Both sexes suffer increased parasitism and reduced energy storage as costs of reproduction in the brown anole, Anolis sagrei
Abstract
Sexual selection theory proposes that males suffer reduced immune function and increased parasitism as costs of expressing sexual signals. Life-history theory proposes that females suffer the same costs because of inherent trade-offs between reproduction and self-maintenance. Mechanistically, each theory invokes an energetic trade-off, although few experiments have directly compared these costs of reproduction between the sexes as a result of fundamental sex differences in the nature of reproductive investment and a tendency for each theory to focus on a single sex. To test whether males and females experience comparable costs of reproduction in terms of energetics, immune function, and parasitism, we used gonadectomy to eliminate most aspects of reproductive investment in wild brown anole lizards (Anolis sagrei) of both sexes. We compared these nonreproductive males and females with intact, reproductive controls with respect to stored energy (fat bodies), immune function (swelling response to phytohemagglutinin), and the prevalence and intensity of infection by four types of parasite (gastric nematodes, intestinal nematodes, faecal coccidia, and ectoparasitic mites). Gonadectomized anoles experienced dramatic increases in fat storage that were accompanied by decreases in the prevalence of intestinal nematodes and in the intensity of coccidia infection. These costs of reproduction were comparable between males and females, although neither sex exhibited the predicted increase in immune function after gonadectomy. Our results suggest that, despite fundamental sex differences in the nature of reproductive investment, both male and female anoles experience similar costs of reproduction with respect to energy storage and some aspects of parasitism.
Introduction
As a result of fundamental differences in their reproductive biology, males and females invest resources in different aspects of reproduction (Trivers, 1972; Clutton-Brock & Parker, 1992; Rolff, 2002; Maklakov & Lummaa, 2013). Males of many species maximize their reproductive success by increasing their mating opportunities, and so they often invest heavily in ornaments, weaponry, mate searching, courtship, and territory defence (Bateman, 1948; Jones, Arguello & Arnold, 2002; Bonduriansky et al., 2008). Females are limited by the time and energy required to provision an embryo, and so they are inherently predisposed to allocate resources to vitellogenesis, egg or embryo production, and parental care (Queller, 1997; Kokko & Jennions, 2008). Despite these fundamental sex differences, total reproductive investment is predicted to be equal for males and females when the adult sex ratio is equal. This is because, at the population level, each sex is engaged in intrasexual competition for the same overall fitness benefit: half of the genes that will be passed on to the next generation (Fisher, 1930; Queller, 1997). However, this prediction is often difficult to assess in wild populations because of inherent sex differences in the nature and seasonal timing of reproductive investment (Cox, 2014). Consequently, the costs associated with reproduction are rarely considered simultaneously in both sexes (Queller, 1997; Bonduriansky et al., 2008; Chu & Lee, 2012).
One reason for this lack of integration between sexes stems from the historical separation of sexual selection theory, which has focused on costs of reproduction that enforce honest signalling in males (Zahavi, 1975; Folstad & Karter, 1992), and life-history theory, which grew from an interest in costs that structure the evolution of demography and population growth via female reproduction (Cole, 1954; Gadgil & Bossert, 1970). This distinction can be illustrated by considering immunosuppression and parasitism as proximate costs of reproduction (Cox, 2014). As an extension of sexual selection theory, the immunocompetence handicap hypothesis predicts that male vertebrates should suffer increased parasitism resulting from the immunosuppressive effects of androgens that coordinate the expression of secondary sex traits, which serve as honest indicators of health and quality (Folstad & Karter, 1992). Although males exhibit lower immune function and higher parasite loads than females in some taxa (Moore & Wilson, 2002; Nunn et al., 2009), the reverse is true in others (McCurdy et al., 1998), and general tests for male-biased parasitism and immunosuppression yield equivocal results (Roberts, Buchanan & Evans, 2004). One reason may be that reproductive females, similar to males, also experience costs of reduced immune function and increased parasitism (Nordling et al., 1998; Ardia, Schat & Winkler, 2003; French, DeNardo & Moore, 2007; Cox et al., 2010; McKean & Lazzaro, 2011). Life-history theory assumes that such physiological costs structure the evolutionary trade-off between reproduction and survival (Rose & Charlesworth, 1981), and reduced immunocompetence in females is considered to result from energetic trade-offs between reproduction and self-maintenance (Sheldon & Verlhust, 1996; Martin, Scheuerlein & Wikelski, 2003; Martin, Hawley & Ardia, 2011). These energetic trade-offs provide a mechanism that is common to both sexes because the immunosuppressive effects of androgens invoked by the immunocompetence handicap hypothesis are considered to derive from their role in coordinating energy allocation between reproduction and immune defence (Wedekind & Folstad, 1994; Cox, 2014). Therefore, both sexes are predicted to face pronounced energy allocation trade-offs during reproduction, which could result in similar costs with respect to reduced immune function and increased parasitism. However, the extent to which these costs are comparable between the sexes is generally unknown.
Experimental manipulations of reproductive investment are often necessary to provide conclusive evidence for proximate costs of reproduction (Reznick, 1992; Landwer, 1994; Cox, 2006). However, experiments can be complicated by the fact that reproductive investment occurs at different times, involves different aspects of reproduction, and incorporates different regulatory pathways in each sex (Cox, 2014). As a simplified example, male vertebrates tend to incur large respiratory (i.e. metabolic) costs related to androgen-mediated courtship, mate-searching, and territory defence at the outset of the breeding season, whereas female vertebrates tend to incur large production (i.e. biosynthetic) costs related to oestrogen- and progesterone-mediated provisioning of eggs and embryos later in the season (Michener & Locklear, 1990; Hoffman et al., 2008; Cox, 2014). Experimental comparisons of reproductive costs are therefore most straightforward in situations where the timing and nature of reproductive investment are closely aligned for males and females. For example, in birds with biparental care, brood manipulations (egg removals and additions) have demonstrated that a loss of body mass and increased parasitism are costs incurred by one or both sexes during offspring provisioning (Allander, 1997; Velando & Alonso-Alvarez, 2003; Christe et al., 2012). However, brood manipulations only address a single aspect of reproductive investment, and their generality is unclear beyond the relatively small proportion of species that provide biparental care (Cox, 2014). Other studies have directly compared the costs of reproduction between the sexes by manipulating mating frequency or the duration of exposure to mates (Kotiaho & Simmons, 2003; Fedorka, Zuk & Mousseau, 2004; Dugas, Wamelink & Richards-Zawacki, 2015), although the costs associated solely with mating likely represent different fractions of total reproductive investment for each sex. An ideal approach would manipulate total reproductive investment and directly compare the magnitude of its associated costs between the sexes.
One way of experimentally assessing the total cost of reproduction is to eliminate most aspects of reproductive investment via gonadectomy. In female brown anole lizards (Anolis sagrei), this method has been used to demonstrate pronounced costs of reproduction with respect to growth, body condition, energy storage, hematocrit, immune function, and survival (Cox & Calsbeek, 2010a; Cox et al., 2010; Cox, Lovern & Calsbeek, 2014b). In principle, castration can also be used to eliminate direct costs of gamete production in males and to reduce or eliminate other indirect costs as a result of androgen-mediated behaviour and physiology. Castration of males abolishes some reproductive and territorial behaviours completely, and reduces the frequency or elevates the stimulus threshold necessary for others (Barfield, Busch & Wallen, 1972; Arnold, 1975; Adkins, 1977; Harding, Sheridan & Walters, 1983; Tokarz, 1986; Tokarz et al., 2002). In the present study, we used gonadectomy to reduce or eliminate most aspects of reproductive investment in a wild population of brown anoles. We then directly tested whether males and females experience similar costs of reproduction with respect to energy storage (wet mass of fat bodies), immune function (swelling response to a novel antigen, phytohemagglutinin), and the prevalence (percentage of infected individuals) and intensity (number of parasites per infected individual) of infection by four different parasites: gastric nematodes (Physalopteridae), intestinal nematodes (Atractidae), feocal coccidia (Eimeriidae), and ectoparasitic mites (Trombiculidae). We predicted that reduction of reproductive investment via gonadectomy would increase energy storage and immune response at the same time as decreasing the prevalence and/or intensity of parasites in both sexes. In accordance with the general prediction that overall reproductive investment of males and females should be equivalent, we predicted that treatment effects would be similar in both sexes (Rolff, 2002; Bonduriansky et al., 2008).
Material and Methods
Study species
The brown anole (A. sagrei) is a sexually dimorphic lizard that is native to Cuba and The Bahamas. Adult males from the closed island population that we studied at Regatta Point on Great Exuma in The Bahamas (23°30′N, 75°45′W) are 32% larger than females, on average, in terms of snout–vent length and 150% larger in terms of mass (Cox & Calsbeek, 2010b). Males fight and display to establish territories encompassing multiple females, and their seasonal reproductive investment extends approximately from the onset of testicular recrudescence and elevated testosterone levels in February until the cessation of mating around September (Tokarz, 1985, 1998; Tokarz et al., 1998). Females repeatedly produce single-egg clutches approximately every 10 days from April to as late as October, with follicular maturation and ovulation continuously alternating between right and left ovaries (Lee et al., 1989; Cox & Calsbeek, 2010a). Genetic analyses reveal a high incidence of multiple paternity across successive eggs produced by individual females (Calsbeek et al., 2007), such that both sexes are polygamous. Because opportunities for fertilization are continuously available and females are constantly gravid, the overall timing of reproductive investment in this species is broadly similar between sexes across most of the lengthy breeding season.
Experimental design and surgical procedures
We used a hand-held noose to capture 220 female and 120 male A. sagrei adults early in the reproductive season (23 May to 4 June), well after the onset of mating activity (February/March) and shortly after the onset of regular oviposition (April/May) but 4–5 months prior to the cessation of mating activity and oviposition (September/October). We measured snout–vent length (nearest 1 mm) and body mass (nearest 0.01 g) for each lizard. We then marked each animal with a unique toe clip and randomly assigned it to one of two treatment groups: (1) bilateral gonadectomy (GDX: removal of both ovaries or testes, N = 110 females, 60 males) or (2) control surgery (gonads manipulated but left intact, N = 110 females, 60 males). During surgery, we confirmed that all experimental animals were in reproductive condition, as determined by enlarged testes with visible seminiferous tubules or enlarged ovaries with vitellogeneic follicles. We followed previously reported surgical protocols (Cox et al., 2009, 2010, 2014b; Cox & Calsbeek, 2010a) that began with the administration of local anaesthesia and analgesia (2–4 μL injection of 0.25% bupivacaine HCl; Hospira Inc.). We then immobilized animals with a 5–8 min exposure to 4 °C and conducted surgeries on top of a chemical ice pack with a slighlty thawed boundary layer. For all surgeries, we made a single 5–8-mm ventral incision into the coelomic cavity, and then ligated, ablated, and cauterized each gonad for the gonadectomy treatment. For control surgeries, we briefly exteriorized the gonads and returned them to the body cavity intact. We closed incisions with VetClose cyanoacrylate surgical glue (Butler Schein Animal Health). We allowed animals to recover overnight in individual containers, and then released each animal at its exact site of capture the next day.
We returned to Regatta Point 10 weeks later (6–15 August) to recapture 50 of 120 males (17 GDX; 33 control) and 51 of 220 females (23 GDX; 28 control), which were used to assess sex and treatment effects on energy storage, immune function, and parasite loads (see below). Although we have previously documented an increase in survival of GDX females, relative to controls, in a nearby population (Cox & Calsbeek, 2010a; Cox et al., 2010), we did not detect any treatment effect on female survival in the present study (logistic regression: χ2 = 0.41, P = 0.52), and survival rates of both GDX and control females (21% and 25%, respectively) were at the low end of natural variation across six previous years at Regatta Point (mean 33%, range 17–46%). By contrast, survival of control males (55%) was significantly higher than that of GDX males (28%) in the present study (logistic regression: χ2 = 9.31, P < 0.01), and higher than in any of nine previous years of study on unmanipulated males at Regatta Point (mean 38%, range 24–46%), whereas survival of GDX males was within the lower range of natural variation across previous years. Consequently, we found a significant sex difference in survival in the present study (logistic regression: χ2 = 11.36, P < 0.001), driven primarily by the atypically high survival of control males (χ2 = 3.56, P = 0.059), as well as an overall treatment effect on survival (χ2 = 7.46, P < 0.01). Because this treatment effect was in the opposite direction predicted from previous studies of GDX and control females (Cox & Calsbeek, 2010a; Cox et al., 2010), we do not interpret these results in the context of survival costs of reproduction. It is unclear whether this indicates that our gonadectomy procedure itself detrimentally affected the survival of males, or that survival of control males was atypically high by chance, or both. Consequently, we focus on inferring costs of reproduction from measures of energy storage, immune function, and parasite levels of recaptured animals.
Energy storage
Anoles and other lizards store energy in paired, abdominal fat bodies (Derickson, 1976). This energy is used for gonadal recrudescence, egg provisioning, and nutrition during periods of low food availability (Chapman & Chapman, 1964; Sexton et al., 1971; Lin, 1979). The size of these fat bodies cycles seasonally in anoles, increasing as reproductive activity decreases toward the end of the breeding season (Licht & Gorman, 1970). Analysis of stomach contents at multiple time points throughout the year suggests that, rather than being driven by patterns of food intake, changes in the mass of fat bodies are instead driven by reproductive activity as stored energy is mobilized to fuel reproduction (Lee et al., 1989). Although the fat bodies are not the sole sites of fat storage, they are a useful index of energetic savings associated with the cessation or elimination of reproduction (Cox et al., 2010, 2014b). To assess the energetic cost of reproduction, we compared the wet mass of abdominal fat bodies dissected from a subset of recaptured males and females in each treatment (N = 23 total: 6 GDX males, 5 control males; 7 GDX females, 5 control females).
Immune function
We assayed immune function by measuring the localized swelling response to challenge with a novel antigen, phytohemagglutinin (PHA), for a subset of recaptured individuals (N = 65 total: 10 GDX males, 22 control males; 12 GDX females, 21 control females). Injection of PHA induces both innate and acquired immune defences (Kennedy & Nager, 2006), including the influx of lymphocytes, heterophils, thrombocytes, basophils, and macrophages, manifesting as localized swelling at the site of injection (Martin et al. 2006). The extent of localized swelling in response to PHA is typically interpreted as a measure of immunocompetence, with greater swelling indicative of a more robust immune response (Goto et al., 1978; Smits, Bortolotti & Tella, 1999; Calsbeek, Bonneaud & Smith, 2008; but see Kennedy & Nager, 2006). We used a dial caliper to measure the thickness of each animal's right hind foot to the nearest 0.1 mm between the first and fifth digits, and then subcutaneously injected 0.1 mg of PHA (Sigma-Aldrich Inc., St Louis, MO, USA), dissolved in 0.01 mL of phosphate-buffered saline, at this same location. We measured the thickness of the foot again 24 h after injection of PHA and calculated the proportional increase in the thickness of the foot as the difference between initial and final thickness divided by initial thickness. We used the mean of three consecutive measurements per individual at each time point in our analysis.
Parasite infection
We used a hand lens to count external mite parasites (Trombiculidae) on a subset of recaptured individuals (N = 80 total: 15 GDX males, 24 control males; 19 GDX females, 22 control females). We then held all animals for 24 h in sanitized plastic containers to collect a faecal sample for later quantification of coccidian oocysts (N = 55 individuals provided fecal samples: 8 GDX males, 19 control males; 11 GDX females, 17 control females). We measured the wet mass of each faecal sample and stored it in 1 mL of 10% formalin. We subsequently placed each fecal sample into a Fecalyzer (EVSCO Pharmaceuticals) with 8 mL of Fecasol (Vétoquinol), ground each faecal sample into solution, and added additional Fecasol (approximately 7 mL) until a meniscus formed on the top of the tube. We then placed a coverslip on the meniscus and let each sample stand for 17 min to allow oocysts to float to the surface before transferring the coverslip to a clean microscope slide for examination at 100 × magnification. We recorded the number of coccidian oocysts on the entire coverslip for each individual and expressed this number per mg of feces. We identified all oocysts as belonging to Eimeriidae, a family of intracellular protozoan parasites within the Coccidia subclass of the phylum Apicomplexa. Coccidians induce cell damage in their hosts, although the degree to which they can be considered pathogenic in wild reptiles is largely unknown (Greiner, 2003).
To quantify both intestinal and gastric nematodes, we dissected the gastrointestinal tracts from the same subset of individuals that we killed to assess fat storage (N = 23 total: 6 GDX males, 5 control males; 7 GDX females, 5 control females). We stored the entire gastrointestinal tract in 10% formalin, then sectioned lower gastrointestinal tracts into 1-cm pieces and counted the total number of rectal nematodes (Atractidae) in all sections under a dissection scope. Atractid nematodes are viviparous, undergo direct development in the lower intestine of their host, and have a venereal mode of transmission between individual hosts (Norval et al., 2011; Langford, Willobee & Isidoro, 2013). For the same subset of animals, we dissected the stomach and counted all visible nematodes (Physalopteridae). Physalopterids parasitize all vertebrate classes and their attachment to gastric mucosa can result in inflammation and excessive mucus production (Levine, 1968; Goldberg & Bursey, 1989).
Statistical analysis
To test for effects of sex and reproductive investment on energy storage, we used analysis of variance (ANOVA) with mass-specific fat mass [fat mass/(body mass – fat mass)] as the response variable and main effects of sex and treatment with a sex-by-treatment interaction. To test for effects of reproductive investment on immune function, we used ANOVA with proportional swelling response to PHA [(final thickness – initial thickness)/initial thickness] as the response variable and main effects of sex and treatment with a sex-by-treatment interaction. To account for scaling of fat mass or swelling response with body mass, we also performed analysis of covariance with either absolute fat mass or swelling as the response variable, treatment as the main effect, and body mass as a covariate. We conducted these analyses separately within each sex because males and females do not overlap in the covariate (body mass). To compare the prevalence of infection by each parasite (i.e. the proportion of lizards infected) between sexes and treatment groups, we used nominal logistic models with the presence of each parasite type (0 or 1) as the response variable, sex and treatment as main effects, and a sex-by-treatment interaction. Because parasite count data were non-normally distributed, we used generalized linear models to compare the intensity of infection by each parasite (i.e. number of parasites per infected individual) between sexes and treatment groups (Alexander, 2012) with untransformed parasite counts (O'Hara & Kotze 2010) as the response variable, fitted with a Poisson distribution and an over-dispersion parameter estimated as the Pearson chi-squared value divided by degrees of freedom. For each of these models, we tested for effects of sex, treatment, and their interaction.
Results
Energy storage and immune function
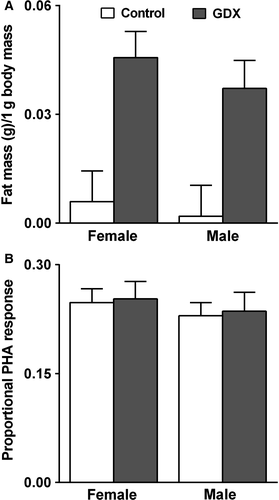
Experimental reduction of reproductive investment via gonadectomy dramatically increased the wet mass of fat bodies (treatment: F1,19 = 21.99, P < 0.001) (Fig. 1A). The treatment effect did not differ between sexes (sex × treatment: F1,19 = 0.08, P = 0.78) and there was no overall sex difference in fat storage per unit body mass (sex: F1,19 = 0.61, P = 0.44). Separate models that included body mass as a covariate also showed that treatment reduced fat body mass for both sexes (females: F1,11 = 14.77, P < 0.005; males: F1,10 = 24.98, P < 0.005). Swelling response to PHA did not differ as a function of sex (F1,65 = 0.67, P = 0.42), treatment (F1,65 = 0.06, P = 0.81) or their interaction (F1,65 < 0.01, P = 0.98) (Fig. 1B). Separate analyses with body mass included as a covariate also indicated no treatment effect on swelling response to PHA for either sex (females: F1,32 = 2.58, P = 0.12; males: F1,31 = 0.39, P = 0.54).
Prevalence and intensity of parasite infection
Experimental reduction of reproductive investment via gonadectomy resulted in a significant decrease in the prevalence (presence or absence) of atractid nematodes in the intestine but did not affect the prevalence of physalopterid nematodes in the stomach, eimeriid coccidia in the faeces or trombiculid mites on the ectoderm (Fig. 2, Table 1). Males and females did not differ in the prevalence of infection by atractid nematodes, physalopterid nematodes, eimeriid oocysts or trombiculid mites (Fig. 2, Table 1). Treatment effects on the prevalence of infection did not differ by sex for any class of parasite (Fig. 2, Table 1).
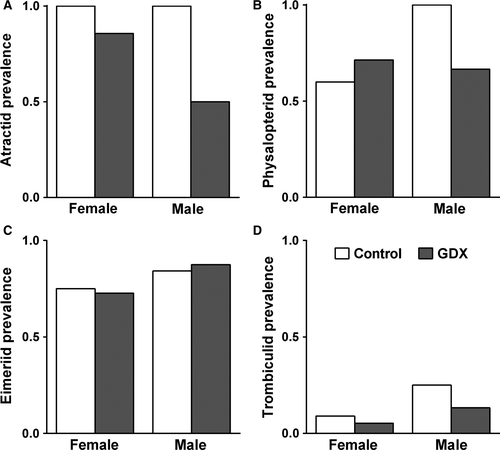
| Parasite family | Model effect | Prevalence (±) | Intensity (number) | ||||
|---|---|---|---|---|---|---|---|
| d.f. | χ2 | P | d.f. | χ2 | P | ||
| Atractidae | Sex | 19 | 0.01 | 0.99 | 15 | 2.83 | 0.09 |
| Treatment | 4.51 | 0.038 | 0.45 | 0.50 | |||
| Sex × Treatment | 0.01 | 0.99 | 1.22 | 0.27 | |||
| Physalopteridae | Sex | 19 | 2.07 | 0.15 | 15 | 1.35 | 0.24 |
| Treatment | 1.47 | 0.23 | 0.56 | 0.46 | |||
| Sex × Treatment | 2.46 | 0.10 | 0.01 | 0.99 | |||
| Eimeriidae | Sex | 51 | 0.98 | 0.32 | 40 | 2.04 | 0.15 |
| Treatment | 0.01 | 0.96 | 4.26 | 0.039 | |||
| Sex × Treatment | 0.10 | 0.76 | 0.38 | 0.54 | |||
| Trombiculidae | Sex | 80 | 2.21 | 0.14 | 7 | 4.50 | 0.034 |
| Treatment | 0.82 | 0.36 | 0.02 | 0.87 | |||
| Sex × Treatment | 0.01 | 0.09 | 0.02 | 0.87 | |||
- Significant (P < 0.05) effects are shown in bold.
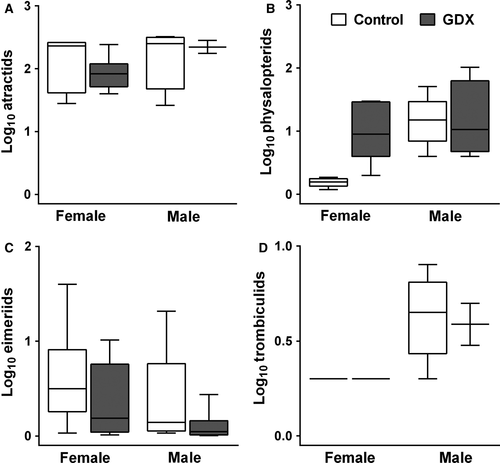
Experimental reduction of reproductive investment via gonadectomy resulted in a significant decrease in the intensity of infection (parasites per infected host) by eimeriid coccidia but did not affect the intensity of infection by physalopterid nematodes, atractid nematodes or trombiculid mites (Fig. 3, Table 1). Infected males had a greater number of mites than infected females (Fig. 3, Table 1), although we did not detect any other sex differences in the intensity of infection, nor did treatment effects on the intensity of infection differ by sex for any class of parasite (Fig. 3, Table 1).
Discussion
Reproductive investment is predicted to be equivalent in males and females because, at the population level, mean reproductive success should be equal for each sex when the adult sex ratio is balanced (Fisher, 1930; Queller, 1997). Hence, each sex stands to gain comparable genetic benefits from reproduction. Whether this means that the myriad costs of reproduction should also tend to be similar in each sex is less clear, particularly when these costs are measured in units (e.g. stored energy, intensity of parasitism) that do not translate directly into fitness (Cox, 2014). In the present study, we found comparable costs of reproduction in each sex with respect to energy storage and several measures of parasite infection, which we view as broadly consistent with the prediction that both sexes should invest similarly in reproduction. In female brown anoles, gonadectomy has previously been used to demonstrate physiological costs of reproduction including reduced energy storage, immune function, and parasite tolerance (Cox et al., 2010; Cox & Calsbeek, 2011). Our experiment extends those results by demonstrating that males also experience similar costs of reproduction. Despite dramatic differences in the behavioural and physiological basis of reproductive investment in each sex, males and females experienced costs of reproduction that were comparable with respect to energy storage and parasite load, although we note that small sample sizes reduced the power of our statistical tests for sex-by-treatment interactions for several parasites (Table 1). An additional caveat to the present study is that our experimental design limited our assessment of costs of reproduction to animals that survived to be recaptured, which could complicate our inferences about treatment effects if, for example, individuals with particularly high or low levels of energy storage or parasitism were more likely to survive in one treatment group than another.
The similar increase in the mass of fat bodies for both sexes following gonadectomy suggests that, for both male and female brown anoles, the energy invested in reproduction is diverted from storage over the course of the breeding season. However, given the divergent reproductive strategies of each sex, different mechanisms probably underlie these otherwise comparable energetic responses. For females, the biosynthetic demands of provisioning eggs are likely to drive the total cost of reproduction. The mass of a single egg is typically close to 10% of the body mass of an adult female brown anole, and individual females repeatedly lay single eggs approximately once every 10 days. Across a 6-month breeding season, a female anole may therefore have a total reproductive output far exceeding its own body mass (Andrews & Rand, 1974). Removal of the ovaries eliminates this large biosynthetic cost and likely results in a substantial energetic savings. However, we cannot rule out additional, non-exclusive explanations, such as an increase in the abdominal space available for food and fat storage, direct effects of ovarian hormones on fat storage and metabolism or indirect effects of ovarian hormones on behaviours that influence energy acquisition and/or expenditure (Cox et al., 2010). Although we cannot rigorously address most of these possibilities, GDX and control females do not differ in their aggressive responses to staged territorial intrusions by females, nor does exogenous oestradiol (which may be reduced by gonadectomy) have any discernable effect on display behaviour in captive females (Cox et al., 2014a, b; E. Parker, N. Brown, R. Cox & R. Calsbeek, unpubl. data).
For male anoles, competitive interactions with other males and the associated metabolic costs of activity and territory defence are likely to contribute heavily to the overall cost of reproduction. Male anoles are highly aggressive towards each other and engage in frequent displays and occasional combat throughout their lengthy breeding season (Evans, 1938; Stamps, 1977; Jenssen, Greenberg & Hovde, 1995). Surgical and chemical castration of male anoles abolishes some aggressive behaviours at the same time as reducing the frequency others (Tokarz, 1986, 1995; Tokarz et al., 2002; Cox et al., 2009), and these behavioural aspects of male reproductive investment have demonstrable metabolic costs in other lizards (Marler et al., 1995; Cox, Skelly & John-Alder, 2005). Mechanistically, many of these energetic effects are likely mediated by a reduction in circulating levels of androgens such as testosterone, which we have previously confirmed as a consequence of our gonadectomy procedure (Cox & John-Alder, 2005; Cox et al., 2005, 2009). These effects could occur directly, given that testosterone reduces body fat in many vertebrate species (Ketterson et al., 1991; Cox et al., 2014a) or indirectly because of the elimination of androgen-mediated behaviours with energetic costs (Marler et al., 1995; Cox et al., 2005). As in the case of females, we cannot rule out additional, non-exclusive explanations for increased energy storage in GDX males, such as an increase in the abdominal space available for food and fat storage (the testes comprise a substantial portion of the coelomic cavity during the breeding season) or the energetic costs associated with maintaining enlarged testes and supporting spermatogenesis. Indeed, one advantage of gonadectomy is that it provides a holistic assessment of the summed contributions of all of these aspects of reproductive investment in each sex.
Irrespective of the exact causative factors underlying the energetic costs of reproduction, our results are consistent with the idea that reproductive anoles have less energy available for functions such as immune defence, which may leave reproductive anoles of either sex vulnerable to parasites if there is an energetic cost associated with parasite defence. Reduced immune defence against parasites and pathogens is frequently implicated as a cost of reproductive investment mediating the ubiquitous trade-off between reproduction and survival (Gustafsson et al., 1997; Lochmiller & Deerenberg, 2000; Norris & Evans, 2000; Harshman & Zera, 2007). By contrast to previous work in this species, we did not detect compromised immune function (reduced swelling response to PHA) as a cost of reproduction (Cox et al., 2010; Cox & Calsbeek, 2011), potentially because our sample sizes were relatively small and the PHA assay is subject to considerable measurement error (Smits et al., 1999). Despite this, we observed reductions in the prevalence or intensity of parasite infection for two of the four parasite types that we quantified (atractid nematodes, eimeriid oocysts), which builds on previous studies of lizards demonstrating that gonadectomy decreases ectoparasite loads in male striped plateau lizards (Cox & John-Alder, 2007) and female brown anoles (Cox et al., 2010). The fact that, in the present study, this treatment effect was seen for some endoparasites but not for ectoparasites is consistent with the ideas that immune responses to parasites can be highly specific and achieved by a variety of underlying mechanisms (Vass, Nappi & Carton, 1993; Schmid-Hempel & Ebert, 2003), and that different modes of defence can vary in the costs that they impose on the host (Coustau & Chevillon, 2000; Rigby, Hechinger & Stevens, 2002). The decrease in the prevalence of atractid nematodes that we observed in nonreproductive males and females also highlights the importance of sexually transmitted parasites as a frequently overlooked cost of reproduction (Hurst et al., 1995; Lockhart, Thrall & Antonovics, 1996). To the extent that castration may have eliminated or reduced the frequency of copulation, castrated animals may have been sheltered from continued reinfection by atractid nematodes (Langford et al., 2013). Although few sexually transmitted parasites are studied in wild populations, animals with high degrees of promiscuity and overlapping generations are particularly likely to harbour such parasites (Webberley et al., 2004).
Although we found that gonadectomy increased energy storage and reduced infection by some parasites, we cannot conclusively determine the causality in this relationship. The reduced energy stores of reproductive individuals could leave them more vulnerable to parasitism or, alternatively, reproduction could directly lead to higher levels of parasitism (e.g. exposure to sexually transmitted nematodes), thereby placing greater demands on the immune system and, in turn, reducing energy stores. Although gonadectomy had no effect on immune function as measured in the present study with the PHA assay, the relationship between immunocompetence and host-parasite interactions is highly complex, and we caution against a simplistic interpretation of our data as evidence against a relationship between immune function and parasite loads (Owen & Clayton, 2007). Regardless of any causal links that may exist between reduced energy storage and increased parasite loads, our results show that both occur as costs of reproduction for male and female brown anoles. This is broadly consistent with the theoretical prediction that total reproductive investment should be comparable for males and females, and it supports the emerging view that males and females share many of the same costs of reproduction (Fedorka et al., 2004; Paukku & Kotiaho, 2005; Penn & Smith, 2007; Hoffman et al., 2008; Cox, 2014; Dugas et al., 2015). The present study also presents a new experimental framework for directly comparing costs of reproduction between sexes by using the same measures of cost and manipulations of total reproductive investment over the same period of time.
Acknowledgements
We thank R. Calsbeek and A. Kahrl for assistance with fieldwork; N. Bottomley for permission to work at Regatta Point; and B. Falk and C. McAllister for assistance with parasite identification. We thank C. Alencar, M. Augat, E. D. Brodie III, R. Costello, H. Donald-Cannon, M. Hague, A. Hanninen, B. Sanderson, and C. Wood for their comments on an early version of this manuscript. Additionally, we thank three anonymous reviewers for their helpful comments. Research was conducted under permits from The Bahamas Environment, Science and Technology (BEST) Commission and Ministry of Agriculture and with approval from the Animal Care and Use Committee of the University of Virginia (protocol 3896). This project was supported by start-up funding from the University of Virginia to RMC.




