Efficacy and Safety of Clopidogrel With and Without a Proton Pump Inhibitor: A Systematic Review and Meta-Analysis
Funding: The study was funded by the Swedish Research Council (2021-01308), and the Swedish state under the ALF agreement between the Swedish government and the county councils (ALFGBG-1005062). The funding sources did not influence the design, methods, data collection, analysis, preparation of the paper, or decision to submit it for publication.
ABSTRACT
Classifications of drug interaction alerts regarding clopidogrel and a proton pump inhibitor (PPI) differ between knowledge resources. In this systematic review, Medline, Embase, and the Cochrane Library were searched for randomized controlled trials (RCTs) applying PICO criteria: P = patients on clopidogrel; I = intervention: PPI (subgroup: [es]omeprazole); C = comparison: no PPI (C1) or a PPI other than (es)omeprazole (C2); O = outcomes, main: a composite of cardiovascular events (efficacy); also: overt gastrointestinal bleeding (safety). Fourteen RCTs fulfilled the PICO criteria, five without high risk of bias and with at least one clinical event per study arm. Regarding efficacy with or without a PPI, the pooled risk ratio (RR) and risk difference (RD) were 1.08 (95% confidence interval (CI) 0.78; 1.50) and 0.2 percentage points (95% CI −0.9; 1.2), respectively (four RCTs; 4341 patients [96% also used aspirin, 98% receiving I used (es)omeprazole]; moderate certainty evidence). Regarding safety, the RR and RD were 0.13 (95% CI 0.03; 0.59) and −0.7 percentage points (95% CI −1.1; −0.3), respectively (one RCT; 3761 patients; moderate certainty evidence). The available evidence did not allow conclusions regarding omeprazole versus pantoprazole. In conclusion, concurrent use of a PPI probably does not largely affect clopidogrel efficacy, but probably reduces the risk of overt gastrointestinal bleeding.
Summary
Some medications that reduce the stomach acid production, to treat, for instance, stomach ulcer, are described to reduce the effect of a blood-thinning medication that is used to prevent heart disease and stroke in patients at high risk. We investigated patient effects of concurrent use of these medications by searching and compiling relevant scientific studies on the topic. Based on five well-designed and well-performed studies, we conclude that the concurrent use probably does not largely affect the heart-preventive effects, but it probably reduces the risk of bleeding in the gut.
1 Introduction
The concurrent use of clopidogrel and a proton pump inhibitor (PPI) triggers clinically significant drug interaction alerts in established knowledge resources such as Stockley's Drug Interactions/Checker (Stockley) [1], UpToDate Lexidrug [2], Micromedex [3] and Janusmed [4]. However, the stated level of documentation and the classification of clinical significance differ to some extent (Table S1).
Elevated gastric pH has been suggested to negatively affect the gastrointestinal (GI) absorption of clopidogrel, which could explain an effect of all PPIs on clopidogrel efficacy [5], perhaps explicating why alerts for all PPIs appear in two of the knowledge resources [2, 4]. However, a possible interaction between clopidogrel and the specific PPI (es)omeprazole has attracted particular interest, since (es)omeprazole has been suggested to substantially inhibit bioactivation of clopidogrel through inhibition of the cytochrome P450 (CYP) 2C19 enzyme, as opposed to other PPIs that have little ([dex]lansoprazole) or no (pantoprazole, rabeprazole) inhibitory effect on CYP2C19 [6]. Therefore, while three of the knowledge resources suggest actions also for PPIs other than (es)omeprazole [2-4], both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) focus on (es)omeprazole only, stating in the product information texts that the combination should be avoided (FDA) and discouraged (EMA) [7, 8].
Given that taking a PPI together with clopidogrel is not uncommon [9, 10], and that alerts triggered by their concurrent use in well-renowned knowledge resources differ regarding categorizations, a thorough compilation of the current evidence base may be useful. Hence, we aimed to investigate whether the concurrent use of clopidogrel and a PPI in general, and (es)omeprazole specifically, affects the risk of cardiovascular (CV) events (efficacy) or GI bleeding (safety).
2 Methods
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies [11]. This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12], and registered with PROSPERO (CRD42024592915). The PICO framework was used to formulate the research question and define the aim. Accordingly, participants (P) were patients on treatment with clopidogrel. The intervention (I) was concurrent treatment with a PPI, with a prespecified subgroup of (es)omeprazole. The comparison (C) was no PPI (C1), or a PPI other than (es)omeprazole (C2). Predefined subgroups of C2 were pantoprazole, (dex)lansoprazole, and (dex)rabeprazole. The main outcomes (O) were a composite of CV events according to study definition (efficacy) and overt GI bleeding (safety). Predefined additional efficacy outcomes were all-cause mortality, myocardial infarction (MI) and stroke (thromboembolic and/or haemorrhagic). The predefined additional safety outcome was major bleeding. Before the data extraction process, but after the PROSPERO registration, we realized that GI bleeding, overt or occult, would be a clinically relevant outcome for the evaluated intervention. This outcome, which was not included in the registration, was therefore added. Publications were restricted to randomized controlled trials (RCTs), and languages were limited to English, Swedish, Danish and Norwegian.
2.1 Literature Search and Study Selection
On 15 May 2024, systematic searches were performed in Medline, Embase and the Cochrane Library. Search strategies are provided in the supplement. Articles cited in four drug interaction knowledge resources (Stockley, UpToDate Lexidrug, Micromedex, Janusmed) were screened for additional references. We also searched for further articles in systematic reviews that were found in the literature search and that fulfilled the PICO criteria.
The titles/abstracts retrieved in the systematic literature search were independently screened by two authors (MABA and NPL or EWJ and SMW) for inclusion according to the PICO criteria. Full texts were retrieved when required for decision making. Discrepancies were resolved in consensus discussions. For articles that were excluded in consensus after full-text reading, the reasons for exclusion were recorded. The remaining studies were included in the systematic review. A similar process, by two independent authors (MABA and NPL or EWJ and SMW) and with subsequent consensus discussions, was applied to identify articles that met our PICO criteria among those included in the identified systematic reviews. We also screened the interaction knowledge resources for potential additional RCTs that met our PICO criteria.
2.2 Data Extraction and Study Assessments
Data from the included studies were independently extracted by two authors (MABA and SMW) and discrepancies were resolved in consensus discussions. Data extraction included the design of the studies, participants studied, the number and characteristics of individuals in the intervention and control groups, as well as the results regarding the outcomes at issue. We did not obtain additional data from the study investigators.
Each study was independently appraised by all authors regarding the risk of bias and directness, after which all authors took part in the subsequent consensus discussions. We used the Cochrane risk-of-bias tool for randomized trials (RoB 2) [13]. Directness was assessed based on a checklist used by the regional Centre for Health Technology Assessment (HTA-centrum, Sahlgrenska University Hospital, Gothenburg, Sweden), including questions regarding each component of the PICO, and the extent to which the assessed study corresponded to this [14]. Reasons underlying the assessments of risk of bias and directness were recorded in consensus. The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [15]. Informative statements according to GRADE guidelines were used to summarize the results [16].
2.3 Statistics
When two or more studies provided data that could be pooled, we performed random-effects meta-analyses using the Review Manager (RevMan) version 5.4.1 software (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) to obtain risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs). Heterogeneity was assessed with I2 statistics. We decided beforehand that studies without a high risk of bias and with at least one clinical event per study arm would form the main basis for conclusions. For comparisons, we also performed meta-analyses including all studies. No ethics approval was required as no sensitive data were handled.
3 Results
After removal of duplicates, the literature search identified 1325 unique publications, 20 of which met the PICO criteria of this systematic review (Figure 1). Publications excluded after full-text reading, as well as the reasons for excluding them, are presented in Table S2. One additional study [17] was identified through the screening of publications in relevant systematic reviews that were identified in the literature search (see p 10 in the Supplement). No additional RCTs were identified in the knowledge resources, which, in all, cited 64 scientific articles, one of which, an RCT that met the PICO criteria of this systematic review and reported clinical events [18], was cited in two resources [1, 2]. Seven RCTs met the PICO criteria but reported zero clinical events concerning our outcomes [19-25].

3.1 Study Characteristics
In all, 14 RCTs contributed data to this review (Table 1) [17, 18, 26-37]. One RCT included patients in 15 countries [18]. The remaining studies were from China [17, 26, 28-34, 36, 37], Taiwan [27] and Japan [35]. In nine studies, clopidogrel was part of dual antiplatelet therapy (DAPT) [18, 26, 29-33, 35, 36], whereas aspirin appeared to be used by most, if not all, patients in another two studies [34, 37] (Table S3). The intervention was omeprazole [17, 18, 26, 30, 32, 35], esomeprazole [27, 29, 31], pantoprazole [28, 33, 34, 37] and lansoprazole [36]. The control was placebo [18, 30, 34], no PPI [17, 27, 28, 33, 36], pantoprazole [26, 32], famotidine [29, 31, 32, 35] or traditional Chinese medicine [37]. Follow-up ranged from ≤ 5 days [34] to 2 years [18]. The primary endpoint was GI events in five RCTs [18, 27, 29, 34, 37] and CV events in two [18, 37]. Neither of the two latter RCTs was powered for the primary CV event [18, 37], and one was stopped prematurely as the funding was lost [18]. Eight RCTs were assessed to have a high risk of bias (Table 2) [17, 28, 30, 32-35, 37]. Out of the remaining six, five reported one or more clinical events per study arm [18, 26, 27, 29, 36]. Reasons underlying the directness and risk of bias assessments are outlined in Table S4.
| Author, year | Design, country | Patients (n) | Age (yrs) | Male sex (n) | I vs. C (substance(s), daily dose, treatment length) | Follow-up | Primary outcome(s) | Definition | |
|---|---|---|---|---|---|---|---|---|---|
| Composite of CV events | Overt gastrointestinal bleeding | ||||||||
| Bhatt et al. 2010 [18] |
Double-blind RCT, 15 countries |
P: With indication for DAPT I: 1876 C1: 1885 |
(median) I: 68.5 C1: 68.7 |
I: 1255 C1: 1308 |
I: Omeprazole 20 mg C1: Placebo Treatment length: maximum 2 years |
Prematurely terminated, median follow-up: 106 days (Maximum follow-up time according to protocol: 2 years) |
Composite of upper GI clinical events including overt/occult bleeding, gastroduodenal ulcers etc. Composite of death from CV causes, nonfatal MI, coronary revascularization, or ischaemic stroke |
Death for CV causes, nonfatal MI, coronary revascularization, or ischaemic stroke |
Overt gastroduodenal bleeding confirmed by upper endoscopy or radiography Overt upper GI bleeding of unknown origin |
| Gao et al. 2009 [17] | Double-blind RCT, China |
P: AMI I: 114 C1: 123 |
(mean) I: 58.2 C1: 57.5 |
NR |
I: Omeprazole 40 mg iv day 1, thereafter 20 mg po for 7 days C1: No PPI |
14 days | NR (focus: clinical events) | — | Hematemesis/dark stools (naked eye) |
| Gu et al. 2016 [26] | Open-label RCT, China |
P: PCI due to NSTE-ACS, and DAPT I: 303 C2: 304 |
(mean) I: 59.15 C2: 58.76 |
I: 209 C2: 215 |
I: Omeprazole 20 mg C2: Pantoprazole 20 mg Treatment length: 30 days (according to Clinicaltrials) |
30 days | Platelet reactivity | MACE: cardiac death, non-fatal MI, ischaemic symptom-driven TVR or non-TVR | — |
| Hsu et al. 2011 [27] | Open-label RCT, Taiwan |
P: Atherosclerotic disease, past history of peptic ulcer I: 83 C1: 82 |
(mean) I: 70.6 C1: 73.3 |
I: 65 C1: 59 |
I: Esomeprazole 20 mg C1: No PPI Treatment length: 6 months |
6 months | Gastric/duodenal ulcer | Unstable angina, MI, ischaemic stroke | Hematemesis or melena documented by the admitting physician, with ulcers or bleeding erosions confirmed on endoscopy, or a haemoglobin decrease of ≥ 2 g/dL in the presence of endoscopically documented ulcers or erosions |
| Lu 2017 [28] | Open-label RCT, China |
P: TIA I: 237 C1: 241 |
(mean) I: 69.5 C1: 68.1 |
I: 140 C1: 149 |
I: Pantoprazole 40 mg C1: No PPI Treatment length: 90 days |
90 days | Not defined | — | — |
| Ng et al. 2012 [29] |
Double-blind RCT, China |
P: ACS I: 163 C1: 148 |
(mean) I: 64.3 C1: 63.1 |
I: 126 C1: 107 |
I: Esomeprazole 20 mg C1: Famotidine 40 mg Treatment length: 4–52 weeks |
4–52 weeks |
Composite of upper GI clinical events including overt/occult bleeding etc. |
Reported in results: Major CV events: CV death, MI, stroke |
Hematemesis and/or melena, with or without endoscopy |
| Ren et al. 2011 [30] |
Double-blind RCT, China |
P: High-risk ACS receiving elective PCI I: 86 C1: 86 |
(mean) I: 62.08 C1: 61.84 |
I: 62 C1: 63 |
I: Omeprazole 20 mg C1: Placebo Treatment length: 30 days |
1 month | NR (focus: platelet reactivity) | — | NR |
| Tunggal et al. 2011 [31] |
Double-blind RCT, China |
P: ACS or elective PCI I: 44 C1: 44 |
(mean) I: 63.2 C1: 63.4 |
I: 37 C1: 37 |
I: Esomeprazole 20 mg C1: Famotidine 40 mg Treatment length: at least 28 days |
28 days | Platelet reactivity | — | NR |
| Wang et al. 2013 [32] | Open-label RCT, China |
P: PCI due to MI; I: 83 C1: 77 C2: 80 |
(mean) I: 62 C1: 63 C2: 64 |
I: 59 C1: 50 C2: 55 |
I: Omeprazole 40 mg C1: Famotidine 40 mg C2: Pantoprazole 40 mg Treatment length: 3 days according to methods, 5–7 days according to abstract |
30 days | NR (focus: clinical events) | — | Hematemesis and positive vomit occult blood test, or hematochezia and positive faecal occult blood test |
| Wei et al. 2016 [33] | Open-label RCT, China |
P: STEMI with PCI I: 123 C1: 84 |
(mean) I: 59.32 C1: 58.47 |
I: 69 C1: 48 |
I: Pantoprazole 40 mg, injection 3–5 days, followed by oral administration until discharge C: No PPI |
GI bleeding: during hospitalization CV event: 6 months |
NR (focus: clinical events) | — | — |
| Wu et al. 2011 [34] |
Double-blind RCT, China |
P: ACS at high risk of bleeding I: 333 C1: 332 |
(> 75 years) I: 81 C1: 88 |
I: 246 C1: 244 |
I: Pantoprazole 40 mg twice daily C1: Placebo Treatment length: 7 days |
GI bleeding: during hospitalization (median: 12 days) Mortality: 30 days |
GI bleeding during hospitalization | — | Hematemesis, nasogastric aspirate containing blood or coffee-grounds material, melena, or hematochezia |
| Yano et al. 2012 [35] | Open-label RCT, Japan |
P: ACS scheduled for stent implantation I: 65 C1: 65 |
(mean) I: 67 C1: 66 |
I: 50 C1: 53 |
I: Omeprazole 10 mg C1: Famotidine 20 mg Treatment length: at least 4 weeks |
CV outcome: at 12 months | Platelet reactivity | Death for CV causes, spontaneous MI, unstable angina, stent thrombosis, TVR, non-TVR, ischaemic stroke | — |
| Zhang et al. 2015 [36] | Open-label RCT, China |
P: NSTE-ACS with DAPT, 6 days after PCI I: 53 C1: 51 |
(mean) I: 65 C1: 61 |
I: 24 C1: 22 (According to the characteristics table; the results text describes 81 males) |
I: Lansoprazole 30 mg C1: No lansoprazole Treatment length: 6 months |
6 months | NR (focus: platelet reactivity) | Death, stroke, MI, rehospitalization due to angina, CV revascularization | — |
| Zhang et al. 2018 [37] |
Double-blind RCT, China |
P: STEMI, NSTEMI, UAP, SAP, treated with PCI I: 58 C1: 59 |
(mean) I: 71.14 C1: 71.83 |
I: 20 C1: 22 |
I: Pantoprazole, dose 40 mg C1: Traditional Chinese medicine Treatment length: 4 weeks |
90 days |
GI bleeding MACE |
MACE: cardiac death, nonfatal recurrent MI, recurrent UAP, in-stent restenosis, TVR | — |
- Abbreviations: ACS = acute coronary syndrome, AMI = acute myocardial infarction, C = comparison, CV = cardiovascular, DAPT = dual antiplatelet therapy, GI = gastrointestinal, I = intervention, iv = intravenous, MACE = major adverse CV event, MI = myocardial infarction, NR = not reported, NSTE-ACS = non ST-elevation acute coronary syndrome, NSTEMI = non ST-elevation myocardial infarction, P = patients, PCI = percutaneous coronary intervention, po = per os, PPI = proton pump inhibitor, RCT = randomized controlled trial, SAP = stable angina pectoris, STEMI = ST-elevation myocardial infarction, TIA = transient ischaemic attack, TIMI = Thrombolysis In Myocardial Infarction, TVR = target-vessel revascularization, UAP = unstable angina pectoris.
| Author, year | Efficacy outcomes | Safety outcomes | Risk of biasa | |||||
|---|---|---|---|---|---|---|---|---|
| Composite of CV events (main outcome) | All-cause mortality | Myocardial infarction | Stroke (thromboembolic and/or haemorrhagic) | Overt GI bleeding (main outcome) | Overt and occult GI bleeding/GI bleeding not further specified | Major bleeding | ||
| I vs. C1 | ||||||||
| Bhatt et al. 2010 [18] | 55/1876 (2.9%) vs. 54/1885 (2.9%) | 5/1876 (0.3%) vs. 5/1885 (0.3%) | 14/1876 (0.7%) vs. 15/1885 (0.8%) | 4/1876 (0.2%) vs. 2/1885 (0.1%) | 2/1876 (0.1%) vs. 15/1885 (0.8%)b | 8/1876 (0.4%) vs. 26/1885 (1.4%)c | — | + |
| Gao et al. 2009 [17] | — | 4/114 (4%) vs. 13/123 (11%) | — | — | 1/114 (0.9%) vs. 6/123 (5%) | 6/114 (5%) vs. 18/123 (15%) | — | — |
| Hsu et al. 2011 [27] | 4/83 (4.8%) vs. 3/82 (3.7%) | 0/83 vs. 0/82 | 2/83 (2.4%) vs. 2/82 (2.4%) | 1/83 (1.2%) vs. 0/82 | 0/83 vs. 1/82 (1%) | — | — | ? |
| Lu 2017 [28] | — | 0/224 vs. 1/222 (0.5%) | — | 26/224 (11.6%) vs. 21/222 (9.5%) | — | 2/224 (0.9%) vs. 3/222 (1.4%) | — | — |
| Ng et al. 2012 [29] | 7/163 (4.3%) vs. 5/148 (3.4%) | — | 4/163 (2.5%) vs. 1/148 (0.7%) | 0/163 vs. 3/148 (2.0%) | 0/163 vs. 3/148 (2.0%) | 1/163 (0.6%) vs. 11/148 (7.4%) | — | + |
| Ren et al. 2011 [30] | — | — | — | — | 0/86 vs. 2/86 (2.3%) | — | — | — |
| Tunggal et al. 2011 [31] | — | 1/55 vs. 0/52 | — | — | — | 0/44 vs. 2/44 (4.5%)d | — | ? |
| Wang et al. 2013 [32] | — | — | — | — | 7/83 (8%) vs. 16/77 (21%) | — | — | |
| Wei et al. 2016 [33] | — | — | 6/117 (5.1%) vs. 4/80 (5.0) | — | — | 2/123 (1.6%) vs. 13/84 (15.5%)d | — | — |
| Wu et al. 2011 [34] | — | 35/333 (10.5%) vs. 34/332 (10.2%) | — | — | 4/333 (1.2%) vs. 12/332 (3.6%) | — | 1/333 vs. 5/332 | — |
| Yano et al. 2012 [35] | NR (8 or 9?)/65 (13%) vs. 11/65 (17%)e | — | — | — | — | — | 3/65 (4%) vs. 1/65 (2%) | — |
| Zhang et al. 2015 [36] | 7/53 (13%) vs. 5/51 (10%) | — | — | — | — | — | 0/53 vs. 0/51 | ? |
| Zhang et al. 2018 [37] | 6/58 (10.34%) vs. 2/59 (3.39%)e | 0/62 vs. 1/62 (1.6%) | 0/58 vs. 0/59e | — | 6/58 (10%) vs. 4/59 (7%)e | 0/58 vs. 1/59f, g | — | |
| I vs. C2 | ||||||||
| Gu et al. 2016 [26] | 7/303 (2.3%) vs. 5/304 (1.6%) | 1/303 (0.3%) vs. 0/304 | 0/303 vs. 0/304 | 0/303 vs. 0/304 | — | — | 0/303 vs. 0/304 | ? |
| Wang et al. 2013 [32] | — | — | — | — | 7/83 (8%) vs. 8/80 (10%) | — | — | — |
- Abbreviations: CV = cardiovascular, GI = gastrointestinal.
- a Assessed using the Cochrane risk-of-bias tool for randomized trials (RoB 2): low risk of bias (+), some concerns (?), or high risk of bias (−). For reasons underlying these assessments, see Table S4.
- b Reported overt events summarized.
- c Reported overt and occult events summarized.
- d Overt or occult events not specified in original study.
- e Not included in the meta-analyses of all RCTs as the number of events was not unambiguously reported.
- f Deceased and quitting patients not included in reported numbers in original study.
- g Type 3 according to BARC (Bleeding Academic Research Consortium).
3.2 Efficacy: PPI Versus no PPI
In all, eleven RCTs provided data related to the efficacy of clopidogrel with and without a PPI [17, 18, 27-29, 31, 33-37]. Four of these were without high risk of bias and reported one or more clinical events per study arm (Table 2) [18, 27, 29, 36].
All four RCTs without a high risk of bias and with one or more clinical events per study arm provided data regarding the main efficacy outcome, i.e., a composite of CV events [18, 27, 29, 36]. In total, 96% of the patients also used aspirin, and 98% in the I group received (es)omeprazole. A total of 73 versus 67 CV events were recorded in 4341 patients. Pooling these results yielded an RR of 1.08 (95% CI 0.78 to 1.50, I2 = 0%; Figure 2) and an RD of 0.2 percentage points (95% CI −0.9 to 1.2, I2 = 0%; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as patients in three RCTs, contributing to 95% of the weighted results, were on DAPT, i.e., took aspirin in addition to clopidogrel for CV prevention. There were some study limitations and uncertain precision but further downgrading due to these aspects was not considered justified, resulting in moderate certainty evidence that a PPI probably does not largely affect the risk of CV events.
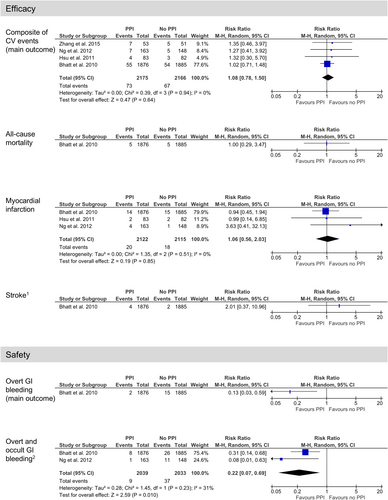
Regarding all-cause mortality, there was one RCT without a high risk of bias and with one or more clinical events per study arm, reporting five versus five events in 3761 patients [18]. The resulting RR was 1.00 (95% CI 0.29 to 3.47; Figure 2) and the RD was 0.0 percentage points (95% CI: −0.3 to 0.3; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT, and two steps because of very serious imprecision (very low certainty evidence). There were some study limitations, but further downgrading was not relevant.
Regarding MI, three RCTs without a high risk of bias and with one or more clinical events per study arm reported 20 versus 18 events in 4237 patients [18, 27, 29]. Pooling these results yielded an RR of 1.06 (95% CI 0.56 to 2.03, I2 = 0%; Figure 2) and an RD of 0.02 percentage points (95% CI −0.5 to 0.6, I2 = 0%; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT, and one step because of serious imprecision. There were some study limitations, but further downgrading was not considered justified, resulting in low certainty evidence that a PPI may not affect the risk of MI.
Regarding stroke, there was one RCT without high risk of bias and with one or more clinical events per study arm, reporting four versus two events in 3761 patients [18]. The resulting RR was 2.01 (95% CI 0.37 to 11.0; Figure 2) and the RD was 0.1 percentage points (95% CI −0.2 to 0.4; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT, and two steps because of very serious imprecision (very low certainty evidence). There were some study limitations, but further downgrading was not relevant.
Figure S2A presents forest plots of all RCTs that include efficacy data.
3.3 Safety: PPI Versus no PPI
In all, 13 RCTs provided data related to the safety of clopidogrel with and without a PPI [17, 18, 27-37]. Two of these were without a high risk of bias and reported one or more clinical events per study arm (Table 2) [18, 29].
One RCT without a high risk of bias and with one or more clinical events per study arm provided data regarding the main safety outcome, overt GI bleeding [18]. A total of two versus 15 events were recorded in 3761 patients, yielding an RR of 0.13 (95% CI 0.03 to 0.59; Figure 2) and an RD of −0.7 percentage points (95% CI −1.1 to −0.3; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT. There were some study limitations and uncertain precision but further downgrading because of these was not considered justified, resulting in moderate certainty evidence favouring the use of a PPI.
Regarding overt and occult GI bleeding/GI bleeding not further specified, two RCTs without a high risk of bias and with one or more clinical events per study arm reported nine versus 37 events in 4072 patients [18, 29]. Pooling these results yielded an RR of 0.22 (95% CI 0.07 to 0.31, I2 = 31%; Figure 2) and an RD of −3.5 percentage points (95% CI −9.7 to 2.7, I2 = 87%; Figure S1). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT. There were some study limitations and uncertain precision but further downgrading because of these was not considered justified. Consistency was not considered problematic as both studies showed a statistically significant difference. Overall, these assessments resulted in moderate certainty evidence favouring the use of a PPI.
Four RCTs provided data regarding major bleeding, all with a high risk of bias and/or zero-event arms [34-37]. A total of four versus seven major bleeding events were recorded in 1016 patients, yielding an RR of 0.62 (95% CI 0.62 to 3.67, I2 = 36%; Figure S2B) and an RD of −0.9 percentage points (95% CI −2.2 to 0.4, I2 = 0%). In the GRADE process, we downgraded one step because of serious study limitations, one step because of serious indirectness, and two steps because of very serious imprecision, resulting in very low certainty of evidence.
Figure S2B presents forest plots of all RCTs that include safety data.
3.4 (Es)omeprazole Versus Other PPIs
In all, two RCTs provided data regarding (es)omeprazole versus another PPI [26, 32]. One of these was without a high risk of bias [26]. Both compared omeprazole with pantoprazole, and subgroup analyses were therefore not relevant.
One RCT, including 607 patients, provided data related to the efficacy of clopidogrel [26]. Regarding the main efficacy outcome, seven versus five CV events were reported in the randomization groups (Table 2). In the GRADE process, we downgraded one step because of serious indirectness as the patients were on DAPT, in line with previous assessments, and two steps because of serious imprecision (very low certainty evidence). There were some study limitations, but further downgrading was not relevant.
Regarding the other efficacy O, one death but no events of MI or stroke were reported. In the GRADE process, in line with the above, we downgraded one step because of serious indirectness, and two steps because of serious imprecision (very low certainty evidence).
One RCT, including 163 patients and with a high risk of bias, provided data regarding the main safety outcome [32]. A total of seven versus eight overt GI bleeding events were reported [32]. In the GRADE process, we downgraded one step because of serious study limitations as the follow-up period of events was considerably longer than the treatment period with a PPI. We also downgraded one step because of serious indirectness as the patients were on DAPT and one step because of serious imprecision due to few events, resulting in very low certainty evidence.
Regarding the other safety O, no study reported results regarding overt or occult GI bleedings. One study reported no major bleeding [26]. As there were no events, we did not apply GRADE to assess the certainty of evidence.
4 Discussion
In this systematic review, concerning efficacy, we found that concomitant use of a PPI and clopidogrel probably does not largely affect the risk of CV events, and that such use may not affect the risk of MI. However, no conclusions could be drawn regarding the risk of all-cause mortality and stroke. Concerning safety, concurrent use of a PPI in patients on clopidogrel probably reduces the risk of both overt GI bleeding and a composite of overt and occult GI bleeding. For the C of (es)omeprazole versus other PPIs, the available studies do not allow conclusions regarding patient O regarding either efficacy or safety.
The main efficacy meta-analysis was largely ( > 95%) based on RCTs where clopidogrel was used together with aspirin, an aspect that could potentially affect the penetrance of a possible interaction. However, the herein compiled data, where 11 out of 14 RCTs indicated concurrent use of aspirin and nine explicitly stated use of DAPT, could reflect that the main question from a clinical perspective is whether concurrent treatment with PPI affects O with DAPT. Indeed, DAPT has for a long time been well established in secondary prevention of CV events. Regarding non-cardioembolic stroke and transient ischaemic attack (TIA), European guidelines recommend that a single antiplatelet is used, with very low certainty of evidence underlying this recommendation [38], and American guidelines specifically recommend aspirin plus clopidogrel in minor non-cardioembolic stroke and high-risk TIA [39].
A recent systematic review focused on potential effects of a PPI on DAPT [40]; regarding our main efficacy outcome, it reported very low certainty evidence and concluded that a PPI does not affect CV outcomes. Further, the reported RR point estimate favoured DAPT plus a PPI (0.93) [40]. This may, however, relate to the high weight in their meta-analysis imposed on an RCT [41] which we excluded because the randomization concerned screening for risk factors for upper GI bleeding rather than a PPI, with pantoprazole prescribed to screened patients at high risk; also, ticagrelor instead of clopidogrel was part of DAPT in almost half of the included patients. In addition, based on our understanding, the mentioned systematic review [40] included patients from two studies [18, 42] from the same RCT in the meta-analysis.
Although the possible effect of PPI treatment on the efficacy and safety of DAPT seems to be the dominating clinical question, it may, from a stroke prevention perspective, be useful to know whether PPIs have a gastroprotective effect in patients treated with clopidogrel without concomitant aspirin. Unfortunately, this question is not answered by the present work. Aspirin is well known to cause GI bleeding through the double mechanism of platelet activity inhibition and impairment of gastric mucosal protection. However, mechanistic reasoning regarding the benefits of PPI treatment on the clopidogrel component of DAPT is not as intuitive. It has, nevertheless, been suggested that clopidogrel may induce recurrent ulcers in previously damaged gastric mucosa at previous ulcer locations where it may provoke bleeding [43]. If this is true, the platelet inhibition by clopidogrel as such may play a role, and in that case, CYP2C19 inhibition would possibly imply fewer bleeding events by (es)omeprazole than by other PPIs. This could theoretically be more notable in patients on clopidogrel without concomitant aspirin than in patients on DAPT. However, the only RCT included herein comparing safety for omeprazole versus pantoprazole showed seven versus eight episodes of overt GI bleeding [32]. Despite our assessment that this study had a high risk of bias and the fact that these patients were on DAPT, this finding may not suggest a difference between CYP2C19 inhibitors and non-inhibitors, against a background of gastroprotection for the aspirin effect.
Interestingly, the meta-analysis underlying our conclusion that use of a PPI probably does not largely affect CV outcomes was primarily based on studies evaluating potential effects of (es)omeprazole [18, 27, 29], the PPI that in the knowledge resources is most consistently suggested to interact with clopidogrel efficacy. The weighted statistically non-significant RD was merely 0.2 percentage points. This finding could be related to the statistically significant absolute reduction of 2.1 percentage points reported for clopidogrel when added to aspirin after acute coronary syndrome (ACS) without ST-segment elevation, in an RCT including 12 562 patients [44]. In that study, however, 11.4% of the patients in the control group had an event [44], compared with only 2.9% in the RCT contributing most to our meta-analysis [18]. Although the patients partly differed between these RCTs, the event rates may reflect that the patient outcome after MI has improved considerably over the years [45]. Therefore, the magnitude of a treatment benefit found in an old RCT may not be fully applicable today. If the benefit of the addition of clopidogrel to aspirin would be more modest in healthcare today, one could speculate that revealing a potential small reduction in the intended effect by an interactive substance would be challenging. Demonstrating non-inferiority would be even more challenging, requiring extreme sample sizes. On the other hand, detecting potential differences of such magnitude may not be a clinical priority.
Two knowledge resources recommend avoidance of concurrent use of (es)omeprazole and clopidogrel [2, 3]. Given the available data on patient O, our evidence synthesis suggests that avoiding clopidogrel may not be fully supported as efficacy loss was not evident. Avoiding a PPI, on the other hand, is likely to imply an increased risk of GI bleeding. Nevertheless, PPIs are not rarely used without a clinical indication [46, 47], and reconsideration of such drug treatment should be encouraged. This may be particularly relevant when clopidogrel is not used as part of DAPT; the benefit of a PPI to prevent GI bleeding in patients on aspirin has been known for long [48].
Surrogate measures, such as measures of active clopidogrel metabolite formation and platelet activity, support that concurrent use of (es)omeprazole attenuates the effect of clopidogrel [22, 49-52]. On the other hand, the data seem to show less tendency of other PPIs to interact with CYP2C19-metabolized drugs [50, 51, 53]. This is in line with the recommendation provided by all knowledge resources, i.e., to consider a switch from (es)omeprazole to, for instance, pantoprazole [1-4]. Indeed, even if we found only very low certainty evidence for this advice as far as patient effects are concerned, the precautionary principle may apply, in particular as switching from one PPI to another implies minor efforts for the prescribing physician, representing a low complexity action [54]. While current pharmacogenetics guidelines recommend avoidance of clopidogrel in CYP2C19 intermediate and poor metabolizers [55], one could expect the strongest effect of (es)omeprazole in CYP2C19 rapid and normal metabolizers. The precautionary principle regarding the choice of PPI may therefore be applicable irrespective of phenotype.
It needs to be mentioned that several studies investigating the potential effect of a PPI on CV outcomes in patients on clopidogrel were not randomized for PPIs. Some of these reported worse CV outcomes for those who used clopidogrel and a PPI concurrently [56, 57], whereas others did not [58, 59]. In general, however, patients on PPI differ from patients not treated with a PPI, making confounding by indication a major issue. For instance, patients taking a PPI are reportedly older [56, 58, 59] although the difference is not always statistically significant [57].
4.1 Strengths and Limitations
A major strength of this systematic review is that it provides a transparent synthesis of currently available RCTs with relevant treatment allocations, i.e., a PPI versus no PPI, and that the certainty of evidence was assessed according to GRADE. Significant efforts have been made to scrutinize study details as well as pharmacological characteristics of potential relevance for clinical practice, including the use of concurrent medication. Another strength is the importance of the subject as such. Concurrent use of clopidogrel and a PPI is fairly common in health care [9, 10], and the knowledge gained in this evidence compilation can be useful in the continuous updating of interaction alerts triggered by the studied drug pairs in the knowledge resources.
Limitations of this study include that although 14 studies were identified that met our PICO criteria, the number of patients and events was low. Further, only six RCTs were without a high risk of bias, limiting the certainty of the compiled evidence. A systematic directness issue, in relation to our prespecified PICO criteria, was that clopidogrel without concurrent aspirin was used with certainty in only one RCT [27]. Finally, because of a lack of data, the pre-planned subgroup analyses could not be performed.
5 Conclusion
This evidence compilation shows that concurrent use of a PPI probably does not largely affect the risk of CV events in patients on clopidogrel, but probably reduces the risk of overt GI bleeding. These results are mainly applicable to patients on both clopidogrel and aspirin, i.e., DAPT, rather than to patients on clopidogrel alone. For the C between omeprazole and pantoprazole, there was only very low certainty evidence.
Author Contributions
S.M.W. conceived the study, and all authors designed it. All authors contributed to the study selection process and performed the assessments. M.A.B.A. and S.M.W. extracted the data. S.M.W. performed the analyses. M.A.B.A. and S.M.W. drafted the manuscript. All authors revised the manuscript for intellectual content.
Acknowledgements
The authors are grateful to Ida Stadig, information specialist at the Medical Library at Sahlgrenska University Hospital, Gothenburg, Region Västra Götaland, Sweden, who performed the literature searches.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
E.W.J. writes texts and is involved in decisions on classifications in Janusmed interactions. The authors declare no other conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.




