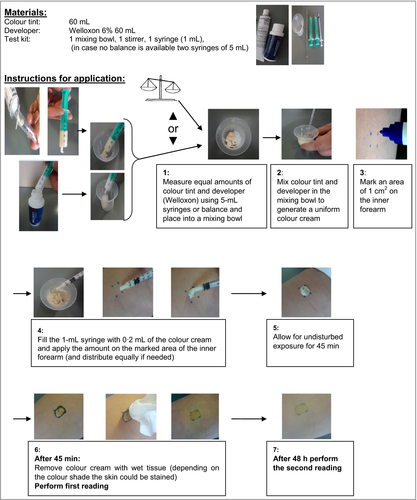
The participants were asked to choose their preferred shade of 10 existing ME-PPD-containing hair dye products, with an ME-PPD on-head concentration range (free base), ranging from 0·15 ± 0·1% to 1·0 ± 0·2%. These shades were grouped into three tonal shade levels (dark to medium-dark, medium-light, light), with appropriate concentration ranges of ME-PPD (Table 1). The formulation base was equivalent to that of conventional hair dye products, comparable with Blömeke et al.12 and Goebel et al.,13 and additionally containing fragrance.
| Shade tone levels | Shade name | Average applied ME-PPD concentration, % |
|---|---|---|
| Dark/medium-dark | Black | 1·0 ± 0·2 |
| Dark brown | ||
| Medium brown | ||
| Light brown | ||
| Light brunette blond | ||
| Medium-light | Dark blond | 0·5 ± 0·1 |
| Medium blond | ||
| Medium brunette blond | ||
| Light | Light blond | 0·15 ± 0·1 |
| Very light blond |
In addition to ME-PPD, the following hair dye precursors were used according to the desired shade: meta-aminophenol, resorcinol, 2-methylresorcinol, hydroxyethyl-3,4-methylenedioxyanline hydrochloride, 1-hydroxyethyl-4,5-diaminopyrazole sulfate, 2,4-diaminophenoxyethanol hydrochloride, 4-amino-2-hydroxytoluene and 2-methyl-5-hydroxyethylaminophenol. Shades and developer (hydrogen peroxide solution 6% w/w, Welloxon) were supplied by Procter & Gamble, Schwalbach, Germany. Materials were sent to the individual dermatologist, who conducted product pretesting with the shade of choice to assess cross-elicitation reactions to ME-PPD, as well as reactions to other dye or nondye ingredients. The ready-to-use colour cream was prepared immediately before testing by mixing equal amounts of the colour tint and the developer.
Having been provided with detailed written instructions (following the protocol of the Allergy Alert Test; http://www.colourwell-colourwise.eu/allergy_alert.html) and syringes (5 and 1 mL), the dermatologist was guided to apply an amount of 0·2 mL of the ready-to-use colour cream on the inner forearm to an application area of 1 cm2 for 45 min (Fig. 2). Readings were performed at 45 min directly after the colour cream had been removed by rinsing (with wet tissue) and after 48 h. Responses were graded into reaction categories according to the German Contact Dermatitis Research Group, comparable with the International Contact Dermatitis Research Group (ICDRG) criteria.14, 15 For documentation of the results an evaluation sheet including a scoring scheme was provided (Table 2).
| Grade | Description of response | Meaning |
|---|---|---|
| − | No reaction | Negative |
| ? | Erythema only, no infiltration | Doubtful reaction |
| + | Erythema, infiltration, possibly discrete papules | Positive, allergic reaction |
| ++ | Erythema, infiltration, papules, vesicles | Double positive, allergic reaction |
| +++ | Erythema, infiltration, confluent vesicles | Triple positive, allergic reaction |
- ‘Irritation’ and ‘not tested’ were removed from the table as these criteria were not considered to be relevant.
Full hair colour treatments with 2-methoxymethyl-para-phenylenediamine-containing hair dye products
Individuals with a negative pretesting result for the shade of choice were sent to their hairdresser to receive a full hair colour treatment with that shade. Supply with the shade through the hairdresser continued for the entire study period between February 2013 and May 2014. For the purpose of continued product supply the individual's hairdresser had to provide feedback (written or by phone call) about the number of colour treatments applied and the corresponding compatibility. All participants were instructed to report to their dermatologist or directly to the study coordinator at any time should any adverse reaction occur.
At termination of the study (April to May 2014) all individuals were queried by telephone about continuous product compatibility. Finally, all individuals and their hairdressers received the information that a corresponding ME-PPD hair dye product is now commercially available in combination with emphasizing once more that careful observation of the product compatibility is of utmost importance for them in collaboration with their dermatologist and hairdresser.
Results
Diagnostic patch test information at study onset and conduct of the study
The study population was comprised of 43 women with a documented history of contact dermatitis to PPD and/or PTD related to hair dyes. The age range was 36–80 years (mean 62), and for one individual no age indication was available. For 17 individuals details of the strength of their diagnostic patch test response for PPD and/or PTD were available: for PPD (13 with +++, two with ++) and/or PTD (three with +++, five with ++, one with +) according to the ICDRG criteria, while for 26 no further specification of the strength of the positive patch test response was available from the allergy card provided by their dermatologist (see Fig. 3 for a flow diagram with an overview of the study).

Shade selection
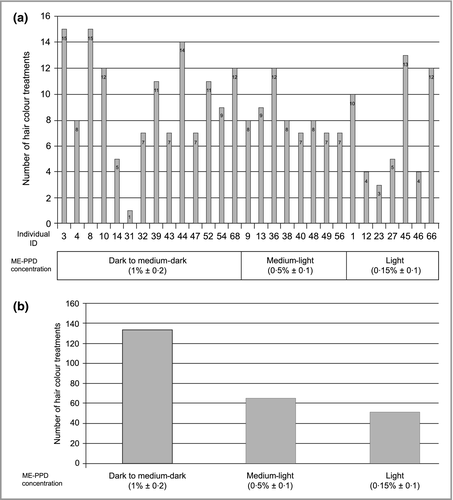
Each individual selected the ME-PPD-containing shade of choice according to tone levels from black to very light blond (Table 1); 26 selected dark to medium-dark shades, 10 selected medium-light shades and seven preferred light shades containing 1·0 ± 0·2%, 0·5 ± 0·1% and 0·15 ± 0·1% ME-PPD, respectively.
Pretesting
The dermatologist of each of the 43 individuals received the selected shade plus developer for the 45-min pretesting on the forearm, together with detailed guidance on how to generate and apply 0·2 mL of the ready-to-use hair dye cream (Fig. 2). Consequently, the applied dose was considered to be 200 mg hair dye product per cm2, equivalent to average applied ME-PPD doses of 2000, 1000 and 200 μg cm2, respectively. The 48-h reading was chosen because it is the current industry recommendation for Allergy Alert Testing on hair colour products, taking into account that readings at further days in practice are considered to affect compliance negatively. In addition, readings at 45 min directly after the colour cream was removed were considered useful to investigate whether immediate contact reactions occur. At the 45-min reading time no reactions were observed. At the 48-h reading time there was a reaction in four individuals (9%) (Table 3). At day 5 after the pretesting exposure, one individual showed a positive reaction that was not obvious at 48 h.
| Individual ID | Initial diagnostic PPD/PTD patch test resulta | Reaction pretestb | |
|---|---|---|---|
| 45 min | 48 h | ||
| 5 | NSP/ND | − | ++ |
| 24 | +++/NSP | − | +++ |
| 26 | +++/+++ | − | + |
| 67 | +++/(+) | − | −c |
| 71 | +++/+++ | − | +++ |
- NSP, no further specification; ND, not documented. aInitial PPD/PTD diagnostic patch test results (if more than one diagnostic patch test result was available, the highest score is presented). bDark to medium-dark tone level with ME-PPD range of 1 ± 0·2% was chosen in all cases. cNo reaction was diagnosed at 48 h, but reaction was reported by the individual at day 5.
These five individuals did not meet the criteria for hair colour treatments with the selected shade. Thus 38 individuals were eligible to receive the first full hair colour treatment at their hairdresser (Fig. 3).
Full hair colour treatments
Upon the first and subsequent full hair colour treatments, 29 of the 38 PPD/PTD-allergic individuals tolerated continuous treatments with the selected ME-PPD-containing hair dye product. The number of full hair colour treatments within the 1-year observation period per individual, related to the shade used (corresponding to the ME-PPD concentrations), is depicted in Figure 4(a). This indicates that up to 15 treatments per individual with the dark to medium-dark shade were well tolerated. The average number of full hair colour treatments per individual was almost nine. For seven individuals detailed results for PPD and/or PTD on their initial diagnostic patch test were available: five were +++ graded to PPD, some also with positive results for PTD (Table 4). For two individuals positive reactions to PTD only were documented, graded ++ and +, respectively.
| Individual ID | Initial diagnostic PPD/PTD patch test result | Average applied ME-PPD concentration (%) | Number of colorations | Interval of colorations (weeks) |
|---|---|---|---|---|
| 52 | +++/++ | 1 | 11 | 4–5 |
| 54 | +++/++ | 1 | 9 | 6–7 |
| 13 | +++/+++ | 0·5 | 9 | 6–7 |
| 12 | +++/NA | 0·15 | 4 | 10–11 |
| 23 | +++/NA | 0·15 | 3 | 16–17 |
- NA, not available.
The total number of full hair colour treatments per ME-PPD concentration level is provided in Figure 4(b), indicating that dark to medium-dark shades were selected for 134 treatments, representing the majority (53%), while medium-light and light shades were used to a lesser degree (26% and 20%, respectively).
Upon the first full hair colour treatment, nine individuals developed allergic contact dermatitis symptoms after first hair dyeing, even though their pretest reaction was negative (Table 5). The symptoms occurred mostly between 8 h and 2 days. In two cases symptoms were recognized either shortly after rinsing or as late as 1 week after the treatment. For six of these nine individuals details of their initial diagnostic patch test strength to PPD were available: four with +++ and two with ++; the latter also reacted ++ to PTD.
| Individual ID | Initial diagnostic patch test result, PPD/PTD | ME-PPD hair dye shade | Severity of symptoms | Time point of first symptoms after rinsing |
|---|---|---|---|---|
| 18 | ++/++ | Medium-light | Mild | 8 h |
| 21 | +++/NSP | Dark/medium-dark | Mild | 2 days |
| 25 | +++/NSP | Dark/medium-dark | Moderate/marked | 1 week |
| 28 | ++/++ | Dark/medium-dark | Mild | 1 day |
| 50 | NSP/NSP | Dark/medium-dark | Moderate/marked | 8 h |
| 51 | +++/ND | Dark/medium-dark | Mild | 1 day |
| 63 | NSP/ND | Medium-light | Mild | Within 15 min |
| 72 | +++/NSP | Dark/medium-dark | Mild | 1 day |
| 74 | NSP/ND | Dark/medium-dark | Mild | 8 h |
- ME-PPD, 2-methoxymethyl-PPD; ND, not documented; NSP, no further specification.
Detailed information about the symptoms was gathered by a telephone interview with each individual. Redness, papules and itching generally occurred on the scalp and adjacent skin areas 8–24 h after the hair colour treatment. In addition four individuals mentioned swelling, with one individual additionally reporting development of vesicles. In two cases a dermatologist and in one case a general practitioner was consulted. They prescribed local treatment. In six cases no physician was consulted. In five of these six cases self-treatment had been applied: three used topical agents, while one had access to oral antihistamines and one treated herself with oral steroids. In one case, antihistamines had been used for other health reasons prior to the hair dye treatment.
The severity of the allergic reaction was evaluated by the clinical symptoms and the intensity of the applied therapy: local therapy (mild reaction), oral therapy (moderate/marked reaction), intravenous therapy and/or hospitalization (severe reaction). Based on this evaluation the degree of the contact dermatitis manifestation was considered to be mild in seven cases and moderate/marked in two cases.
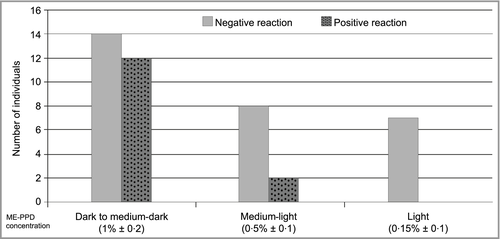
A comparison of all responding and nonresponding individuals regarding the ME-PPD shades used, combining pretesting and full hair colour treatment results, is provided in Figure 5. Positive reactions occurred in 46% (12 of 26) of the individuals exposed to dark to medium-dark shades, in 20% (two of 10) exposed to medium-light shades and in none of the individuals (0 of 7) exposed to light shades.
Discussion
With the introduction of ME-PPD into commercial hair dye products, a PPD/PTD alternative has become available that is considered to avoid de novo generation of hair dye contact allergy for hair dye users.3 However, despite warnings not to use ME-PPD hair colorants in case of hair dye allergy, individuals with existing allergy to PPD and/or PTD are still likely considering the use of ME-PPD-containing hair dye products as an alternative. Consequently, in our present study we extended our previously reported analysis of the cross-elicitation response to ME-PPD in PPD-allergic individuals.12 We asked whether PPD/PTD-allergic individuals tolerate full hair colour treatments with their preferred ME-PPD-containing shade upon negative pretesting on their forearm. Through requests reaching our customer service, we recruited 43 PPD- and/or PTD-allergic individuals with a documented history of hair dye-related allergic contact dermatitis to participate in this study.
Continuous exposures with ME-PPD-containing hair dye products with concentrations up to 1 ± 0·2% were tolerated by 67% (29 of 43) of the individuals in intervals as short as 4 weeks over the 1-year study period (Fig. 4). The limited cross-elicitation rate to the full hair colour treatments of 33% (14 of 43) is comparable with the cross-elicitation rate of 30% to a 30-min forearm exposure simulating hair dyeing with the maximum ME-PPD concentration of 2%.12
Among the 38 individuals with negative pretesting results, an additional nine individuals showed skin reactions, with symptoms ranging from mild in seven cases to moderate/marked in two cases following the first full hair colour treatment with their selected ME-PPD-containing hair dye product (Table 5). The reasons for this difference are not clear, but one likely explanation may be that the pretesting challenge on the forearm was not sufficient to trigger an elicitation response. When subsequently the scalp and hairline skin were exposed upon the first hair colouring it appears likely that the repeated availability of the antigen was sufficient to overcome the (cross-)elicitation threshold for memory T cells, leading to an allergic contact dermatitis in these individuals.16, 17
Furthermore, the individual sensitivity, as well as the exposure concentration, affects the time until an allergic individual (cross-) reacts, as was shown for isoeugenol.18 Thus, differences regarding the reactivation threshold may also have impacted the individual onset of the (cross-) elicitation responses observed in two individuals with symptoms occurring within 15 min or as late as 1 week after the hair colour treatment (Table 5). This is in line with the recently described variability of time intervals between hair dyeing and dermatitis onset (1 day up to 1 month).19
Ho et al.20 analysed the tolerance of PPD-containing hair dye products in PPD-allergic individuals based on the strength of their previous response to PPD under diagnostic patch testing: tolerance was recorded in 73% of 55 cases with a mild strength (+) and 49% of 61 patients with a moderate strength (++), but in none of 14 with a severe strength (+++). In contrast, we found tolerance to ME-PPD-containing hair colorants in five cases with a strong diagnostic patch test reaction to PPD (+++), for three of them with a frequency of full hair colour treatments of every 4–7 weeks for dark to medium-dark and medium-light shades (Table 4). These findings indicate that the addition of the methoxymethyl side chain to PPD yielding ME-PPD can strongly reduce or completely prevent T-cell recognition even in strongly PPD-sensitized individuals.
Apart from limited T-cell recognition, low exposure (e.g. through short contact time and professional colouring techniques, as reported by Chan et al.)11 may have been relevant for the clinical tolerance observed (Fig. 4). This is further supported by our finding that the percentage of positive elicitation reactions increased with the increasing ME-PPD concentrations of the shades used (Fig. 5). For the individuals without specified reaction strength, low PPD contact sensitivity may be additionally considered a reason for the clinical tolerance observed.
So far, our results indicate that hair dyeing with ME-PPD-containing hair colour is associated with a certain risk for PPD/PTD-allergic hair dye consumers, with a (cross-)elicitation rate of approximately 30%. Based on this experience with the small number of 43 allergic individuals, pretesting with the hair colour product of choice can be considered helpful to provide an alert of a strong (cross-)reactivity, but it is insufficient to provide an indication of the absence of mild to moderate/marked symptoms.
A commercially available pretesting kit containing solely PPD reportedly failed in a PTD/PPD-allergic individual with a past 48-h diagnostic patch test strength of +++ on PTD and ++ on PPD.21, 22 Although PPD is considered an acceptable screening agent,9 other hair colour precursors23, 24 and formulation ingredients25 were described as further responsible allergens in allergic contact dermatitis to hair dye products. Therefore we consider pretesting with the hair colour product selected by the consumer as advantageous, because it contains all possible allergens.
In summary, we found that pretesting with the ME-PPD-containing hair colour product of choice was predictive for 77% of PPD/PTD-allergic individuals (33 of 43), and 67% (29 of 43) tolerated continuous hair colouring during the observation period of approximately 1 year. This indicates that due to its structural difference, the recognition of ME-PPD by the immune system of a considerable number of allergic individuals is reduced, or possibly completely lacking in some cases.12 This is in line with the primary prevention benefit of ME-PPD in terms of significantly reduced skin sensitization potency for all hair colour consumers.3
In terms of secondary prevention, the recommendation for hair-dye-allergic individuals to avoid hair colouring, including PPD/PTD alternatives such as ME-PPD, is a relevant measure because of a (cross-)elicitation rate of approximately 30%. However, avoidance of hair dyeing by allergic individuals is known to be a challenge, as patients have been described who do not want to avoid hair dyeing, disregarding their dermatologists’ advice and continuing conventional hair dyeing ignoring their symptoms.10, 20, 26 Our study for the first time correlates two critical steps for hair-dye-allergic individuals: forearm (pre-)testing with the selected hair dye shade at the individual's dermatologist, and in case of a negative result subsequent professional hair colour treatment(s) (Fig. 3). We consider this approach as providing relevant guidance for the allergic individuals, helping to avoid the underestimation of the risks associated with hair dyeing, and that consultation with the dermatologist is of major importance for them prior to considering the use of hair colourings.
Acknowledgments
We would like to thank Harald Schlatter, Maike Seib, Angela Schmenger and Maria Castan for their support. For administrative support we would like to thank Erika Bitsch and Andrea Haselwanger.




