The flexible lek: Phymatopus hecta the gold swift demonstrates the evolution of leking and male swarming via a hotspot (Lepidoptera: Hepialidae)
Abstract
Leking moths present an evolutionary problem in their apparently simultaneous reversal of male–female attraction. The mating system of Phymatopus (or Hepialus) hecta embraces an unusually wide range of procedures. Both males and females use medium-range olfactory attractants (also probably visual signals), and both sexes will lure, and both will approach, the other, either when flying or perched. This produces an ‘infinite variety’ which includes the classic moth mating procedure (males fly to sessile female); a typical lek procedure (female flies to sessile male); a mating swarm (hovering male follows passing female); and intermediates such as a mutual courtship dance. Male behaviour includes a flying display, two sessile displays, and an escalating war of attrition. The system is versatile, persistent, and probably evolutionarily stable. The lek site has a high density of perches suitable for copulation which facilitate predator-escape by means of a dead drop. The whole supports a model for the evolution of resource-based leks which commence with a concentration of females on a hotspot, leading to a concentration of males; and then an escalating process of sexual selection as males become increasingly attractive over a distance to females, and females use the males as a way of locating the resource. The main stabilizing pressure may be selection for efficient mate acquisition, and as with grouse leking systems, the precondition for evolution was probably having travelling females that actively sought a reproductive resource or a predator free space. © 2014 The Linnean Society of London, Biological Journal of the Linnean Society, 2014, 114, 184–201.
Vespere in aere fluctitat motu in pendulo, at solitarius Fabricius 1781
Introduction
The term lek, originally used by Darwin (1871: 100) to denote the play-like courtship of various grouse species (English to leyk, Swedish leka), is sometimes used to describe any system in which males use distance-signalling to lure females: a reversal of the canonical procedure in which females attract males (Greenfield, 1981). In insects particularly, the term embraces both classical leks in which females select mates from a group of sessile males, and ‘mating swarms’ in which flying males aggregate and individually pursue females that are tempted to fly past (Shelly & Whittier, 1997; Sivinski & Peterson, 1997). But this reversal of the usual procedure, for instance in moths which normally have sedentary females that lure travelling males, appears to require coordinated reciprocal changes in both sexes. Moreover, this doubles the difficulty of kick-starting the evolutionary change: if males need to aggregate to appeal to females, they would already need to be individually attractive. And if aggregation increases attractiveness (Alem et al., 2011), we might expect that the already attractive females would be the ones who evolved the aggregations. This presents a challenge for evolutionary theory.
The present study proposes a solution following from the discovery of a species that has not so much reversed the role of the two sexes, but allowed both sexes to adopt both roles.
Male mating aggregations are widespread in insects, but rare in moths (Greenfield, 1981; Phelan, 1997), where they are mostly found in a few families (Willis & Birch, 1982; Alem et al., 2011) but chiefly in the Hepialidae: a dozen species of moth in the genus Hepialus s.l. (see Supporting infromation, Doc. S1; Nielsen, Robinson & Wagner, 2000; Grehan, 2012) are well known to indulge in lek-like behaviour (Mallet, 1984). Among these, the behaviour of Phymatopus hecta gold swift is a rich ground for examining ways in which such behaviour could evolve. The crepuscular male swarms of this species and its relative Hepialus humuli ghost moth are spectacular and usually the only aspect reported by passing observers (Carolsfeld-Krausé, 1959; Ruckdeschel, 2006). They were noted by Fabricius (1781: 208) and Gmelin (1790: 2617) and a century later were understood to involve female attraction to males (Chapman, 1876; Tutt, 1896: 335). The leking behaviour of P. hecta has been described piecemeal (Barrett, 1886, 1895: 155; Robson, 1887; Cockayne, 1912; Cockayne & Jackson, 1913; Hanson, 1938; Turner, 1976, 1988, 2014; Speidel, 1994; Lepidopterologen-Arbeitsgruppe, 2000; Post, 2006; Picozzi, 2012, 2013), but with uneven detail and some mistaken observations, and in total reporting only a dozen enumerable couplings. The present study represents the first comprehensive investigation, based on approximately 170 h of observation including approximately 50 h of detailed filming (plus approximately 50 h of continuous monitoring), and around 70 matings. An unpublished study (Wagner, 1985) of the three North American Phymatopus species describes behaviour similar in many ways to the present results. The simpler male-swarming behaviour of H. humuli is known in some detail (Chapman, 1876, 1886; Leuschner, 1970; Reynolds, 1973; Turner, 1976; Mallet, 1984; Andersson, Rydell & Svensson, 1998; Kaaber, Kristensen & Simonsen, 2009; Picozzi & Espie, 2011).
The present study provides an overview of the new observations. Videoclips are provided in the Supporting information (Videos S1 to S18).
Material and Methods
The lek site is a dense, species rich, roughly 40 m2 stand of Luzula sylvatica greater woodrush in a woodland clearing near Inverness in the Scottish Highlands (57°27′N 4°24′W) (see Supporting information, Doc. S2; Turner, 2013). Its architecture is crucial to the lek behaviour: above the Luzula foliage, there are emergent Luzula inflorescences, and emergent plants of Dryopteris filix-mas male fern, Heracleum spondylium hogweed, Stachys sylvatica hedge woundwort, Urtica dioica stinging nettle, Geum urbanum herb Bennett and Bromus ramosus hairy brome, as well as overhanging branches of a young Corylus avellana hazel, all of which are used as perches.
Observations were made for a total of 136 evenings, variously within the period 14 June to 3 August over 7 years, 2008 to 2014 (the analysis of 2014 is not yet complete), commencing up to 2 h before the start of lek activity, and ending after activity had ceased. Activity was recorded variously by note taking and voice recording, and filmed on two digital camcorders, one of them able to operate in the infrared with its own floodlight. It was necessary sometimes to use a visible spectrum LED headlight over a distance of around 2 m to follow the moths in the dark, additional to the camcorder screen. There was no detectable change in behaviour during trials in which the lamp was switched on and off while trained on a pendulating moth, unless the light was used at extremely close quarters when taking visible-colour records of perching or copulating moths; this sometimes caused them to become agitated, as did the camera's integral heat-emitting lamp. Behaviour off the site was monitored by one or two timed MV light traps operating at up to 40 m from the site boundaries; moths were retained during the next day and subject to observed releases from their previously opened boxes at dusk. Local times of sunset were obtained from a GPS, verified against astronomical sources on the Internet. The recordings have been transferred to DVD, and analyzed, in stopped- or slow-motion when necessary, on a plasma television. Further details and technical specifications are provided in Turner (2013).
Distinguishing males from females
The sexes of this species can be distinguished in the field by colouring, morphology and behaviour. In daylight the male and female patterns are usually distinctive; males are a bright warm or rich dark brown with white spots, females are a dull bluish brown with a subtle pattern (Video S16). In flight the male colour is much the warmer and more saturated, the female colour additionally becoming faded and ‘bled’ when the wings are spread (either when flying or as a pinned specimen) because they are more translucent. A minority of females have a male-like pattern (Barrett, 1895: 153) arising from a variable scatter of white or reflecting scales, producing a set of silvery spots that vary in apparency according to the intensity and angle of the light; they are prominent under intense illumination, but on close inspection the pattern usually differs in detail from the male (Video S17). In infrared all colour and some pattern distinctions disappear. Only males have scent brushes (Video S18), these being the modified tibiae of the metathoracic legs (Bertkau 1882, Deegener, 1902; Lepidopterologen-Arbeitsgruppe, 2000; Picozzi, 2012); they are bright yellow, visible in flight and in infrared, and deployed continuously during the lek. Fighting behaviour is unique to males, but most other behaviours are exhibited by both sexes. The members of a copulating pair are unambiguous, but there is some homoerotic behaviour. Thus although some features are decisive in themselves (scent brushes, fighting, the obesity of a gravid female), assignment of gender requires caution, and a small percentage of observations mostly in the infrared must remain unassigned.
Recognizing moths
Individual colour patterns appear to be unique, but are somewhat asymmetrical. Individuals may therefore be tracked over several days by repeat photography, although unfortunately it is not always possible to film both sides of individuals who are perched or copulating in the natural vegetation.
Experiments
Two experiments were performed by disturbing a pair a short time before copulation. For experiments on anti-predator defence, see Turner (2013).
Results
In the considerable filmed and otherwise recorded observations, most of the behavioural elements described below have been noted multiple times: male displays and flights occur every night, fighting on approximately 50% of nights, and matings on approximately 40%. A few events (noted as ‘unique’ in the text) have been observed clearly only once, and the male–male and female–pair attractions only three or four times.
Coming and going
The spatio-temporal diel cycle is described in detail by Turner (2013). Twenty-four hours of quiescent roosting are interrupted by two relatively short bursts of activity in both the twilight periods: the lek itself occurs only in the evening. Moths enter the lek from promiscuous roosting sites ranging from the tree canopy to the base of the lek vegetation, and return there at the close. They also enter and leave during the whole lek period to and from, it is conjectured, eclosion and oviposition sites, other lek sites, and solitary satellite display positions. Relatively few moths roost during the day in the upper and emergent vegetation on the lek site, but copulating pairs and sessile displaying males in that layer remain there all night and relocate only at dawn.
Timing (Fig. 1)
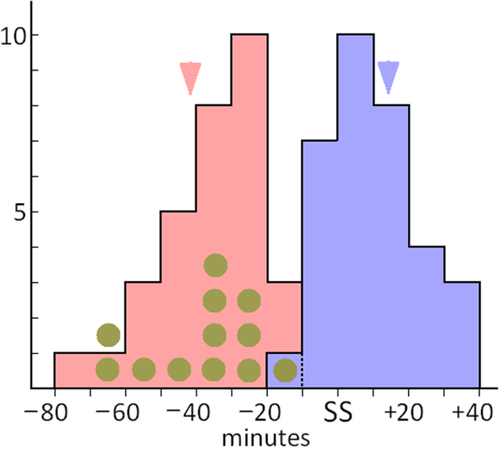
Start and finish times of lek activity (male pendulation or exceptionally last female flight) in relation to sunset (SS), for the years 2008 and 2010. The circles plot the additional times of other preliminary male activity, when this starts before pendulation. The arrows are the extreme start and end times for a smaller number of evenings at Banchory (57°3′45′′N, 2°29′41′′W) 120 km from the present site in 2013 (Picozzi, 2013). The bird-free ‘window’ (Andersson et al., 1998; Rydell, 1998) starts upwards of 1 h before sunset (Turner, 2013).
Lek activity commences with one or two males taking up pendulating flights over the top of the Luzula, often by flying up from the base of the plants (Turner, 2013), and is deemed to close when the last male ceases to fly, either by perching or leaving the site, or rarely later when the last female flies in. There is some preliminary activity, with individual males making short flights in the vegetation to take up perches, always observed to be in the tented display posture; this is not observed on all nights, and may have had its timing truncated by occurring before observations commenced. Time at close is approximate because it is not possible to know for certain that the last male has disappeared until a few minutes have elapsed, and impractical to perform continual time checks. There is then an indeterminate ‘wind down’ period as the sessile males gradually fold away their scent brushes. Activity starts up to 80 min (and at least 10 min) before and ends up to 40 min after (and no earlier than 20 min before) sunset (Fig. 1); the duration on any one night ranged from over 80 min to less than 10 min.
Flight
Males have broadly three flight modes: ‘rapid forward flight’, particularly when leaving the lek or traversing it at heights of 2 m or more; ‘progressive or searching flight’, in which they move forward quite gently sometimes in line ahead, but more usually with a sinuous motion, which they use to move around on the lek and explore perching places (Video S1); and ‘pendulation’, in which the moth appears to swing from side-to-side ‘as though attached to the extremity of an invisible pendulum’ (Stainton, 1857: 109, and most monographs passim) (Video S2). The apparent large side-swings are both an aerodynamic impossibility and a powerful optical illusion produced by the moth executing a rapid figure of eight pattern (Slashchevskiy, 1929a, b). This has been confirmed by slow-framing on video. The ‘8’ is laid flat, and is somewhat higher at the ends than at the crossover in the middle. This flight is executed about 2–5 dm over the top of the Luzula leaves, with an amplitude of 3–4 dm and a period of about 1 s. Pendulation is momentarily over the same spot of ground, but frequently is progressive, the insect moving around the site while pendulating. Females employ at least the searching flight and pendulation.
Male display and fighting
While pendulating, males dangle their metatibial scent brushes, creating a visual and probably olfactory ‘flying display’. When several are pendulating they frequently appear to start moving in parallel with each other, 1–4 dm apart; they may then simply draw apart again, or continue their mutual approach (Cockayne, 1912) until the pattern develops into a ‘fight’ (Turner, 1988). The males fly at each other while executing the ‘8’ pattern, more or less head on. Actual physical contact seems to be rare, but occasionally one of the males will be destabilized and take a plunge into the vegetation. This incurs some risk because they are then prone to intersect with orb webs, which are plentiful (Turner, 2013). In one case, a male was filmed diving directly down for about 0.5 m and striking the other in flight.
Fights usually end with one moth leaving, or the pair pulling apart again and continuing to pendulate independently. Or they may escalate into a ‘fighting dance’ (Video S3), which often starts also without the preliminary fight: the males fly in close proximity, both facing in the same direction, with the body often closer to the vertical than in normal pendulation and the abdomen flexed strongly dorsad, and only a few centimetres apart. They hover up and down, and can appear to hang almost stationary in the air while quivering intensely: the action is actually a very short back and forth patrol with a sharp turn at each end. They may loop upwards to a height of 2–3 m. There are close approaches, probably sometimes with physical contact. These dances last for 1–2 min, and the invariable outcome is for one of the males, on no perceptible cue, to fly rapidly away, usually horizontally but on one occasion upwards, or rarely to take a direct downward nose-dive, maybe as the result of a collision. The departure flight is too rapid to track, and appears usually to be off-site, but occasionally the departure (or nose-dive) is followed by a male (probably the same) reappearing from the same direction and continuing the fight. The fighting dance is probably initiated by one of the males first taking up the quivering flight: one clear instance was observed in which the challenge was not accepted, and the second male continued with normal pendulation. Fights and dances normally involve two or less often three males, and have been filmed with up to five participants.
Males also display statically from perches at the same time as deploying their scent brushes. There are two such ‘sessile display’ postures. In the ‘tented display’ (Video S6) (Cockayne & Jackson, 1913; Speidel, 1994), the moth is perched, ventral side upwards if from a horizontal support, with the wings folded downward in a triangular formation over the body (as is normal for most moths when resting), with the brushes protruding from underneath the wings (illustrated in Speidel, 1994; about 50% of the images of males on the Internet are in this position); the position differs from that of a resting P. hecta in that the wings are not so closely applied around the body (Video S6) (for Sthenopis auratus, see also McCabe & Wagner 1989). In the ‘spread eagle display’ (Video S7) (Cockayne & Jackson, 1913; Picozzi, 2012: plate 16), the forewings are spread out sideways and flat at right angles to the main axis of the body (a visually arresting display of the bright brown colour with white flecks), whereas the black hindwings are folded down laterally along the side of the abdomen, with the scent brushes protruded ventrad. A single female was also filmed using this posture: the semivestigial metathoracic legs, which lack scent brushes, were carried in the same position as that employed by the males. Sessile displays are normally placed at about 0.3–0.5 m on prominent features of the lek vegetation, either the emergent plants or the upper leaf blades and flower stalks of the Luzula; the moth holds on with its four functional legs to the support overhead, but in the tented display and particularly if the support is on a diagonal, may have the body more closely applied to the substrate, as in a normal resting position. There is normally a minimum of several centimetres of clear air between the moth and any vegetation beneath. During both displays, the scent brushes may be vibrated, pulsated or waved about. Perching also occurs lower down on Luzula leaves.
Males make all transitions between the three display modes (Video S14), although tented to spread eagle is unusual. At the close of activity, spread males fold into the tented position, the tented position very slowly contracts into the full resting posture (Video S6), and all males slowly retract the brushes, which are stowed in grooves in the abdomen (Bertkau 1882; Picozzi, 2013).
Displaying males are usually completely still apart from slight movements of the brushes, but in a minority of cases various degrees of wing-fanning (see below) are used, usually for periods of up to 1 min (but rarely reaching 3 min) and often between flights (Video S14). Males when perching may fan for a short time, or immediately adopt one of the stationary poses.
Territoriality versus togetherness
Pendulating males frequently take several minutes to select a sessile display position, sometimes colliding repeatedly with potential sites, notably the drip tips of Heracleum and other leaves, before finally settling (Video S4). Picozzi (2013) has observed that H. humuli males sometimes drag their scent brushes across the surface of leaves, and suggests that P. hecta also may be scent-marking. Sessile males occasionally fly away from their perch for less than 30 s and up to 3 m, and then return to (almost) exactly the same place. Otherwise males do not appear to defend a narrow air space, often pendulating gently away from the site of a victorious fight, and do not clearly defend the perch positions themselves. Rather, they appear to be somewhat drawn to each other, frequently perching in pairs or even triplets on the same support (Video S8), and often making repeated approaches to an already sessile male. Analogous behaviour was also observed in females, who sometimes use a fanning display while perched close to an already copulating pair, sometimes flying off and then returning, or flying to another pair (Video S9). This observation is (pre-)confirmed by the (no longer) puzzling finding by Robson (1892: 78) that when he collected copulating pairs with a sweepnet, he sometimes found a supernumerary female in the net. The detailed behaviour suggests that the females are being attracted by (the scent of) the male in the pair and are (futilely) soliciting a mating, although the female attempted a mating with the male in only one case; but they might be attracted to the same high quality roosting sites, or use the presence of a mating pair as an indicator of a high quality roosting site. These explanations clearly are not mutually exclusive. This clustering results in a similar under-dispersal of copulating pairs.
Rarely, the male–male interaction appears to be aggressive: a flying male will repeatedly collide with a sessile male, causing him to swing passively but generating no other response (Video S5). It is impossible so far to tell in these cases whether they are reacting to the sessile male or treating him as part of a potential perch. In a unique incident, a flying male was seen to land on a vertical grass stalk, and although continuing to flutter, to slide down the stalk until he knocked off a male that was already perched lower down.
A simple summary is that males tend to defend an as yet undefined air space when pendulating, but in sessile display tend to be tolerant and can select the same prime quality perches and/or perches next to other males, and that females are similarly attracted to copulating pairs.
Male homoerotic attraction
Occasionally males attempt to mate with other males (Video S14). Usually both partners are active and appear to be treating the other as a female. The attempt always fails and lasts only a few seconds, the pair usually taking up resting postures a few centimetres apart. In two out of the three cases so far analyzed on film, there was also a virgin female within 1 m, and one of the males after a minute or so flew to her and mated. In one case (Video S14), the female had flown close to both males, one pendulating and the other a neighbouring sessile male, before flying down and perching in the ground vegetation. After their failed pseudocopulation, the males rested for a few seconds, before the previously pendulating male flew down and mated with the female.
Copulation
Copulation is rapid and often partly obscured by the wings of the moths: both partners fan vigorously. The male and female both hang vertically by their four front feet from the support overhead, while manoeuvring into position. Two procedures have been recorded. In procedure 1 (‘venter to venter’) (Video S13), the male holds the female's thorax with his legs and swings into a venter to venter position. Coupling is achieved by a ventrad bending of his abdomen tip; he then releases his footing, so that he hangs head down held only by the grip of the genitalia, with the female continuing to hold on to the support. This last is the standard hepialid position (Videos S7, S16, S17; see also illustrations in Slashchevskiy, 1929b; Speidel, 1994; Lepidopterologen-Arbeitsgruppe, 2000) and the whole procedure is identical to that of H. humuli (Turner, 1976; Mallet, 1984) and Hepialus schamyl great Caucasian swift (Slashchevskiy, 1929b). Procedure 2 (‘back to front’) (Video S9) (see also Hanson, 1938; and possibly also G. Ebert in Speidel 1994 for K. lupulina common swift) involves an unusual form of contortion. Starting with the moths close together but continuing to hang individually from the support; the male presents his dorsal surface to the female's ventral surface; and consummation is achieved by mutual bending of the abdomens, dorsad in the male, ventrad in the female, and in mutually opposite directions laterally, allowing the abdomen tips to lock in the correct orientation. The male then releases his grip, executing a half circle while undoing the twist in the abdomens, so that he is hanging head down in the final copulation position. It is likely that there are intermediate procedures involving a lateral orientation, which are used especially when the mating takes place on a vertical structure like a grass or Luzula stem. On overhanging supports, females normally perch and mate in a vertical position, holding on with their four front feet.
Oviposition
Females separate from the males at the following dawn, and are assumed to start oviposition during the next twilight (Turner 2013). Oviposition has been studied very little in hepialids, which lack ovipositors and drop their non-adhesive eggs while flying or perched. Various studies claim to have observed oviposition in flight for various species, but usually give no evidence that the moths were actually dropping eggs. There is full verification for only half a dozen species (see Supporting information, Doc. S3). Phymatopus hecta has been described, without evidence or attribution, as scattering eggs while the female ‘flies swiftly a few inches above the [ground] surface’ (Barrett, 1895: 155), perhaps paraphrased by Heath (1976) and Manley (2009) as flying over/above the food-plant/bracken. Personally I have not seen either behaviour, but in 2013 two females, one a released captive and one freshly arrived, flew slowly around part of the site, mostly on the margin, very low over the vegetation and making repeated descents, disappearing from sight in the base of the Luzula before coming up again after a few seconds. This is plausibly the oviposition flight. Over all years a few other gender-ambiguous or female moths have been filmed making slow flights through the lek, sometimes briefly settling or descending into the vegetation base.
Wing fanning and fluttering
Both males and females can rapidly fan or more slowly flutter (or even twitch) their wings while perched (Videos S1, S9, S11) (Speidel, 1994; Andersson et al., 1998; Picozzi, 2013). The independent action of the fore- and hindwings in hepialids means that this can involve both pairs of wings, albeit in different amplitudes (Korscheltellus fusconebulosa map-winged swift; J. R. G. Turner, pers. observ.) or the hindwings may be held stationary alongside the abdomen as in the spread eagle display (e.g. Gazoryctra sp.: McDunnough, 1911; Korscheltellus gracilis pine swift: Wagner & Rosovsky, 1991; Wiseana copularis porina: Allan & Wang, 2001). This distinction in the present study awaits analysis; both postures are used. Females fan the wings when calling males, males fan in response to a nearby female, and both sexes fan prior to copulation. Females have also been observed in the tented position with the abdomen tip exposed dorsad to the wings (the normal resting pose) but pulsing gently, and something similar is sometimes seen in males (Hanson's (1938) gender-ambivalent report of a ‘small dark tube at the tip of the abdomen [my translation]’ which may be the structure often visible in resting males (e.g. Post, 2006: fig. 1) and in the difference between a displaying and resting male in S. auratus (McCabe & Wagner, 1989). Because abdominal scent glands are not known in hepialids (Wagner & Rosovsky, 1991; Phelan, 1997), the significance, if any, is unclear.
Courtship and mating
The diverse mating procedures can be conceived as a spectrum between two extremes: the classical moth ‘sessile female lures flying male’ procedure (procedure 1) and a leking ‘sessile male lures flying female’ procedure (procedure 2b). For full citations of previous observers, see the Supporting information, Doc. S4.
Procedure 1 (classic moth) (Video S9): flying male(s) approach(es) sessile female. The female flies through or alongside the lek, without apparently being detected by any males, perches, and then starts wing-fanning. This results in every pendulating male on the lek turning and flying rapidly toward the female, followed by a ‘mating frenzy’ in which a cloud of up to possibly a dozen males fly agitatedly around the female and most or all try to settle and copulate with her, up to five males simultaneously. Occasionally only one male is involved. After some minutes of intense scramble, one male succeeds and most of the others fly gently away, although one or three may attempt, always unsuccessfully, to mate with the already mated female.
Procedure 2: female approaches displaying male. Females are seen to fly towards males from a distance of approximately 1 m, with the following variants:
Procedure 2a (male mating swarm): the male is pendulating, usually with others. The female flies past and lands on a perch, usually within approximately 0.3 m and normally under a more or less horizontal leaf blade, and starts fanning. A single male then immediately flies to her, and lands; both moths fan their wings, and copulation quickly follows.
Procedure 2b (classic lek) (Video S9): the male is sessile. The female lands directly next to the male where he is perched, and copulation proceeds as before. Both tented and spread eagled males are approached.
Procedure 2c (male mating swarm, variant) (Video S10): a pendulating male follows a passing female to a perch. This version of 2a is much reported (Barrett, 1886, 1895: 155; Robson, 1887; Turner, 1976; Picozzi, 2013). Here it has been filmed with groups of two or more males in pursuit, and also in a variant in which the moths circled round each other before the female, followed by the male, perched under an adjacent leaf. The distinction from 2a depends on observing the timing of both the male and the female, and misdescription in either direction is quite probable. If there are half a dozen males, the process ends with a mating frenzy, as above (Video S15). This procedure (2c) is normal in H. humuli (Barrett, 1895: 168; Leuschner, 1970; Turner, 1976; Mallet, 1984).
Procedure 2d (aerial strike): not observed in the present study, but reported by Speidel (1994). The female strikes a pendulating male in flight and the couple plunge to the ground or the subtending vegetation, copulation occurring immediately. However, these might have been pseudocopulations (or aggressive interactions) between males (W. Speidel, pers. comm.). R. Leverton (pers. comm.) reports one such mating; after the collision, the pair then used a following flight as in 2c. In H. humuli, this is the minority procedure (Mallet, 1984), although there is an apparently unverified tradition that it is the norm (Edwards, 1964); it could be considered the extreme lek procedure (no allurement by female) or could be interpreted as a male pouncing on a passing female (Phelan, 1997).
Procedure 3: there is simultaneous or alternating attraction between the two sexes.
Procedure 3a (prolonged negotiation) (Video S11): the female lands some distance (approximately 10 cm) from a sessile male, and commences a fanning/vibrating spread display. The male may take off and fly to the female to mate with her (short version), but if he fails to do this, she flies over to him, settling directly adjacent but not in a copulating position, and continuing to vibrate while (almost) nudging the male. The male then takes off, flies round, and lands back next to the female before finally mating (long version).
Procedure 3b (mutual courtship) (Video S12): a female slowly flies toward a pendulating male, and the couple then pendulate together, rather as two male moths might, with the female eventually settling and the male landing next to her before copulating. Sometimes two males are involved, and sometimes the process is prolonged, with the male unable to find the female when she first perches, and the couple taking flight at least once more before they successfully land together. This process clearly resembles 2c, and might blend into it.
There are various intermediates between these procedures; for example, a sequence, seen once, which could be classified as 3a or a medley of 2b and either 2a or 1 (Video S13).
Although flying males will attempt to mate with a copulating pair (in some cases making repeated returns), sessile males remain impassive and on the whole do not respond to females that have landed next to a neighbouring male. This tends to apply also to males that are perched at a distance of decimetres away from a calling female, giving rise to the escalating negotiated procedure 3a.
Flexibility of female behaviour
When courtship was interrupted in two cases which had both commenced with procedure 2b, the females both continued, after a short pause, with their appetitive behaviour, but changed respectively to procedure 1 and procedure 3b; both mated successfully. This shows that female procedures are not rigidly innate, but are subject to contextual change.
Other lek sites, satellite male
No other leks have been found in the immediate vicinity (within 200 m) of the current site, and significantly none adjacent to the positions of the MV traps. Two have been found 400 m and 700 m to the south, but not observed in detail. Each had no more than three males when observed. They were on low herbage in woodland clearings, one on a grassy riverbank with some Luzula, the other in a clearing with mixed broad-leaved herbs. A solitary sessile male was found 50 m to the west of the main site, displaying on the leaf of a well-grown Corylus approximately 2 m above ground.
Flight in the periphery
Moths caught during the night, variously encompassing the evening twilight, the dawn and the periods of full darkness (Table 1) appear to show a female-biased sex ratio in traps approximately 40 m from the site, but an approximately equal sex ratio in traps at 10 m. The difference in sex ratio is above the formal 0.05 significance level.
| Approximately 10 m from lek site | Approximately 35–50 m from lek site | |||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | Total | 2011 | 2012* | 2013 | Total | |
| Females | 7 | 15 | 4 | 26 | 4 | 17 | 13 | 34 |
| Males | 5 | 20 | 2 | 27 | 0 | 7 | 9 | 16 |
- *See also Turner (2013); the data here include three moths on two further nights not previously reported because these records did not differentiate between dawn and dusk.
- The placing and timing of the traps before 2012 was dictated by general questions of moth periodicity and was not specifically targeted at P. hecta. Clearly, the data do not distinguish the effect of the position of the traps from the years of trapping (a temporal change in the sex ratio), although the former is more likely than the latter.
- Analysis of contingency χ2 (values in bold border on significance with 0.1 > P > 0.05): within full data set (d.f. = 5) 8.02; within 10 m group 1.70, within 35–50 m group 2.78 (d.f. = 2 for both); between group heterogeneity by difference (d.f. = 1) 3.5.
Mutiple mating
This has yet to be investigated fully, both for males and females, from the filmed record, where both sexes can be individually identified from portrait-shots. So far from 2012, one male with a very distinctive pattern has been noted mating on two consecutive nights.
Discussion
Attraction mechanisms
There is no doubt that, in this species, as reported by Hanson (1938) (but probably unsoundly) and Picozzi (2013) (see also Mallet, 1984; for North American Phymatopus sp., see Wagner 1985), males lure females and females lure males. This is shown clearly by procedure 1 (classic moth) and procedure 2b (classic lek). The balance is maintained to the point where in some matings there is a mutual and apparently equal courtship negotiation (procedure 3a, b). Both sexes use a pheromonal lure. The male pheromone is produced from the metatibial brushes, which are prominently deployed by all males during the lek, has been chemically characterized in this and in a North American congener (Francke et al., 1985; Kubo et al., 1985, Sinnwell et al., 1985, Uchino, Yamagiwa & Kamikawa, 1985) and shown to attract individual females from a distance of approximately 2 m (Schulz et al., 1990). The female attractant has been investigated in only two nonleking hepialids, K. gracilis (Kuenen et al., 1994) and W. copularis (Allan & Wang, 2001): virgin females attract males by olfaction while fanning their wings. In K. gracilis the scent was shown to emanate from the hindwings and in W. copularis is known to be one of four detected volatiles (Allan, Jimenez-Perez & Wang, 1999). In the absence of evidence to the contrary, this can be taken as applying to female fanning in P. hecta. Males probably control the release of their pheromone in part by protruding and retracting the metatibial brushes; the relatively brief periods of fanning probably help to disperse the pheromone (as does the flying display), but sessile males normally keep their wings motionless and simply move the brushes. The withdrawal of the brushes into the abdominal grooves presumably aids the switch off at the close of the lek. Males that are in copula likewise show no visible trace of scent brushes. Females can switch on their pheromone by wing fanning, as illustrated by the more or less simultaneous response of a swarm of pendulating males to the commencement of fanning, but males do sometimes follow flying females (as in procedures 2c and 3b), probably within a trail of scent. The attention of unmated males and females to copulating pairs suggests a residual scent from one or both moths. An unexplored further possibility is that one or both of the pheromones serves as a short-range aphrodisiac during the final stages of mating.
During their collisions with perching places and other vegetation, males might also, as suggested by Picozzi (2013), scent-mark the vegetation, often in the neighbourhood of a suitable copulation perch. This may be for their own guidance, or to mark the perch for incoming females, or to warn off other males, or all of these, or even may not be a scent-marking procedure at all but merely a method of inspecting or memorizing a potential perch. It was noteworthy that after one failed mating (two males with a female) on a vertical Heracleum stem, one male returned and made repeated flying contact with that part of the plant. Judging from the spread eagle posture, displaying the striking male pattern of bright white spots on a dark ground, it is likely that females also use visual cues to locate individual males, analogous to the attraction of the brilliant white colour of male H. humuli (Kaaber et al., 2009). Most of the lek behaviour occurs while there is still strong visible light, with twilight being prolonged at this high latitude (Fig. 1). Clearly visual orientation, as well as detection of air and wing movement, is likely to be used by both sexes during the final stage immediately before copulation.
It is also clear that both males and females are attracted, when perching, to members of their own sex and/or to copulating pairs.
The infinite variety of courtship procedures
At first sight the mating repertoire of this species appears to be a highly diverse set of mating strategies. It is tempting to propose a complex theory of co-existing evolutionarily stable strategies. Alternatively, from the published small scale observations (see Supporting information, Doc. S4) conducted in locations as far apart as the UK, Sweden, southern Germany and Switzerland, it would be possible to suggest that these strategies were geographical variants. All of the previously noted behaviours, and then some more, have been shown here to coexist at this single location, and there is good evidence for males (which switch readily between the three display routines) and preliminary evidence for females (which will change their mating procedure if interrupted) that they are deployed flexibly, rather than being fully determined by genotype or juvenile environment.
A simpler interpretation of this ‘infinite variety’ is that it is a consequence of two basic facts: that either sex can allure the other (and be allured), and that either sex can carry out these operations while flying or while perched (Fig. 2). The apparently enumerable (or innumerable) individual mating strategies are therefore actually a continuum of occurrences, arising in (almost) all possible combinations, a result merely of the vagaries of who has detected whom first, and where, and by which channel, and also by the hazards of sight lines and the direction and turbulence of scent plumes. Such hazards are exemplified by the homoerotic episodes, which are most easily explained as an erroneous reading of two sensory channels: both the males detect the female pheromone but misinterpret the other male, which may be the only other moth they can see, as its source. A more nuanced, but analagous, crosstalk between olfaction and vision during sexual identification has recently been demonstrated in humans (Zhou et al., 2014).

Both sexes (M, male; F, female) can attract, and both can be attracted, all when flying and when sessile, causing approaches in the direction of the arrows. This generates the variety of courtship and other procedures: 1, classic moth; 2b, classic lek; 3a, prolonged negotiation; 3b, mutual courtship; H, homoerotic attraction (probably resulting from confusion between olfactory and visual signals); S, social attraction of females to copulating pairs (probably to the male pheromone, possibly to the female). Of the other procedures (see text) 2d, which is problematic in this species, involves a female flying to a flying male, and 2a and 2c (male mating swarm) involve a flying female approaching a flying male, and the male then approaching the female after or while she flies to a perch. 3a involves some flight by both sexes from the perched positions.
Mate choice
It is widely proposed that female mate choice drives the evolution of leks (Bradbury & Gibson, 1983; Höglund & Alatalo, 1995; Picozzi, 2013). This can be neither demonstrated nor disproved here without much refined work, but there clearly are opportunities for mate choice. Repeat mating by males has been briefly demonstrated both here and in H. humuli (Picozzi, 2013), suggesting routinely enough that some males are more successful at mating than others. The actual act of copulation clearly presents opportunities for refusal by both sexes because (particularly in procedure 2) it can be achieved only by a mutually coordinated and tricky alignment. There are opportunities for mate choice by females, both when approaching several flying or sessile males, and when they ‘call’ a mating frenzy of all flying males simultaneously. One 3a courtship with both individuals fanning in close proximity lasted for more than 13 min without success, the male finally flying away. The female in one homoerotic event could be said to have chosen a pendulating male when she failed to fly, as would be normal, to an adjacent perch which was occupied by a sessile male, and instead dropped down to where the pendulating male eventually followed her (Video S14). It is common for sessile males to ignore a female that is fanning a decimetre or two away from them (even very occasionally a female that is directly adjacent), and only to become alerted and (sometimes) attempt to mate after another male has arrived and commenced copulation (also Picozzi, 2013; Turner, 2014). A simple explanation would be that the first male has too high a threshold (quantitative or qualitative) to respond to the signals from the female; these rise to his threshold only when both members of the mating pair start fanning, possibly also adding the pheromone of the successful male. Thus many of these apparent ‘choice’ situations may simply be the result of the limited competence of the neurosensory apparatus (as shown by homoerotic events), or by the vagaries of sight lines and the direction of turbulent pheromone plumes. The distinction between sensory incompetence and ‘choice’ is of course extremely subtle.
Resources offered by the lek
It is uncertain whether the site doubles as a breeding site. Oviposition, previously noted as never having been seen (Lepidopterologen-Arbeitsgruppe, 2000; Turner, 2013), has now been plausibly observed here, but the number of females suspected of ovipositing is nowhere near to the number seen mating, and most released captive females have flown away from the site (Turner, 2013). Furthermore, compared with the moment to moment excess of males on the site (not quantifiable, but on almost all evenings there are more males than there are mating pairs) the light traps at 40 m in the adjacent woodland show an excess of close to 2 : 1 of females over males, and traps at approximately 10 m show a nearly equal sex ratio (Table 1). Some of the peripheral females are known to be mated, from direct identification or laying fertile eggs. Within the site, males did not concentrate in the areas where females were suspected of ovipositing. The general picture is that males are aggregated in and around the lek site, while females are flying widely in the general landscape. This argues against the lek site being a concentrated breeding site, and against the possibility that females could remain crawling and ovipositing on site in the base of the ground-layer.
The local distribution of plants is not of much help because the larvae are root-feeders and the host range is imperfectly known. The only properly verified hostplants are the two fern species Pteridium aquilinum bracken (Porter, 1997; Post, 2006) and Dryopteris dilatata broad buckler-fern (Post, 2006), but the species is believed to be polyphagous, and over a dozen herbs have been quoted, entirely without traceable supporting evidence (see Supporting information, Doc. S5). In northern Switzerland, the Lepidopterologen-Arbeitsgruppe (2000) made the important observation that they could find no larvae in the soil beneath stands of Urtica dioica much frequented by the adult moths. The species can clearly lek on sites that are not breeding sites and any designations of hostplants based solely on an association with adult moths are unreliable. A similar deduction can be made forcibly from the observation of a small swarm on Salix branches over standing water and mud (Post, 2006). The lek site does contain Dryopteris, Urtica nettle and Taraxacum dandelion (but not Pteridium) from the supposed host genera (see Supporting information, Doc. S2), but again there is no strong reason to believe that breeding is preferentially concentrated there. There is plentiful Pteridium in the neighbourhood (Turner, 2013) and in May 2014, in one of seven test digs in a stand 100 m from the lek site, a single fully grown larva, recently dead, was found at the base of a Pteridium plant. Leking has not been observed on this stand. No larvae were found in five test digs around various plants on or adjacent to the lek site. Thus, although there is no clear evidence that this is not a breeding site, it appears at least to be less concentrated as a breeding site than as a site for mating.
Alternatively, the resource offered appears to be a safe copulation perch. Phymatopus hecta uses an instant dead drop as a defence against predators: the moth releases the grip of its feet (prothoracic feet first) and falls into the subtending vegetation (Turner, 2013; see also Lepidopterologen-Arbeitsgruppe, 2000). This has been seen twice on this site (one pair, one sessile male) as a defence against wasps. Hence roosting P. hecta can be collected by ‘beating’ vegetation: the moths will drop and lie ‘as if dead’ in the beating tray (Freyer, 1856: 96). Both males and females have this response but, in copulating pairs, which hold the support by the female's four front feet, only she can initiate the drop: touching the male produces no response by either moth (Turner, 2013). Copulating male H. humuli can likewise be painted without disturbance, although the female response is unknown (Picozzi, 2013). The pair are therefore well protected against flying and crawling predators (e.g. wasps, arachnids), but not (and this is the Achilles' heel) if the male is in contact with or within reaching distance of the surrounding vegetation. Matings conspicuously occur only rarely in such a way that the male is touching anything, are normally under diagonal or horizontal supports with a clear subtending air space, and are frequently high in the ground layer or on the leaves of emergent plants or the low-hanging Corylus. In a dropped copulating pair, the female will then climb up again to a suitable perch, and on vertical but curving grass- or Luzula-blades the female will move upwards after mating, until the male hangs clear of the support. When matings occur on (sub)vertical supports such as Luzula leaves, the male is often somewhat protected by the angling of the abdomens, which cause the male's head and thorax to be held about 1 cm away from the support, and thus less likely to be contacted by a predator crawling upwards. These observations are supported by reports elsewhere that matings and male displays are on Pteridium leaves (Hanson, 1938; Turner, 1976; Picozzi, 2012, 2013), Symphytum (Cockayne, 1912), Molinia inflorescences (Harper, 1959), Heracleum leaves (Turner pers. obs. 1990, Dornoch Sutherland, 57°53′9′′N, 4°4′44′′W), low hanging sapling branches or Salix bushes (Hanson, 1938; Post, 2006), or near the tops of Urtica plants and under Corylus leaves (Lepidopterologen-Arbeitsgruppe, 2000). Juncus stems are also used (Cockayne & Jackson, 1913; Turner, 2014).
Both males and females show intense interest in perch positions, both directly and as represented by sessile or copulating individuals. Pendulating males frequently make repeated approaches to potential perches before finally selecting one of them. Males appear also to defend perches, but in an ambivalent fashion, because it is not clear what is being disputed during the fights: perhaps an aerial space around a copulation perch, perhaps an area with a concentration of perches, or perhaps simply a part or all of the lek site. Sometimes they butt, perhaps scent mark, other sessile males, but mostly assemble next to them without any further aggression (Videos S5, S8). There is probably a trade-off between the advantages of being near other males, and the disadvantage of having too many immediate competitors. It is notable that H. humuli, which has a much more subdued form of fighting (Robson, 1892: 55–56; Picozzi, 2013), has no sessile displays.
Sessile males have effectively chosen and then advertised a copulation perch. Pendulating males are probably flying near to a potential perch (and sometimes have perhaps marked it), with the final perch selection apparently left to the female. In procedures 1 and 2c, the female makes the choice, apparently rather rapidly: females have never been seen testing the vegetation.
The value of this particular site for the lek therefore appears to be its high density of emergent plants. This architecture is in fact quite rare: stands of Luzula are normally monocultures, and in the immediate area herb layers with clear emergents are not frequent. In conclusion, it is possible that the males on the lek are offering oviposition sites as a resource, but clearer that they are offering safe mating perches.
Evolutionary origins
The specialized flight behaviour of P. hecta is readily derived from the general flight patterns. The extremely rapid flight exhibited by nonleking species (Harper, 1959) has apparently been lost: this correlates well with the morphology because it is known that speed of flight (thoracic temperature) correlates positively with wing loading, and P. hecta has the lowest such loading ratio of any of the five north European hepialids tested (Rydell & Lancaster, 2000). The more moderately-paced more or less linear flight used for flying in and out of the lek, is modified during passage through the lek into the sinuous searching flight. This readily condenses from an ‘S’ to an ‘8’, rapidly executed to produce the apparent pendulum flight, allowing the male in effect to hover over a limited area of ground. The fighting dance is clearly a further condensation. The moths do not feed and so cannot replenish their resources: in terms of expenditure the sessile displays are low cost, pendulation and the first grade of fighting are costly, and the escalated dancing must incur a high cost indeed. It is most easily interpreted as an asymmetrical war of attrition, as seen in some damselflies (Marden & Waage, 1990; Marden & Rollins, 1994), in which the individual with the smaller fat resources resigns, presumably in anticipation of total exhaustion. In the present case, the departing male still has sufficient energy to fly rapidly away. The spread eagle display appears to be a ritualization of the intention-movement that males exhibit while temporarily perching, or an energy-saving modification of a fanning display. The fact that the sessile males do not move while displaying also suggests that they are avoiding gleaning bats by remaining motionless and silent, as well as conserving energy. The fanning displays, which are of surprisingly short duration, would be expected to attract females from the greater distance by impelling the perfume.
The male attractant could have originated as a close-range androconial signal, or as a defence compound, and the scent brush from pre-existing scales on the legs, more or less as they are still found in H. humuli. However although the female metathoracic legs are usually described as unmodified, they are in fact much reduced in size and functionality compared with those of nonleking hepialids, are seldom used when perching, and have only limited use in locomotion (J. R. G. Turner, pers. observ.). This may indicate that the brushes originated on an already redundant leg, or of course may simply be the result of correlated evolution in the two sexes (as with the retention of nipples in male mammals).
Versatility, flexibility, stability
Clearly, the mating system of this species is a lek in the sense of an ‘assembl[y] of adult males which females visit solely for the purpose of copulation’ (Bradbury & Gibson, 1983: 109). It has persisted on this particular spot for at least 7 years. The system is resource-based, versatile, flexible and, it will be argued below, evolutionarily stable.
Signals are clearly olfactory and by implication visual. The variety of mating procedures probably arises from the simple rule ‘both sexes attract/are attracted, both when perched and when flying’ and from the demonstrated flexibility of both males and females as to what procedure they will employ. The most likely resource is an abundance of safe copulation perches, making this a clear case of a lek operating on a hotspot.
The procedures employed are unusually versatile (Fig. 2) and embrace strategies which could be regarded as the classic male moths assembling to a calling female, a male mating swarm, or a classic lek. A general expectation from the theory of leks (Bradbury & Gibson, 1983; Bradbury, 1985; Höglund & Alatalo, 1995) might be that such a versatile system would be evolutionarily unstable because of the selective benefits of female mate choice, which might favour the full lek procedure. But following the argument of Phelan (1997), one could argue that this is instead a unified mate-acquisition procedure with considerable failsafe provisions, and that natural selection for mate acquisition is often more powerful (if only because shorter-term) than natural selection for choosing good genes (you cannot choose between cereal brands if you never reach the supermarket). Any tendency by either sex to decrease the effectiveness of its pheromone would confer an immediate individual disadvantage by reducing the probability of acquiring a mate. This would substantially stabilize the present system. The only obvious major trigger for instability would be for the males to abandon the high-energy and predation-risky flying display, in which case females would no longer need to lure them and the whole system could turn into a straightforward lek. Paradoxically, it might be the original classical procedure which gives the greatest opportunity for female choice because it allows the female to inspect most of the males on the lek simultaneously.
Evolutionary dynamics
The mating system of P. hecta has the appearance of a completely preserved set of ‘living fossils’: the considerable versatility from classic moth to full lek strongly suggests an evolutionary sequence (without necessarily implying a direction), or at least can be used as an illustration of various evolutionary models.
The evolution of lek behaviour is initially harder to explain in moths than in game birds (Fig. 3). In these birds, a classic system in which females seek or are attracted to male territories or to the males that possess them (Wynne-Edwards, 1986), changes into a system in which females seek an aggregation of males (Wiley, 1974; Höglund & Alatalo, 1995). Only one initial change is needed: males have to change from holding dispersed territories to aggregating (although there can be substantial discussion about the cause and the role of the intermediate exploded lek strategy). In moths, there is an additional problem, created by the initial use of female olfactory lures (‘pheromones’) in the vast majority of moths (Svensson, 1996; Phelan, 1997). Thus males that travel to seek alluring females have to change to being sedentary and luring the females (before or after they have adopted aggregation), and females that remain in one place have to change to travelling in search of the alluring males. This last pair of changes has to be coordinated simultaneously between the males and the females. It can be shown that male aggregation could be favoured because there is a trade-off between increased detection by females versus predators (Alem et al., 2011; Brunel-Pons, Alem & Greenfield, 2011; Cordes et al., 2014) but, for this to be the initial trigger for the evolutionary change, the males must already have changed to using a lure. We have in short a double chicken-and-egg problem, in which it is hard to understand how the change can start on the reasonable Darwinian assumption that two or more adaptively interdependent characters with independent genetic bases cannot be changed simultaneously.
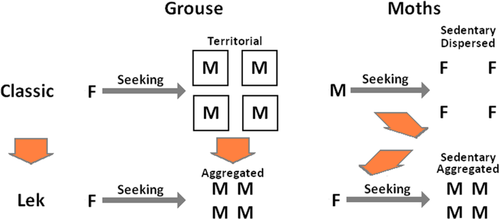
The origination of lek behaviour in grouse/game birds seems to require only one relatively simple (although not necessarily simply-explained) evolutionary change: males must evolve from being dispersed to aggregating. In moths, three changes are needed, which appear to be interdependent: females must change from sedentary to seeking, males must change from seeking to sedentary, and also from dispersed to aggregated. Dark arrows, behavioural movement; orange/white arrows, evolutionary change. M, male, F, female.
The aggregation could however have commenced with the females. Alluring females do not normally aggregate, attributable to the ‘asymmetry’ between the sexes (Phelan, 1997) which ensures, that because multiple mating in males versus single mating in females results in males having a higher variance in offspring number and therefore a greater opportunity for and intensity of selection (Bateman, 1948; Crow & Kimura, 1970), there is a stronger bias in females than in males against high risk strategies for mate acquisition, like aggregating (Phelan, 1997). However there is no such asymmetry during copulation (both sexes have equal investment and equal risks) and during oviposition the pressure reverses in favour of female risk-taking. Thus, as is well recognized, if oviposition sites are spatially under-dispersed, the necessary aggregation of females on these sites can act as the trigger for the evolution of lek-like behaviour (Parker, 1978). The same would be true for spatially clustered copulation sites. Although the evolution of male mating swarms in insects is usually attributed to their ability to intercept females travelling toward oviposition sites (Phelan, 1997), this is somewhat problematical, as it is a priori unlikely that unmated females will be strongly drawn to such sites (feeding sites are irrelevant in hepialids). Furthermore, it is likely in herbivorous insects that oviposition sites are also eclosion sites: if one is clustered then so is the other. But in that case both males and females are already aggregated on the eclosion site, and there is no motor for evolutionary change.
A model for evolution on a hotspot
The behaviour of P. hecta strongly suggests a solution to this paradox: there is substantial attention to obtaining perches which carry a reduced risk of predation during copulation. [The perches are relatively safe against invertebrate predators and nonflying mammals: aggregation, in itself risky for both males and females against bats and birds, has been facilitated by the use of the bird-bat-free window at twilight (Fig. 1) (Andersson et al., 1998; Rydell, 1998). A general assessment of predation risks from all sources is provided by Turner (2013).] Moreover at least in current ecological conditions these perches appear to be spatially aggregated (to clearings, river banks, woodland rides, compared with the general woodlands that may act as breeding territory). Speidel (1994) describes a strong affinity with herb-rich woodland edges and perennial-rich Sphagnum moor.
Thus, we can apply Parker's spatial heterogeneity model, as developed into the ‘hotspot’ hypothesis (Parker, 1978; Bradbury & Gibson, 1983; Bradbury, 1985). If initially females tended to congregate on sites with safe perches, there would here be a considerable individual advantage to males in congregating there in anticipation. Male hecta still effectively hover in a mating swarm on such sites. Once this situation pertains, there can be two mutually re-enforcing selective processes: it is individually advantageous to males to lure individual females in their direction, and it is efficient for females to orient towards the males (collectively and individually) rather than choosing their own perch by making a rapid assessment of the complex geometry of the vegetation. This orientation to other, perched or copulating, individuals is indeed observed in both sexes. The male lures would be expected to be, in these apparently deaf moths, some combination of visual signals and olfaction, developing probably from pre-existing androconial scents in the males.
Why swift moths are like grouse
Such a mutually escalating process of natural and sexual selection effectively produces evolution in the direction of a strictly-defined lek, with a full reversal of sex roles when it comes to allurement, and with competitive fighting between males as an unsurprising addition. Because hepialid moths do not feed as adults, they have a limited energy supply. The sessile displays, with no aggressive component, are likely to have evolved as an energy-saving alternative to the pendulating, fighting display, with the additional benefit of being undetectable by bats if the moth remains motionless; they could be derived variously from a perched fanning display or intention movement, and from the regular resting pose. The tented display is perhaps a low-risk, low-benefit ‘satellite’ strategy. One would predict that males will increase their use of the sessile displays as they age, and also (Lepidopterologen-Arbeitsgruppe, 2000) at lower temperatures or higher wind speeds.
The final evolution of pure lek versus mating-swarm behaviour is probably shown by the other leking or like-leking hepialids (Table 2), where there seem to be species which use only the male-swarm procedure, or only the sessile full lek (‘seem’ because, apart from H. humuli and the American Phymatopus species, the observations are still limited).
| Species | Flying display | Sessile display | References |
|---|---|---|---|
| Phymatopus hectoides, Phymatopus californicus, Phymatopus behrensii | +* | + | Williams (1905); Wagner (1985); Wagner & Rosovsky (1991) |
| Sthenopis auratus | – | + | McCabe & Wagner (1989) |
| Sthenopis purpurascens | – | ?a | Schmidt & Laurie (1999) |
| Sthenopis argenteomaculatus | + | – | Holland (1903); Keith (1915) |
| Sthenopis thule | + | – | Lyman (1893); Winn (1909) |
| Hepialus humulib | + | – | Literature |
| Hepialus schamylc | + | – | Slashchevskiy (1929a, b) |
| Endoclita excrescens | + | – | Kan et al. (2002a, b) |
- *The mating systems of P. hectoides and P. californicus resemble those of P. hecta; the system in P. behrensii appears to be simpler (Wagner, 1985)
- a Negative for flying display, sessile display can be postulated by default
- b The predominant mating in H. humuli (combined data of Chapman, 1876, 1886; MacArthur, 1895; Manders, 1908; Leuschner, 1970; Turner, 1976; Mallet, 1984) is procedure 2c [male flying display, males follow passing female to perch (singly in Britain, several males in Germany)], with a minority (four out of 26 matings enumerated for Britain) being procedure 2d (female strikes flying male); there is a single report apparently of procedure 1 (male flies to sessile female) (Harper, 1960). A briefly perched male photographed by Picozzi & Espie (2011) may have been indulging in a transitory sessile (tented) display.
- c Mating in H. schamyl is described as a male mating swarm; procedures include mutual male–female pendulation, and a male flying to a female after she has perched.
Clearly we cannot finally eliminate two other hypotheses: first that the aggregation itself is an anti-predator defence by satiation during periods of high density like that observed by Carolsfeld-Krausé (1959) or by chemical defence, possibly employing the scent attractants (communal watchfulness and full aposematism are ruled out by the species' biology); or that lek evolution has been favoured because females prefer aggregated males that they can assess for ‘good genes’. However, these alternatives still fall foul of the problem of initiation, and the escalated hypertrophy of display structures from ‘good gene’ dynamics that characterizes some bird leks appears to be missing, although the scent brushes (Video S18) and sexually dimorphic colours might qualify.
There is still one question to answer in the above scenario: how/why does a sessile alluring female become a female that actively seeks a safe copulation perch? Many but not all nonleking hepialid moths already have such a prenuptial flight (Wagner & Rosovsky, 1991); the females fly away from their eclosion position, perch, and start wing fanning [Triodia sylvina orange swift (Blair, 1918), K. gracilis (Wagner & Rosovsky, 1991), K. fusconebulosa (J. R. G. Turner, pers. observ.); probably the very first report of male moths assembling to a calling female is of this last species (Hill, 1859)]. A possible explanation is that moths in general tend to expand their wings while perched on a vertical surface, which does not provide the diagonal or overhead support needed for safe copulation. Wagner & Rosovsky (1991) point out that species without this prenuptial flight, where the females simply remain at their emergence point to call the males (e.g. Wiseana: Allan & Wang, 2001: K. lupulina: Robson, 1891) largely occupy the basal clades of the provisional phylogeny, with the prenuptial-flight species occupying the middle clades before the leking species at the apex. They therefore suggest that the prenuptial flight was a precursor of the lek behaviour. The scenario thus reduces the ‘problem’ of lek evolution in hepialids to something very similar to that in game birds (Fig. 3). In both cases, it is an apparent precondition of the evolutionary sequence to have females that are already travelling seekers: of a safe place to mate in the one case, and in the other of a good male in a good breeding territory. This means that, in both cases, the evolution of leking behaviour can commence with the aggregation of males.
The proposed evolutionary sequence is therefore: males seeking sessile females; females adopting prenuptial flight; females aggregating on a hotspot; males aggregating on the hotspot; males attracting females (individual sexual selection); and females orienting to males (natural selection); hence a development of a lek-like mate acquisition procedure, with further male display and fighting, while retaining the original procedures because all strategies are evolutionarily stable.
Whether these explanations apply in other cases is left for further consideration. Most moths have sessile alluring females without prenuptial flights, which may explain why leking in moths is so rare. Among the other families involved (see Greenfield, 1981), the Arctiidae (Willis & Birch, 1982) are generally aposematic: male aggregation in this group might therefore be explained initially as a predator defence.
Conclusions
Phymatopus hecta has probably ‘the most complicated mate acquisition procedures known in any insect’ (G. A. Parker, pers. comm.). A highly diverse set of mating rituals is generated by the simple fact that both sexes use scent and probably vision to lure each other, as is also seen in the simpler system of Estigmene (Willis & Birch, 1982). The system (Fig. 2) is probably evolutionarily stable.
The sequence of lek evolution in game birds and hepialid moths may be very similar, commencing with females that are seekers, and a relatively simple evolutionary step: the aggregation of males (Fig. 3). In the case of hepialid moths, this is on a hotspot of spatially aggregated safe mating positions. The further evolution of the lek behaviour in the moths can be rather easily explained from a combination of inter-male sexual selection and natural selection on female orienting behaviour.
Acknowledgements
I thank Malcolm and Kathy Fraser for indispensible facilitation, ambience and encouragement; my wife Sandra for support and infinite patience; Deane Waters for the initial loan of a camcorder; Sandy Payne for coaching in fern identification; the British Comparative Literature Association for an award which facilitated a camcorder purchase; the librarians of the Royal Entomological Society (Valerie MacAtear), the Natural History Museum, London (Lorainne Portch) and the Biodiversity Heritage Library for accelerated literature access; John Grehan, Geoff Parker, Wolfgang Speidel and David Wagner for essential expert correspondence; David Wagner and an anonymous referee for suggestions; and the University of Leeds for facilities under an Honorary Visiting Fellowship. I also thank Roger Butlin and the late Jocelyn Crane-Griffin for previously educating me in the theory and practice of studying insect mating systems. The research is self-funded.




