Proximate mechanisms of colour variation in the frillneck lizard: geographical differences in pigment contents of an ornament
Abstract
Animal coloration has evolved in contexts such as communication, camouflage, and thermoregulation. Most studies of animal coloration focus on its adaptive benefits, whereas its underlying mechanisms have received less attention despite their potential influence on adaptive benefits. In fish and reptiles, for example, colour variation from yellow to red can be produced by carotenoid and/or pteridine pigments, which differ dramatically in the way they are obtained (carotenoids through diet and pteridines synthesized de novo). Hence, potential adaptive benefits could differ greatly depending on the relative contribution to coloration of different pigments. In the present study, we investigate the mechanisms underlying colour variation in the frill of the Australian frillneck lizard (Sauropsida: Chlamydosaurus kingii). Frill colour varies between populations across the species' range (red, orange, yellow or white). We argue that this geographical variation results from different concentrations of carotenoids and pteridines in the frill. Frill carotenoid concentrations were lower in eastern populations (yellow and white forms), and pteridines were present only in the red and orange forms, thereby explaining their redder hues. The observed geographical variation in frill carotenoids suggests variation in carotenoid availability across the species' range, which is backed up by the finding that plasma carotenoid concentrations were higher in the red (western) compared to the yellow (eastern) form. Although no correlations were found between individual colour measurements, frill pigments and plasma carotenoids, our results suggest that selective pressures vary across the species' range and we speculate that predation pressures and/or intrasexual signalling context differ between forms.
Introduction
Animal coloration has evolved to facilitate a great diversity of behavioural and physiological functions, such as communication (Senar, 2006), camouflage (Stuart-Fox & Moussalli, 2009; Klomp et al., 2014), and thermoregulation (Watt, 1968; Fan, Stuart-Fox & Cadena, 2014). The mechanisms underlying colour production are also diverse (Kemp, Herberstein & Grether, 2012) but have received less attention compared to studies focusing on the adaptive benefits they confer.
The primary mechanistic classes of colour production (across animal species) are structural reflectance and pigmentary absorption (Hill & McGraw, 2006; Kemp et al., 2012). Structural reflectance depicts the light-scattering properties of integument nanostructure (Doucet et al., 2006) or cell layers (San-Jose et al., 2013), whereas pigmentary absorption is facilitated by the light absorbance properties of pigments sequestered in the integument (e.g. fish scales: Grether, Hudon & Millie, 1999; lizard skin: Weiss, Foerster & Hudon, 2012).
Coloration in animals can be produced by a range of pigments, such as carotenoids, melanins, porphyrins, psittacofulvins, and pteridines. Some pigments, such as carotenoids, are present in a wide range of taxa (e.g. fish: Grether et al., 1999; birds: McGraw et al., 2002; lizards: Steffen & McGraw, 2009), whereas others are group-specific (e.g. psittacofulvins in parrots: McGraw & Nogare, 2005). Some pigments can produce a wide range of hues, whereas the same hue can be produced by different pigments (Hill & McGraw, 2006). For example, pteridines can produce a range of colours, including white, yellow, orange, and red (Johnson & Fuller, 2014). However, hues from yellow to red can also be the product of carotenoids (Hill, 1993), pheomelanins (McGraw et al., 2004), psittacofulvins (McGraw & Nogare, 2005), and/or a combination of two (e.g. carotenoids and pteridines: Steffen & McGraw, 2009). Moreover, pigments differ in their production pathways: some can be synthesized by animals (e.g. melanins and pteridines), whereas others cannot (e.g. carotenoids; Hill & McGraw, 2006). This may inflict different constraints on the function of colourful traits and their influence on adaptive benefits. Therefore, the identification of the pigments responsible for observable colour is a vital step to understand the evolution and function of these traits.
In reptiles, colours ranging from red to yellow can be produced either by carotenoids (Fitze et al., 2009), pteridines (Kikuchi & Pfennig, 2012) or a combination of the two (Weiss et al., 2012). However, these two pigment types differ significantly in the way they are obtained. Because animals cannot synthesize carotenoids de novo, they therefore need to acquire them via diet (Olson & Owens, 1998). Carotenoids have been shown to enhance immune function and to act as antioxidants (Simons, Cohen & Verhulst, 2012) and, as such, carotenoid based-ornaments are considered to be honest indicators of health and condition, particularly in birds (e.g. trade-offs between ornamentation and physiological functions: Blount et al., 2003; Pérez-Rodríguez, Martínez-Padilla & Mougeot, 2013). However, whether these trade-offs occur in reptiles is less clear; to date, most studies that have tried to experimentally manipulate coloration via carotenoid supplementation have been unsuccessful (Olsson et al., 2008; Steffen, Hill & Guyer, 2010; San-Jose et al., 2013; but see also Kopena, López & Martín, 2014). Pteridines, in contrast, can be synthesized de novo (from guanosine triphosphate in pigment cells: Ziegler, 1965; McGraw, 2005) and are known to reduce oxidative stress (Oettl & Reibnegger, 2002) and to be linked to the immune response (Huber et al., 1984), although this has not been shown for pteridine pigments directly involved in ornamentation. Honest signalling relies on the idea that signals are costly (Johnstone, 1997) but, in contrast to carotenoids, there is no evidence of any cost being associated with pteridine-based colour expression. However, there is evidence that pteridine-based ornaments signal some aspects of individual quality (e.g. yolk antioxidants: Weiss et al., 2011; parasite load: Johnson & Fuller, 2014), although the means by which their honesty is maintained remains unclear.
Given the fundamental differences in the underlying mechanisms of carotenoid- and pteridine-based ornament production, their costs and constraints are likely to differ significantly as well. Because similar colours can be produced by either one, or a combination of carotenoids and pteridines (Steffen & McGraw, 2009; Weiss et al., 2012), identifying the relative contribution of each pigment type is crucial for understanding the function of colour signals. A recent study showed that variation in dewlap colour among subspecies of the lizard Anolis jubar in different regions of Cuba is a result of differences in the relative proportion of carotenoids and pteridines (Alfonso et al., 2013). This suggests that geographical variation in the relative contribution of pigment types can produce colour variation among subspecies/populations. However, studies investigating whether the mechanisms of colour production vary geographically within a species are rare (Hill, 1993). One example is that of the male orange spots (a sexually selected trait: Endler & Houde, 1995) of guppies (Poecilia reticulata). In this species, carotenoids vary along an environmental gradient, and pteridines covary with carotenoids to maintain the hue of the orange spots (Grether et al., 1999; Grether, Cummings & Hudon, 2005). Given the scarcity of similar studies, there is a need for more research investigating intraspecific colour production mechanisms across populations.
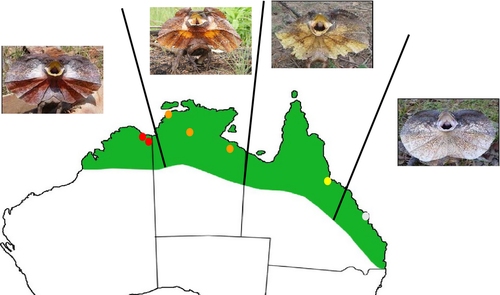
In the present study, we aim to add data to this limited body of work and identify the pigmentary basis of geographical variation in frill colour across the Australian range of the iconic frillneck lizard (Chlamydosaurus kingii). In this species, individuals have similar frill colour within populations. However, frill colour varies geographically and includes red, orange, yellow, and white forms, at least according to human vision (Fig. 1) (Cogger, 2014). Given the dramatic differences in colour, it is likely that there are divergences in the pigmentary make-up of the species across its range. A previous study identified carotenoids in individuals with red frills and suggested that frill colour is used to signal male fighting ability (Hamilton, Whiting & Pryke, 2013). However, tests for the presence of pteridines were inconclusive and the pigmentary basis of the orange, yellow, and white colour forms remains unknown. If frill colour is expressed by different relative ratios of pigment types among different phenotypic forms, the consequences for the function of the ornament across the species' range may vary. To disentangle the mechanisms behind frill coloration across the different forms, we (1) quantified frill colour variation as perceived by the lizard visual system; (2) identified and quantified the underlying pigment types for the different colour forms; (3) investigated whether frill carotenoid content had an environmental basis through quantification of plasma carotenoids (i.e. a proxy of carotenoid availability in their diet) in individuals of two colour forms (red and yellow) and (4) analyzed the correlations between individual colour measurements (colour contrasts), frill pigment concentrations, and plasma carotenoids in the red and yellow forms.
Material and Methods
Study system and sampling
The frillneck lizard is a large agamid with a distribution ranging across the northern tropical savannahs of Australia (Cogger, 2014). Its habitat is characterized by a strongly seasonal monsoonal climate consisting of distinct dry (May to September) and wet seasons (October to April). Fieldwork was conducted during the wet season because this is when the lizards are reproductively active and males actively defend territories (Christian & Bedford, 1995). Lizards were located by driving slowly along roads in appropriate habitat and captured either by hand or by noosing (Shine, 1990).
Data were collected over two field seasons (November 2012 to February 2013 and November 2013 to March 2014) from populations in and around the towns of Wyndham and Kununurra (red form; 15.49°S, 128.12°E and 15.77°S, 128.74°E, respectively), Darwin, Katherine, and Cape Crawford (orange form: 12.27°S, 130.50°E; 14.28°S, 132.16°E and 16.40°S, 135.48°E, respectively), Townsville (yellow form; 19.26°S, 146.82°E) and Rockhampton (white form: 23.22°S, 150.30°E), spanning the Australian distributional range of the species (Fig. 1). Because both sexes exhibit coloured frills, we sampled both males and females (red form: 15 females, 30 males; orange form: 10 females, 18 males; yellow form: 10 females, three males; white form: four females, five males). Lizards were sexed by head and snout–vent length measurements (males are larger and have larger heads than females: Christian, Bedford & Griffiths, 1995), whereas hemipene eversion (Harlow, 1996) was used in cases where body measurements were ambiguous. For each individual, we took spectrometric measurements of the frill (see 2.2) and sampled the colour patch of the frill tissue using a hole punch (approximately 5 mm; see Pigment identification and quantification). During the second field season only, we also acquired a blood sample from individuals of both red and yellow forms (see Supporting information, Data S1) captured within a 2-month period (February to March 2014).
Visual modelling
During the first field season, we quantified frill coloration using a USB2000 spectrometer (Ocean Optics) with illumination from a PX-2 light source (Ocean Optics) and, for the second field season, we used a Jaz spectrometer (Ocean Optics) with the same light source. The measurements recorded were the same in both field seasons, as detailed in Hamilton et al. (2013). Briefly, we took six measurements (three from each side) of the frill colour and cheek patches, and three measurements of the throat and the surrounding brown frill background, which were then averaged within patches over the range 300–700 nm before analyses (see Supporting information, Fig. S1).
To quantify frill colour variation as perceived by conspecifics, we used reflectance spectra from the first field season to calculate the relative stimulation of lizard photoreceptors (receptor quantum catches) for the colour patches (colour, cheek, and throat) using the equation detailed in Vorobyev et al. (1998), sensu Teasdale, Stevens & Stuart-Fox (2013). This analysis resulted in five variables (quantum catches) for each patch: one for each of the four single cones used for colour perception and the double cone assumed to be used for luminance perception (Osorio & Vorobyev, 2005). Spectral sensitivities are not known for frillneck lizard, but visual systems are phylogenetically conserved within lizard families (Olsson, Stuart-Fox & Ballen, 2013). So, we used spectral sensitivities data of a closely-related agamid, Ctenophorus ornatus, where three single visual pigment types (short, medium, and long wavelength-sensitive; see Supporting information, Fig. S1) have been identified (Barbour et al., 2002). Barbour et al. (2002), in contrast to other studies on related lizards (Loew et al., 2002; Bowmaker, Loew & Ott, 2005), were not able to identify ultraviolet-sensitive (UVS) photoreceptors. However, UVS photoreceptors have recently been discovered in another closely-related agamid, Ctenophorus decresii, because four opsin genes were expressed (Yewers et al., 2015). Therefore, we considered an additional fourth UVS photoreceptor (λmax = 360 nm; see Supporting information, Fig. S1, sensu Chan, Stuart-Fox & Jessop, 2009). We also used an irradiance spectra representing full sunlight (see Supporting information, Fig. S1) because frillneck lizards are diurnal and live in open habitat (Stuart-Fox et al., 2003). We applied the von Kries transformation to the cone catches to normalize them to the background, thus accounting for receptor adaptation to the light environment (Vorobyev & Osorio, 1998).
To investigate correlations between frill colour, skin pigmentation, and plasma carotenoids (in the red and yellow form only), we used the receptor quantum catches (see above) to quantify frill colour variation as changes in the contrast between frill colour (red or yellow) and the surrounding brown frill background. In accordance with previous work on frill coloration (Hamilton et al., 2013), we used the Vorobyev–Osorio model to estimate these contrasts (Vorobyev & Osorio, 1998; Osorio & Vorobyev, 2008). This model estimates the discriminability of two colours in units of just noticeable differences and assumes that visual discrimination is limited by photoreceptor noise. It produces a measure of the chromatic (colour) and achromatic (luminance) contrasts (see Supporting information, Data S1). All analyses were carried out using the pavo package (Maia et al., 2013).
Frill tissue pigment identification and quantification
Chemical pigment extraction from frill samples was performed in accordance with a modified protocol of Steffen & McGraw (2009) (for more details, see Supporting information, Data S1), which separates the pigments into two chemical liquid phases. This extraction protocol was used for three separate analyses: presence/absence of the pigments in the four forms, identification of the carotenoid pigments in the four forms, and pigment quantification in the red and yellow forms.
To test for the presence/absence of pigments, we combined samples from multiple individuals within the same form to ensure that colour would be visible. The presence of colour in the upper phase signifies the presence of carotenoids in the frill, whereas coloration in the lower phase is a sign of the presence of pteridines. Visual diagnostic was further confirmed by reading absorbance over a 400–700 nm spectrum of 200 μL of each phase using a microplate reader (Tecan Infinite M1000Pro; Tecan Australia). The occurrence of carotenoids was confirmed by the presence of the typical three-peak absorbance spectra with λmax = 450 nm, whereas, for pteridines, the absorbance spectra has one peak at λmax = 490 nm, which was matched with the absorbance spectra of Drosophila serrata heads (Weiss et al., 2012).
To identify frill carotenoid pigments across the range of the species, we ran 18 pooled samples from the four colour forms on a high-performance liquid chromatography (HPLC) (six from the red form; six from the orange form; four from the yellow form; and two from the white form, equal ratio males and females). The samples were analyzed on a Shimadzu LC-10-VP Series (Shimadzu Corp.) using an Alltech Prevail C18 column (5 μm; 250 × 4.6 mm). The mobile phase consisted of mixtures of acetonitrile : water : triethylamine (90 : 10 : 0.1, v/v/v) (solvent A) and ethyl acetate (solvent B). After pigment extraction (see above), we evaporated 200 μL of the upper phase under nitrogen and then reconstituted in 100 μL of solvent A before injection (50 μL) onto the HPLC column. The gradient elution started at 100% A (hold for 3 min) then decreased linearly to 66.7% B over 22 min, at a flow rate of 1 mL min–1. Sample components were detected with a photodiode array detector SPD-M10-VP (190–800 nm) and the chromatogram was monitored and integrated at 450 nm. Details on carotenoids identification are given in the Supporting information (Data S1).
To quantify pigment concentrations in individuals of the red and yellow forms, we followed the same extraction protocol as explained above (with individual frill samples in this case) and then applied the formula in McGraw et al. (2002) and Weiss et al. (2012). Further details are provided in the Supporting information (Data S1).
Plasma carotenoids identification and quantification
Total plasma carotenoid content in individuals of the red and yellow forms was quantified using a spectrophotometric method (see Supporting information, Data S1) (Costantini et al., 2005). To assess the reliability of this technique for this species, and to identify the carotenoid pigments, HPLC was performed on six samples (three from each sex) using the same protocol as described above and compared with the colorimetric measurements. Total plasma carotenoid concentrations calculated with the HPLC were highly positively correlated with concentrations calculated with the spectrophotometric quantification (r = 0.92; P < 0.0001).
Statistical analysis
We used a linear discriminant analysis (discrimin function in ade4 package: Dray & Dufour, 2007) to test whether the objective measures of frill colour from a lizard visual system correlate with the differences in frill colour observed by human eyes (sensu Teasdale et al., 2013). This analysis generates canonical variables (linear combination of the response variables) maximizing the probability of correctly assigning observations to their predetermined groups (here the four frill colour forms). It was run on the five quantum catches (four single cones and a double cone) of the colour patch, cheek patch, and throat (15 variables in total). A jackknife approach was used to determine how accurately individuals were assigned to their colour group.
For all subsequent analyses, we used corrected Akaike information criterion (AICc) model selection (Burnham & Anderson, 2002) using the MuMIn package (Barton, 2012). Input variables were standardized before model selection (Gelman, 2008) and we selected models with a ΔAICc < 4 (Burnham, Anderson & Huyvaert, 2011) or models with a lower AICc than the null model (if the null model was in the subset of models). We also calculated a weight (ωAICc) for each model, which represents the probability that a given model is the best approximating model among the subset of models (Symonds & Moussalli, 2011). When this approach resulted in multiple models that were equally likely, we computed model-averaged parameter estimates, SEs, and confidence intervals without shrinking the parameters (Burnham & Anderson, 2002). This allowed us to take model selection uncertainty into account.
We used a linear regression on the total areas of the frill sample HPLC chromatograms to investigate how frill carotenoids varied among forms. To meet model assumptions, HPLC total areas were square-root transformed. In addition, we used frill pigment individual concentrations (obtained by the absorbance method) to compare the red and yellow forms and corroborate the pattern obtained with a smaller sample size on HPLC data. Analysis of variance (ANOVA) was used to test for the interaction between sex and frill colour and its main effects on carotenoid, pteridine and total pigment (carotenoid + pteridines) concentrations. Variables were log-transformed to meet model assumptions.
Similar to the analyses on frill pigment concentrations, we used ANOVAs to investigate whether sex and frill colour form (red or yellow), or their interaction with one another, influenced plasma carotenoid concentrations. Because we had repeated measurements for individuals from the red form, we measured plasma carotenoid repeatability with a linear mixed-effects based method using the rptR package (Nakagawa & Schielzeth, 2010). Plasma carotenoid concentrations were repeatable within an individual [r = 0.647; 95% confidence interval (CI) = 0.319 to 0.820], we thus used averaged plasma carotenoid concentrations for individuals for which we had multiple measurements.
Finally, analysis of covariance (ANCOVA) was used to investigate the links between individual colour contrasts (chromatic and achromatic; see 2.5), frill pigments, and plasma carotenoids. Because the red and yellow forms differed in all these variables, we standardized them within form to be able to compare the effect of sex and frill colour. In each case, we considered the two interactions between a dependent variable, and sex and frill colour, respectively. We ran three sets of ANCOVAs. First, we investigated whether individual frill pigment concentrations (carotenoids, pteridines or total pigments) varied with plasma carotenoid concentrations; second, whether individual colour contrasts (chromatic or achromatic) varied with plasma carotenoid concentrations; and, finally, whether colour contrasts varied with individual frill pigment concentrations (carotenoids, pteridines or total pigments). All analyses were run using R, version 3.1.0 (R Core Team, 2014).
Results
Could frill colour variation be perceived by the lizards?
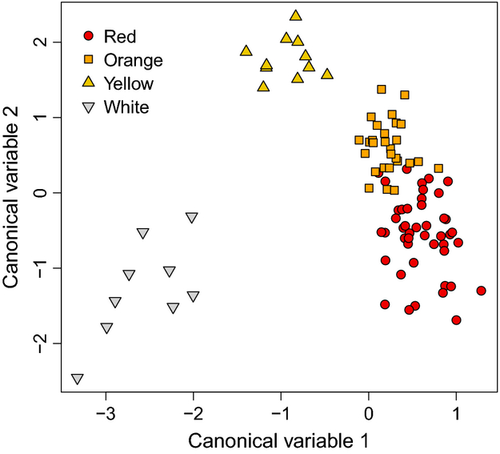
Lizards were categorized according to frill colour form, with linear discriminant analysis demonstrating that the four perceived colour forms were also separated into four groups (red, orange, yellow, and white) based on the lizard visual system (Fig. 2). The first canonical variable explained 42% of the variance and discriminated between three colour groups: white, yellow, and red and orange together; whereas the second canonical variable explained 36% of the variance and discriminated between three different colour groups: yellow, orange, and white and red together. This resulted in individuals with white and yellow frills being clearly differentiated, whereas individuals with an orange or red frill were more similar but still separated (Fig. 2). Hence, based on the lizard visual system, an individual was correctly attributed to its form in 92.4% of the cases, indicating that lizards are, in theory, able to discriminate among the four frill colour forms.
Are differences in colour a result of different pigments in the frill tissue?
The biochemical tests on frill samples revealed yellow colour in the upper phase of all samples, thereby indicating the presence of carotenoids in the frill of all forms, which was further confirmed by the typical three-peak absorbance spectra (see Supporting information, Fig. S2). The lower phase had a red colour (indicative of the presence of pteridines) only for individuals from red and orange forms but not from the yellow or white forms. Moreover, the match of the absorbance spectra with that of D. serrata heads suggests that these pigments were drosopterins (see Supporting information, Fig. S2), as found in other lizard species (Steffen & McGraw, 2007; Weiss et al., 2012).
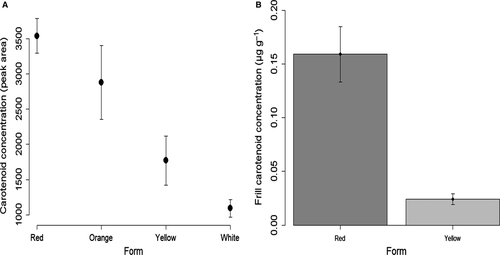
All frill samples contained carotenoids, although carotenoid concentrations significantly decreased from west to east (AICc model with Form: 299.96 versus null model: 310.26). The intensity of the yellow colour in the upper phase decreased linearly from a bright yellow in the red to very pale yellow in the white form, as shown by the HPLC data (Fig. 3A). This was further confirmed by frill carotenoid concentrations (as measured by the absorbance method), where concentrations were eight-fold higher in individuals from the red form compared to those from the yellow form [red: 0.16 ± 0.02 (μg g−1 of skin; mean ± SE); yellow: 0.02 ± 0.005] (Fig. 3B). The best-fitting model contained colour form alone, the three models with colour form had a ΔAICc < 4 and represented 100% of the cumulative ωAICc. However, models with the sex × colour form interaction and sex alone were much less likely to explain the data (ΔAICc = 3.77 and 38.93, respectively). The results were similar when considering total pigments (carotenoids + pteridines).
Chromatograms were similar across forms, showing that all frill samples predominantly contained the same three carotenoids (see Supporting information, Fig. S3): dehydrolutein (retention time: 13.92 min), lutein (15.15 min), and zeaxanthin (15.92 min). Furthermore, there were three small peaks with retention times between 17 and 22 min, which are probably carotenoid esters (San-Jose, Granado-Lorencio & Fitze, 2012). Finally, there were two unidentified minor compounds: one that was not a carotenoid (based on absorbance spectra) with a retention time of 10.2 min and λmax = 433 nm, and another that was a carotenoid with a retention time of 13.3 min and λmax = 448 (see Supporting information, Fig. S4).
Because pteridines were not present in the yellow form, we compared carotenoid and pteridine contents in the red form only: carotenoids were in higher concentrations than pteridines (mean ± SE: carotenoids = 0.16 ± 0.026 μg g−1 of skin; pteridines = 0.047 ± 0.010; paired t-test: t20 = 3.66; P = 0.0016). Moreover, we found no sex differences in pteridine concentrations in the red form (null model had a lower AIC than the model with sex; ΔAICc = 2.36).
Is variation in frill carotenoids a result of differences in plasma carotenoids?
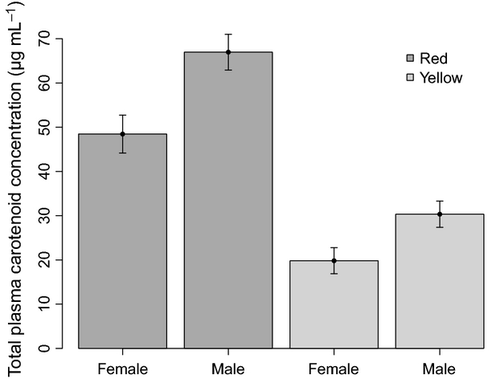
Plasma carotenoid concentrations were correlated with colour form and sex independently but not their interaction. Individuals with red frills had plasma carotenoid concentrations more than twice as high compared to individuals with yellow frills, whereas males had higher overall plasma carotenoid concentrations than females [red males: 67.0 ± 4.0 (μg mL−1; mean ± SE); red females: 48.46 ± 4.3; yellow males: 30.34 ± 2.9; yellow females: 19.82 ± 2.9] (Fig. 4). The subset of models contained the additive model ‘sex + colour form’, which was the best-fitting model, and the model with the interaction ‘sex × colour form’ (ΔAICc = 1.23). All the other models had a ΔAICc > 10. However, only sex and frill colour appear to influence plasma carotenoid concentrations because the confidence interval of the interaction term overlapped zero (estimate: 0.159; 95% CI = −0.147 to 0.465).
There were no differences in the identity of the carotenoid pigments in the plasma between the red and yellow colour forms because all chromatograms were very similar. In addition, the type of plasma carotenoid pigments were the same as in the frill tissue: dehydrolutein, lutein, and zeaxanthin.
Are frill colour, frill tissue pigments, and plasma carotenoids correlated?
There was no statistical relationship between individual colour contrasts (chromatic and achromatic), frill pigments, and plasma carotenoids. The null model was the best-fitting model in four out of 11 cases and was equally likely as another model in six cases. However, the results changed for the correlation between achromatic contrasts and frill carotenoids when we excluded two outliers with very high carotenoid concentrations. In this case, individuals with a higher contrast between frill colour and the frill brown background had fewer carotenoids in the frill (estimate: −0.324; 95% CI = −0.616 to −0.031). Three of the four models in the subset contained the variable frill carotenoids and the null model was much less likely (ΔAICc = 2.68). The result was the same when total pigments were considered (estimate: −0.377; 95% CI = −0.665 to −0.089).
Discussion
Despite the substantial body of work on the evolution of intrapopulation (Sinervo & Lively, 1996; Healey, Uller & Olsson, 2007) and interpopulation colour variation (Klomp et al., 2014; McLean, Moussalli & Stuart-Fox, 2014), studies investigating the underlying mechanisms are rare (Steffen & McGraw, 2007; Alfonso et al., 2013). In the present study, we aimed to reduce this scarcity by investigating the underlying mechanisms of the geographical variation in frill colour across the distributional range of the frillneck lizard. First, our visual model analyses revealed that the lizards appear able to distinguish between the four colour forms (Fig. 2), thereby indicating that the observed colour variation is biologically relevant. We also found that frill carotenoid concentrations strongly decreased from west to east, being the highest in the red (western) form and the lowest in the white (eastern) form (Fig. 3A). Furthermore, we found that pteridines were only present in individuals with a red or orange frill, albeit in lower concentrations than carotenoids (tested in the red form only). We suggest that the observed differences in frill carotenoid concentrations were modulated by geographical differences in diet carotenoid availability. The finding that plasma carotenoid concentrations (in the red and yellow forms) (Fig. 4) followed the same pattern as frill carotenoid concentrations in these forms supports this suggestion. However, we cannot exclude the possibility that carotenoids are equally available throughout the species' range but that individual absorption capacities (i.e. how efficient they are at extracting carotenoids from their food) differ among forms. Moreover, the specific type of carotenoid pigments present in both frill tissue and plasma samples did not vary with frill colour form. We discuss our findings in relation to other studies on the mechanisms of animal colour variation, temporal effects of sampling, and tissue structural components.
Geographical variation in frill colour, pigmentation, and plasma carotenoids
The present study demonstrates that in the frillneck lizard, variation in frill colour is determined by the interaction between carotenoids and pteridines. Frill carotenoids were present in all colour forms and their concentrations strongly decreased from the red to the white form, whereas pteridines were present in the red and orange forms only. Hence, the redder hue of frills in the red and orange forms is attributed to red pteridines (most likely drosopterins) in addition to the yellow colour of carotenoids.
Because frillneck lizards are mainly insectivorous (Shine & Lambeck, 1989; Griffiths & Christian, 1996) and insects can be a good source of carotenoids (Eeva et al., 2010), we suggest that the observed variation in frill carotenoid concentrations could be a result of variability in available carotenoid-bearing insects in the diet. This suggestion may also be further implied by the observation that plasma carotenoid concentrations are higher in individuals of the red form (western populations) than in those of the yellow form (eastern populations). Similarly, variation in plasma carotenoid concentration among land iguana (Conolophus subcristatus) populations has been documented and, as in the present study, the identity of the carotenoid pigments present did not differ across populations (Costantini et al., 2005). Also, the three main carotenoids we identified were also present in land iguanas: lutein and zeaxanthin are obtained directly from the food and dehydrolutein is metabolically derived from zeaxanthin (Costantini et al., 2005). The observed similarities in pigment composition may suggest that the lizards are feeding on similar prey (termites, ants, and butterfly larvae: Griffiths & Christian, 1996), although we cannot exclude the possibility that different prey have similar pigment composition. Moreover, lutein and zeaxanthin are known to be prime targets for absorption in the intestine of lizards (Raila et al., 2002). Thus, the plasma concentrations of these pigments should closely match diet concentrations (Olsson et al., 2008; Fitze et al., 2009; San-Jose et al., 2013). This suggests that variation in plasma carotenoids among the different forms in the present study is a result of variation in the abundance of carotenoid-rich insects and/or prey carotenoid content. However, sampling of lizard stomach contents across sites would be necessary to confirm this.
An alternative explanation to the hypothesis that variation in frill carotenoid content (and hence frill colour) is determined by dietary components is that there are differential absorption capacities among forms. Although this has been shown interspecifically in poultry (Surai et al., 1998), to our knowledge, no studies on birds or reptiles have observed intraspecific differences in absorption capacities to the extent observed within our study species. Other alternative explanations include among-form differences in background-related reflectance of the iridophores (Grether, Kolluru & Nersissian, 2004; San-Jose et al., 2013) and/or genetic variation among forms (Hubbard et al., 2010). In the frillneck lizard, genetic analyses based on two mitochondrial and three nuclear loci indicate that the red form is genetically divergent from the three others forms, which are clustered together (M. Pepper & J. S. Keogh, unpubl. data). If differences in coloration were purely the result of underlying genetic differences, we would expect individuals with similar frill colours (i.e. red and orange) to be more closely-related across evolutionary time. However, we cannot exclude the possibility that some specific genetic markers involved in coloration processes have different expression levels in the different forms.
In both red and yellow forms of frillneck lizards, we found that males had higher plasma carotenoid concentrations than females. By contrast to the present study, Costantini et al. (2005) found a reversed sex effect in the Galápagos land iguanas. In frillneck lizards (as in land iguanas), males are larger and heavier than females (frillneck lizard: Christian et al., 1995; land iguana: Snell, Snell & Tracy, 1984), and so the higher plasma carotenoid concentrations in males could be explained by different feeding requirements between sexes. However, this does not appear to be the case given that food volume in stomach flush samples were similar in both sexes, at least among individuals of the orange form (Christian, Griffiths & Bedford, 1996). If this scenario is true across the different forms, one likely explanation for the sex difference in plasma carotenoids could be that males are able to absorb more carotenoids than females from the same food resource. However, we would then expect to find sex differences in frill carotenoids and individual colour contrasts, which we did not. Males could be allocating relatively more plasma carotenoids toward physiological functions (i.e. immune and antioxidant defences) than females, potentially explaining the absence of differences in frill carotenoids and colour contrasts. Male–male fights are frequent and often lead to injuries in frillneck lizards (Shine, 1990), which may lead to higher metabolic rates and disease susceptibility in males. This could in turn trigger higher oxidative damages and immune activity, potentially counteracted by higher levels of carotenoids, although the role of carotenoids as antioxidants remains controversial (Hartley & Kennedy, 2004; Kopena et al., 2014).
Absence of correlations between colour, frill pigments and plasma carotenoids
Most carotenoid supplementation studies on lizards recognize a positive effect on plasma carotenoid concentrations, although this does not necessarily translate into changes in pigmentation/coloration (Olsson et al., 2008; Weiss et al., 2012). The present study concurs with this result because we found no correlations between plasma carotenoids, frill pigments, and colour contrasts. Indeed, in the present study, we found only one significant correlation among the 11 we tested for. Achromatic contrasts and frill carotenoids were negatively correlated: individuals with a higher contrast between frill colour and the frill brown background had fewer carotenoids in the frill (Butler, Toomey & McGraw, 2011). However, this result was only apparent after excluding two individuals with very high carotenoid concentrations, thereby suggesting a weak relationship. One explanation for the absence of correlations could be that variation in frill colour is a result of the background-related reflectance of the iridophores (Grether et al., 2004; San-Jose et al., 2013). If this is the case, detection of a correlation between pigments (in plasma or skin) and coloration would be markedly less likely.
Conclusions
The underlying mechanism of frill colour variation in the frillneck lizard is explained by the interaction and variation in carotenoid and pteridine contents in frill tissue. Frill carotenoid concentrations strongly decreased from west to east (i.e. from red to white form), whereas pteridines were only present in red and orange forms. The results of the present study suggest that variation in frill carotenoid concentrations is a result of variation in carotenoid availability (i.e. food resource availability) because plasma carotenoids [measured in the red (east) and yellow (west) forms only] followed the same pattern as that observed in the frill tissue. However, although we separated individuals in four groups based on frill colour, we cannot exclude the possibility that frill colour variation is actually continuous across the range of the species, as suggested by the linear gradient in carotenoid concentrations. A larger and more systematic sampling effort across the distributional range of the species would be required to answer this question. Given the differences in the underlying mechanisms of colour production between the western and eastern frillneck lizard populations, it is possible that the function of the ornament varies across the range of the species. Habitat is more open across the range of the western populations (red/orange forms) compared to the eastern populations (yellow/white forms) (D. G. Hamilton, pers. comm.), which may suggest a higher predation pressure in the western populations. Because the frill has been suggested to have evolved for predator deterrence and for intraspecific communication (Shine, 1990), having redder frills may be more effective to deter predators in the western populations. However, because the colour red has been linked to intraspecific intimidation in a variety of species (Pryke, 2009), the addition of pteridines to carotenoids in the red and orange forms may also be related to intraspecific communication. Building on the work from Hamilton et al. (2013), comparing the signalling properties of frill colour across forms and sexes would provide further insight regarding this issue
Acknowledgements
We thank Anika Immer and Tobias Hayashi for their help in the field. We are also grateful to Charles Hocart for his help with the HPLC protocol and to Julie Collet and Jessica McCarrol for providing Drosophil serrata specimens. We thank Scott Keogh and three anonymous reviewers for valuable comments on the manuscript, and to Devi Stuart-Fox for advice on the visual modelling analyses and comments on the manuscript. Funding for this project was provided by a grant from the Australian Research Council to Sarah Pryke. Thomas Merkling was supported by a Fyssen Foundation Post-doctoral Fellowship and David G. Hamilton was supported by an Australian Geographic Seed Grant.
References
Shared Data
Data deposited in the Dryad digital repository (Merkling et al., 2015).




