Rutin-Associated Hepatoprotection: A Review of Mechanisms and Therapeutic Prospects
Yanting Feng and Lanchun Peng contributed equally to this work as first authors.
Funding: This work was supported by the National Natural Science Foundation of China, grant number 81973593; Hunan Provincial Natural Science Foundation, grant number 2024JJ8175; the Graduate Innovation Project of Hunan University of Chinese Medicine, grant number 2024CX173.
ABSTRACT
Background
Liver disorders pose a considerable global health challenge, accompanied by rising mortality rates. Current therapeutic strategies, though effective, often face limitations due to adverse effects and therapeutic resistance, prompting the exploration of alternative treatments, particularly safer natural compounds. Rutin, a widely available bioflavonoid, has emerged as a promising candidate owing to its varied pharmacological properties.
Methods
We conducted a comprehensive search on PubMed and Web of Science using the following keywords: ‘rutin’, ‘liver diseases’, ‘hepatoprotection’, ‘clinical observations’, ‘mechanisms, pharmacology’ and various combinations of these terms.
Results
This review systematically examines rutin's therapeutic potential in hepatic disorders, focusing on its molecular mechanisms, particularly its effects on inflammatory pathways, oxidative stress and hepatocellular protection.
Conclusion
We analyse existing evidence supporting rutin's hepatoprotective efficacy, identify its cellular and molecular targets and evaluate its potential applications in various liver diseases. Our systematic analysis provides theoretical support for developing rutin-based therapies in hepatic disease management and identifies future research directions and clinical applications.
Summary
- Rutin exerts its protective effects through the activation of the NF-κB signaling cascade and the Nrf2/HO-1 pathway.
- The discussion addresses the challenges associated with the bioavailability of rutin, exploring potential solutions to enhance its absorption and efficacy.
- A brief overview highlights the inadequacies present in clinical trials involving rutin, indicating the need for more rigorous and comprehensive studies.
1 Introduction
The liver represents the most pivotal metabolic organ within the human system, orchestrating an intricate network of metabolic processes. Nevertheless, contemporary lifestyle modifications and environmental perturbations have precipitated a substantial escalation in the prevalence of metabolic hepatic disorders [1], primarily nonalcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD), with global prevalence reaching 32% [2, 3]. Furthermore, mortality rate attributed to end-stage hepatic complications (encompassing hepatic insufficiency, cirrhosis and hepatocellular carcinoma) associated with hepatitis B/C viral infections demonstrate an upward trajectory [4]. Hepatocellular carcinoma demonstrates global incidence and mortality rates of 4.3% and 7.8%, respectively [5], with 5-year survival rates ranging from 5.0% to 30.0% [6]. Consequently, the proliferation of hepatic pathologies has emerged as a paramount contributor to global morbidity and mortality indices. Current therapeutic approaches show notable limitations. While nucleotide analogues, entecavir, effectively suppress viral replication, they may induce drug resistance [7]. Similarly, silymarin [8], a conventional hepatoprotective agent, can exacerbate hepatorenal dysfunction during prolonged use [7, 9]. However, botanical drugs have attracted the attention of researchers and scholars because of their pharmacological activity and few toxic side effects.
Rutin (3,3′,4′,5,7-pentahydroxy flavone-3-rutinoside), alternatively designated as rutinoside, quercetin-3-rutinoside or sophoroside, a flavonoid compound prevalent in various botanical sources, including Prunella vulgaris, Fagopyrum esculentum and Asparagus officinalis [10-12]. Its molecular architecture is characterized by a polyphenolic framework incorporating a C6-C3-C6 skeleton, distinguished by a glycosidic linkage between the 3-position hydroxyl moiety and a rutinose disaccharide [13]. This distinctive structural configuration, featuring multiple phenolic hydroxyl groups, confers significant biological activities [14]. Moreover, research has indicated that irreversible ultraviolet irradiation can induce the degradation of rutin in solution [15]. In contrast, employing a novel green extraction system significantly enhances the extraction efficiency of rutin. This system is primarily composed of natural deep eutectic solvents and propanol, demonstrating that rutin maintains a high level of stability within this formulation [16].
Contemporary investigations have illuminated rutin's diverse pharmacological properties, encompassing antioxidative capacity [17], anti-inflammatory potential [18], lipid metabolism modulation [19] and neuroprotective effects [20], establishing its therapeutic significance in hepatic disorders (Figure 1).
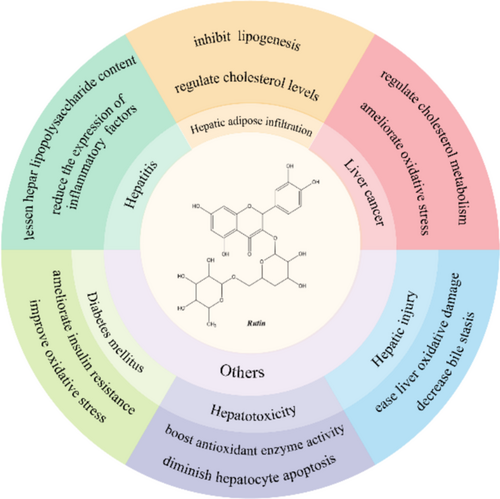
Given these compelling attributes, rutin has emerged as a focal point in pharmaceutical research. This review aims to examine the molecular mechanisms underlying rutin's hepatoprotective effects and explore its therapeutic potential in liver disease management.
2 Methods
We conducted a comprehensive search on PubMed and Web of Science using the following keywords: ‘rutin’, ‘liver diseases’, ‘hepatoprotection’, ‘clinical observations’, ‘mechanisms, pharmacology’ and various combinations of these terms. The search was not restricted by time. Initially, we screened the eligible literature by reading abstracts and organized the relevant studies for further examination. Throughout this process, we focused on those works that provided comprehensive insights into rutin and elucidated its mechanisms of action in liver diseases. Ultimately, the selected literature not only clarified the chemical properties and biological activities of rutin but also systematically analysed its protective effects on liver diseases at the cellular and animal level, along with the potential molecular mechanisms involved. This foundation provided significant information and contextual support for our subsequent paper writing.
3 Result
3.1 Anti-Inflammatory Mechanisms of Rutin in Hepatitis
Inflammatory severity exhibits detrimental effects on hepatic function [21]. When hepatocytes and adipocytes undergo damage or necrosis induced by deleterious lipid metabolites, particularly lipopolysaccharide (LPS), they trigger the activation of inflammatory signalling cascades. This activation precipitates the secretion of various proinflammatory mediators, including interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α), subsequently inducing hepatic inflammatory responses and exacerbating the progression of diverse hepatic pathologies [22, 23]. Thus, mitigation of hepatocellular inflammation represents a crucial therapeutic strategy in hepatoprotection.
Rutin has garnered considerable scientific interest due to its potent anti-inflammatory properties [24]. Castor leaf extract containing rutin demonstrates significant hepatoprotective effects against d-galactosamine(d-gal)-induced hepatic injury through reduction of serum hepatic enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and the reduction of malondialdehyde (MDA) levels [25] (Table 1). In murine models, rutin ameliorates Zearalenone-induced hepatic inflammation via enhancement of short-chain fatty acid production, intestinal microbiota modulation and reduction of hepatic lipopolysaccharide content [26]. In obesity-induced hepatic inflammation, rutin's therapeutic efficacy operates through nuclear factor-κB (NF-κB) pathway modulation, reducing inflammatory markers TNF-α and IL-6 [27]. The compound's anti-inflammatory mechanism encompasses suppression of endoplasmic reticulum stress, ROS inhibition and downregulation of proinflammatory cytokine expression, particularly monocyte chemoattractant protein-1 (Mcp1) and TNF-α [28], thereby attenuating inflammation-related pathologies (Figure 2).
| Experimental models | Dose of rutin | Signalling pathways | Pharmacologic effects | Ref. |
|---|---|---|---|---|
| d-galactosamine–induced male albino Wistar rats | 200 mg/kg for 14 days |
↑GPX ↑GSH ↓MDA |
↓Hepatitis | [25] |
| Zearalenone-induced Kunming female mice | 500 mg/kg for 28 days |
↓IL-1β ↓IL-6 ↓NF-κB |
↑SCFA ↑Intestinal function ↓Liver damage |
[26] |
| Male albino rats feed a HFD and streptozotocin | 80 mg/kg for 21 days |
↓TNF-α ↓IL-6 ↓NF-κB ↑SOD |
↓Hepatitis | [27] |
|
RAW 264.7 cell HFD-induced male C57BL/6 mice |
200 μM for 4 h 50 mg/kg for 56 days |
↓IL-1β ↓TNF-α ↓Mcp1 |
↓Fat mass ↓Insulin resistance ↓Inflammation |
[28] |
| CCL4-induced hepatotoxicity in male Wistar rats | 70 mg/kg for 28 days |
↓IL-6 ↓MEK5 ↓FADD ↓STAT3 ↓JAK |
↓Hepatotoxicity | [29] |
| Male Swiss mice feed paracetamol | 30/100/300 mg/kg for 21 days |
↓TNF-α ↓IL-1β ↓GSH |
↓Lethality ↓Hepatotoxicity |
[30] |
| Male Wistar albino rats feed high-cholesterol-diet | — |
↓IL-6 ↑IL-3 ↓TGF-β ↓Smad-2 |
↓TG ↓TC ↓Hepatotoxicity |
[31] |
| Male albino CD-1 mice infected S. mansoni cercariae | 20/30/40 mg/kg for 14 days |
↓TNF-α ↓IL-17 ↓IL-4 ↓Th17 ↑ROS |
↓Granulomas ↓Fibrosis ↑Immune |
[32] |
| Lead acetate–induced in male Wistar albino rats | 10 mg/kg for 30 days |
↓TNF-α ↓IL-1β ↑IL-10 ↓MDA ↓Caspase-3 |
↓Oxidative stress ↓Inflammation |
[33] |
| Female Sprague–Dawley rats by subcutaneous injection of 17α-ethinylestradiol | 100 mg/kg for 14 days |
↓NF-κB ↓TNF-α ↓HO-1 ↓MDA ↑SOD |
↑Hepatic regeneration ↑Antioxidant system ↓Inflammation |
[34] |
- Note: ↓ indicates inhibition; ↑ indicates promotion.
- Abbreviations: FADD: fas-associated death domain protein; GPX: glutathione peroxidase; GSH: glutathione; HO-1: heme oxygenase-1; IL-1β: interleukin 1β; IL-10: interleukin 10; IL-17: interleukin 17; IL-3: interleukin 3; IL-4: interleukin 4; IL-6: interleukin 6; JAK: Janus kinase; Mcp1: monocyte chemoattractant protein-1; MDA: malonaldehyde; MEK5: mitogen-activated protein kinase 5; NF-κB: nuclear factor-κB; ROS: reactive oxygen species; SCFA: short-chain fatty acid; Smad-2: recombinant mothers against decapentaplegic homologue 2; SOD: superoxide dismutase; STAT3: signal transducers and activators of transcription 3; TGF-β: transforming growth factor-β; Th17: T helper cell 17.
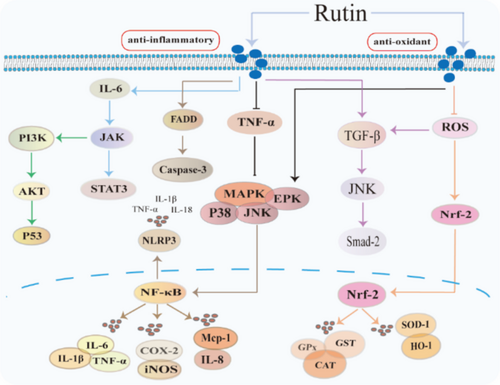
Drug-induced hepatotoxicity: Rutin demonstrates remarkable hepatoprotective features through the attenuation of inflammatory responses and oxidative stress. In tetrachloride (CCL4)–induced liver injury, rutin attenuates hepatotoxicity through downregulation of IL-6/signal transducer and activator of transcription 3 (STAT3) signalling cascade and associated molecular mediators, such as mitogen-activated protein kinase 5 (MEK5), epidermal cell growth factor (EGF) [29]. The compound demonstrates efficacy against paracetamol-induced hepatotoxicity by suppressing proinflammatory cytokines, such as IL-1β, TNF-α and interferon γ (IFN-γ), and reducing serum aminotransferase levels [30]. In hypercholesterolaemia models, rutin modulates transforming growth factor β (TGF-β)/recombinant mothers against decapentaplegic homolog 2 (Smad-2) signalling, affecting hepatocyte caspase-3 and tumour protein 53 (P53) expression while enhancing cell cycle protein–dependent kinase inhibitor and interleukin-3 (IL-3) levels [31]. The rutin's therapeutic potential extends to parasitic infections, where it reduces inflammatory markers, including TNF-α, interleukin-17 (IL-17), interleukin-4 (IL-4) and granuloma formation [32]. Furthermore, rutin ameliorates lead-induced hepatotoxicity through cytokine modulation and antioxidant enhancement, including serum interleukin-10 (IL-10), glutathione reductase (GSH) levels and enhancement of superoxide dismutase (SOD) and catalase (CAT) [33], while demonstrating efficacy in intrahepatic cholestasis via regulation of bile acid homeostasis and farnesol X receptor (FXR) agonism [34].
In conclusion, rutin demonstrates multifaceted hepatoprotective mechanisms through proinflammatory mediator suppression, inflammatory response attenuation, NF-κB pathway modulation and hepatic microenvironment optimization. While its therapeutic potential in hepatic disorders is evident, further research is warranted to fully elucidate the molecular mechanisms underlying its anti-inflammatory effects in hepatic tissue. The comprehensive pharmacological effects of rutin in hepatitis amelioration are summarized in Table 1.
3.2 Antioxidative Properties of Rutin in Hepatic Disorders
Perturbation of redox homeostasis precipitates oxidative stress [35], wherein the accumulation of reactive oxygen species (ROS) and associated free radicals in hepatic tissue catalyses the development of various liver diseases, including hepatic ischaemia–reperfusion injury, acute hepatic injury and nonalcoholic hepatitis [36-39]. In this background, rutin has occurred as a potent antioxidant with extensive applications in hepatic disorders.
Dietary rutin supplementation demonstrates significant antioxidant effects by normalizing the oxidized glutathione (GSSG)/GSH ratio and restoring SOD and CAT activities in hygromycin-induced hepatic oxidative stress [40] (Table 2). In T-butyl hydroperoxide–induced liver injury models, rutin enhances antioxidant enzyme activities, including SOD, glutathione peroxidase (GPX) and glutathione S-transferase (GST), while modulating nuclear factor erythroid 2–related factor-2 (Nrf-2) and inducible nitric oxide synthase (iNOS) pathways [41] (Figure 2).
| Experimental models | Dose of rutin | Signalling pathways | Pharmacologic effects | Ref. |
|---|---|---|---|---|
| Silver catfish feed oxytetracycline-diet | 1.5 g/kg for 14 days |
↑SOD ↓GSSG/GSH ↑LOOH ↓NO ↓HSP70 ↓Caspase-3 |
↓Lipid peroxidation ↓Apoptosis ↓Oxidative stress |
[40] |
|
Erythrocytes T-butyl hydroperoxide–induced in Swiss albino female mice |
16.3–1.63 uM for 1 h 10/50/100 mg/kg for 7 days |
↓MDA ↓CO ↑GPX ↑Nrf2 ↓iNOS |
↑Erythrocyte morphology ↓Oxidative stress |
[41] |
| H2O2-induced hepatocytes damage |
10/50/100/200 μg/mL for 24 h |
↓ROS ↑SOD-1 ↑HO-1 ↑CAT ↑GSH |
↓Lipid peroxidation ↓ROS generation ↑Cell viability |
[42] |
| Cadmium-induced Isa Brown chickens | 500 mg/kg for 60 days |
↓PIPK1 ↑Caspase-8 ↓JNK ↓NF-κB ↓EPK |
↓Cadmium accumulation ↓Hepatocellular necrosis ↓Oxidative stress |
[43] |
| Bile duct ligation–induced male Sprague–Dawley rats | 25 mg/kg for 28 days |
↓TGF-β1 ↓TNF-α ↑Nrf-2 ↑HO-1 |
↑Hepatoprotective ↓Inflammation ↓Oxidative stress |
[44] |
| Doxorubicin (DXR)-induced male Wistar rats | 50 mg/kg for 35 days |
↓AST ↓ALT ↑SOD ↑LPO ↑TNF-α |
↓Oxidative stress ↓Inflammation ↓Apoptosis |
[45] |
| BALB/c mice were intraperitoneally injected CCl4 | 16.4 mg/kg for 42 days |
↓TGF-β1 ↓TNF-α ↓MDA ↑SOD |
↓Collagen accumulation ↓Inflammatory |
[46] |
| Schistosoma mansoni in Balb/c mice | 100/200/400 mg/kg for 28 days |
↓MDA ↑SOD |
↑Anti-inflammatory ↑Antioxidant |
[47] |
| ↑GSH | ||||
| CCL4-induced male Kunming mice | 10/20/40 g/kg for 42 days |
↓AST ↓MDA ↑GSH-Px |
↑Antioxidant activity ↓Lipid peroxidation ↓Free radical |
[48] |
| CCL4-induced male BALB/CN mice | 10/50/150 mg/kg for 5 days |
↑Cu/Zn ↓NF-κB ↓iNOS ↓3-NT ↓TGF-β1 |
↓Free radicals ↓Inflammatory ↑Antioxidant ↑Antifibrotic |
[49] |
| Female Qingyuan partridge chickens | 0/200/400 mg/kg for 30 days |
↓MDA ↑CAT ↑Nrf-2 ↑HO-1 |
↓TG ↓TC ↑Antioxidant |
[50] |
|
Male C57BL/6 mice feed ethanol CCL-4–induced male Sprague–Dawley rats |
11.5 mg/kg for 8 weeks |
↓AST ↓MDA ↓TNF-α ↑GPX ↑SOD |
↓Hepatic injury ↓Antioxidant ↑Antioxidant |
[51] |
| CCL4-induced male Wistar rat | 200/400 mg/kg for 14 days |
↓AST ↓LDH ↓TNF-α ↓TBARS |
↑Antioxidant ↑Hepatoprotective ↓Hepatotoxicity |
[52] |
| CCL4-induced male Sprague Dawley rats | 200/400 mg/kg for 28 days |
↓LDL ↑SOD ↑POD |
↓Oxidative stress ↓DNA damage ↓Anti-inflammatory |
[53] |
| Carbofuran-induced in Swiss albino rats | 250/500/1000 mg/kg for 28 days |
↓MDA ↑SOD ↑GPX ↑CAT |
↓TG ↓TC ↓Oxidative stress |
[54] |
| Alloxan-induced female Swiss albino mice | 250 mg/kg for 12 days |
↑GPX ↑SOD ↓TNF-α |
↑Antioxidative ↑Antidiabetic |
[55] |
| Male C57BL/6J mice feed HFD diet | 50 mg/kg for 16 weeks |
↑GPX ↑SOD ↑Glut4 |
↑Antioxidant ↓Inflammation |
[56] |
|
Red blood cell Male Wistar rats feed a HFD/cholesterol |
100 mg/mL 400/800 mg/kg for 28 days |
↓MDA ↑GSH |
↓TG ↓TC ↑Antioxidant |
[57] |
| Streptozotocin-induced male albino Wistar rats | 100 mg/kg for 45 days |
↑SOD ↑GPX ↓TBARS |
↓Tissues damage ↑Antioxidant |
[58] |
| d-galactose-induced male Institute of Cancer Research mice | 3/10/30 mg/kg for 35 days |
↑T-AOC ↑T-SOD ↑CAT ↓MDA |
↓Senescence ↓Oxidative stress ↑Immune |
[59] |
| d-galactose-induced male C57BL/6J mice | 100/200/400 mg/kg for 30 days |
↓MDA ↑SOD ↑GSH |
↑Antioxidant | [60] |
| Old rats | 25/50 mg/kg for 42 days |
↑p-AKT ↑SOD ↓IL-1β ↓ATF3 |
↓Lipid accumulation ↓Inflammation ↓Oxidative stress |
[61] |
| d-galactose–induced Kunming mice | 0.3 mmol/kg for 8 weeks |
↓MDA ↑SOD ↓IL-6 |
↑Antioxidant ↑Anti-inflammatory |
[62] |
| Male Wistar rats feed corn starch-rich diet | 1.6 g/kg for 8 weeks |
↓MDA ↑CAT ↓AST ↓ALT |
↓Steatosis ↓Blood glucose ↓Oxidative stress ↓Inflammation |
[63] |
| Wistar rat intraperitoneal injection poloxamer | 50 mg/kg for 30 days |
↑CAT ↑GST ↑SOD |
↑Immune ↓Oxidative damage |
[64] |
| Mercury chloride (HgCl2)-induced male Sprague–Dawley rats | 100 mg/kg for 7 days |
↑SOD ↓MDA ↓NF-κB ↓IL-1β ↓caspase-3 |
↓Oxidative damage ↓Apoptosis ↓Inflammation |
[65] |
| Triptolide-induced male Kunming mice | 12 mg/kg for 14 days |
↑SOD ↑Nrf-2 ↓MDA ↓NLRP3 |
↓Organ injury ↓Ferroptosis |
[66] |
| Male Wistar rats feed d-galactosamine and lipopolysaccharide | 5/10/20 mg/kg for 6 days |
↑SOD ↑GSH ↑CAT |
↓Hepatic glycogen ↓Oxidative stress |
[67] |
|
Malathion-induced Male Sprague Dawley rats |
50/100 mg/kg for 28 days |
↑SOD ↑GPX ↓MDA ↓PERK ↓Caspase-3 |
↓Oxidative stress ↓Autophagy ↓Apoptotic |
[68] |
- Note: ↓ indicates inhibition ↑ indicates promotion.
- Abbreviations: 3-NT: 3-nitrotyrosine; Akt: protein kinase B; ALT: alanine aminotransferase; AST: aspartate transaminase; ATF3: activating transcription factors 3; CAT: catalase; CO: carbon monoxide; EPK: extracellular signal regulated kinases; GPX: glutathione peroxidase; GSH: glutathione; GSH-Px: glutathione peroxidase; GSSG: oxidized glutathione; GST: glutathione S-transferase; HO-1: heme oxygenase-1; HSP70: heat shock protein70; IL-1β: interleukin 1β; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase; JNK: c-Jun N-terminal kinase; LDH: lactate dehydrogenase; LDL: low-density lipoprotein; LOOH: lipid hydroperoxides; LPO: lactoperoxidase; MDA: malonaldehyde; NF-κB: nuclear factor-κB; NLRP3: NACHT, LRR and PYD structural domain protein 3; NO: nitric oxide; Nrf2: nuclear factor erythroid-2–related factor 2; PERK: protein kinase RNA-like endoplasmic reticulum kinase; PIPK1: phosphatidylinositol phosphate kinase 1; POD: peroxidase; ROS: reactive oxygen species; SOD: superoxide dismutase; T-AOC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances; TC: total cholesterol; TGF-β1: transforming growth factor-β1; TG: triglycerides; TNF-α: tumour necrosis factor α.
Rutin displays hepatoprotective effects through multiple molecular mechanisms. It enhances Nrf-2-mediated antioxidant protein expression, including SOD-1, CAT, heme oxygenase (HO-1), while suppressing ROS generation [42]. In cadmium-induced hepatic necrosis, rutin functions via antioxidant enhancement, suppression of stress-activated protein kinase (JNK), tumour protein 38 (P38) expression and the mitogen-activated protein kinase (MAPK)/NF-κB pathway modulation [43]. The compound demonstrates efficacy in hepatic ischaemia–reperfusion injury through HO-1 upregulation and lipid hydroperoxide (LOOH) reduction [69], and attenuation of dimethylamine hydrolase 1 (DDAH1) protein expression [70], while protecting against cholestatic liver injury via extracellular signal-regulated kinases (ERK) inhibition and Nrf2/HO-1/MAPK activation [44]. In azithromycin-induced hepatotoxicity, rutin reduces hepatic enzyme markers (alpha-fetoprotein [AFP]) while preserving antioxidant enzyme activities [45]. Furthermore, it shows prophylactic potential against CCL4-induced fibrosis and Schistosoma mansoni infection through antioxidant system activation and TGF-β1 pathway inhibition [46, 47].
Rutin exhibits powerful antioxidant activity through dual mechanisms: reducing MDA content and enhancing antioxidant enzyme activities, including SOD, GPX and cyclooxy-genase-2 (COX-2), thereby protecting against CCL4-induced hepatotoxicity [48, 49]. Mechanistically, rutin modulates redox status via Nrf2 and CAT upregulation while downregulating AMPKα to attenuate lipid accumulation [50]. Its antioxidant effects involves all kinds of pathways, counting catalase enhancement [51], peroxidase (POD) and GSH normalization [52] and suppression of oxidative stress, conferring protection against various hepatotoxic agents, including CCL4 [53] and furan [54]. In metabolic disorders, rutin shows therapeutic potential in diabetic models [55], by restoring hepatic SOD activity and protein thiols (PT) levels [71]. It ameliorates HFD-induced insulin resistance through enhanced antioxidant systems [56]. Notably, Prunus spinosa L. extract, enriched with rutin, exhibits dose-dependent antioxidant properties against HFD and streptozotocin-induced oxidative stress [57]. In streptozotocin-induced diabetes, rutin elevates antioxidant enzymes, reduces lipid peroxidation and protects multiple organs, improving systemic antioxidant status [58].
Recent investigations have established rutin's efficacy in attenuating d-gal–induced oxidative stress and aging-associated manifestations in murine models [59], through multiple mechanisms: attenuation of antioxidant enzyme suppression [60], reduction of MDA and nitric oxide (NO) concentrations, enhancement of total antioxidant capacity (T-AOC) [61] and modulation of inflammatory at mediators (downregulating IL-1β, IL-6 and TNF-α while upregulating IL-10) [62]. In metabolic disorders, rutin normalizes hepatic oxidative stress markers [63] and alleviates hyperlipidaemia-induced lipotoxicity through restoration of redox homeostasis [64]. Against environmental toxicants, rutin exhibits hepatoprotective properties by attenuating mercuric chloride and malathion-induced hepatotoxicity through enhancement of antioxidant systems [65]. Notably, rutin's synergistic administration with quercetin activates the protein kinase B/mammalian target of rapamycin (AKT/mTOR) signalling pathway, augments CAT and GSH activities [66] and provides protection against triptolide-induced multiorgan injury and d-gal–induced acute organ dysfunction [67]. Furthermore, rutin demonstrates anti-neoplastic efficacy through inhibition of HepG2 cell invasion and upregulation of detoxification enzymes, effectively suppressing hepatocellular carcinoma [72].
In conclusion, rutin reveals in many ways therapeutic effects through enhancement of antioxidant enzyme systems, attenuation of oxidative stress and protection against drug-induced hepatotoxicity. However, careful consideration of dosage is paramount, as high-dose rutin administration may precipitate trace mineral deficiencies (including iron, zinc and copper) and diminish metalloenzyme activities, despite enhancing antioxidant status [73, 74]. Furthermore, excessive rutin supplementation potentially exacerbates hepatic injury, necessitating the establishment of optimal therapeutic dosing regimens [75]. The antioxidant mechanisms of rutin in liver disorders are summarized in Table 2.
3.3 Rutin-Mediated Lipid Metabolism in Liver Disease
Diverse lipid manifestations, which consist of intracellular lipid droplets and triglycerides, are ubiquitous in biological systems, and aberrant adipose tissue accumulation demonstrates strong associations with various chronic pathologies [76], encompassing hepatic disorders [77], diabetes mellitus [78] and cardiovascular diseases [79].
Significantly, rutin exhibits therapeutic potential in ameliorating these chronic conditions through dual mechanisms: suppression of lipogenesis and lipid accumulation, while simultaneously promoting homeostatic lipid metabolism.
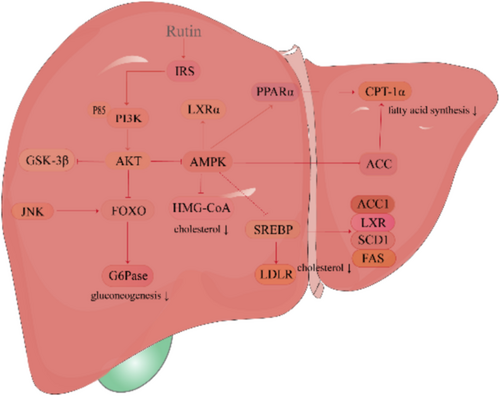
Rutin regulates lipid homeostasis through downregulation of lipogenic genes (sterol regulatory element-binding protein-1C [SREBP-1C], acetyl-CoA carboxylase α [ACACα] and stearoyl-CoA desaturase 1 [SCD1]) and upregulation of fatty acid oxidation genes, such as carnitine palmitoyl transferase 1 (CPT1), peroxisome proliferator–activated receptor α (PPARα) and FXR, resulting in reduced hepatic and visceral triglyceride (TG) and total cholesterol (TC) concentrations in avian models [80] (Table 3). In NAFLD models, rutin exhibits therapeutic efficacy by attenuating triglyceride accumulation, enhancing fatty acid catabolism and inhibiting de novo lipogenesis [81]. Additionally, rutin reduces circulating lipid profiles while downregulating key molecular mediators—monooxygenase cytochrome P450-2E1, c-Jun N-terminal protein kinase 1 (JNK1) and inducible nitric oxide synthase (i-NOS)—demonstrating comprehensive regulation of lipid metabolism and hepatoprotective effects [82] (Figure 3).
| Experimental models | Dose of rutin | Signalling pathways | Pharmacologic effects | Ref. |
|---|---|---|---|---|
| Taihang chicken | 0.3/0.6/09/1.2 g/kg for 8 weeks |
↓MDA ↓ACC ↑T-AOC |
↓TG ↓TC ↓Serum lipid |
[80] |
|
Male C57BL/6 mice feed HFD OA-induced HepG2 and RAW 246.7 |
200 mg/kg for 28 days 10–40 μM for 2 h |
↓MDA ↓SREBP-1c ↓TNF-α ↑SOD |
↓TG ↓Lipid accumulation ↓Oxidative injuries |
[81] |
| Male Albino mice feed diet and fructose | 50/75/100 mg/kg for 14 days |
↓LDL-C ↓CYP2E1 ↓JNK1 |
↓Oxidative stress ↓Inflammatory steatosis |
[82] |
| 3T3-L1 cell | 5/10 μM for 48 h |
↑p-AMPK ↑p-Akt |
↓Lipid accumulation ↓Weight |
[83] |
| Male mice C57BL/6J feed HFD-diet | 100 mg/kg for 7 weeks |
↓AST ↓ALT ↓LDL-C |
↓TG ↑Antiobesity ↓Hypolipidaemic |
[84] |
| Male Wistar Kyoto rats feed HFD diet | 250/500 mg/kg for 28 days |
↓MDA ↑SOD ↑GSH |
↓TG ↓Oxidative stress ↑Antiobesity |
[85] |
| HFD and STZ-induced male C57BL/6 mice | 200 mg/kg for 35 days |
↓LDL-c ↓TG ↓MDA |
↓Lipid accumulation ↓Oxidative injury ↓Inflammation |
[86] |
|
Male db/db mice feed 60Co radiation mice granule feedstuff High glucose stimulating |
120/60 mg/kg for 8 weeks 8/16/32/64 μg/mL |
↑IRS-2 ↑p-PI3K ↑p-GSK-3β |
↑Hepatocyte proliferation ↓Blood glucose |
[87] |
| STZ-induced male Sprague–Dawley rats | 100 mg/kg for 28 days |
↓TG ↓TC |
↓Blood glucose ↓Blood lipid |
[88] |
| Male Wistar rats feed oil orally and cocktail | 10/100 mg/kg for 28 days |
↓LDL-C ↓AST ↓ALT |
↓Plasma lipid ↓Cholesterol |
[89] |
| Sprague–Dawley male rats feed 10 g/kg of 5% dextrose | 200/400 mg/kg for 21 days |
↓LDL ↑SOD ↑GPX |
↓Oxidative stress ↓Blood glucose |
[90] |
| STZ-induced Wistar albino rats | 10/50/200 mg/kg for 45 days |
↓MDA ↓AST |
↓TG ↓Cholesterol |
[91] |
|
T2DM db/db mice HepG2 cells |
200 mg/kg for 12 weeks 100/200/300 mg/mL for 24 h |
↓LDL-C ↓G6Pase ↓PCK1 ↑p-Akt ↑p-FOXO1 |
↓Gluconeogenesis ↓Hypoglycaemic |
[92] |
| Male Syrian golden hamsters feed HFD diet | 0.5/1/2 g/kg for 12 weeks |
↑LDL/HDL ↑CPT-1 ↓FFA ↓HMG-CoA |
↓TG ↓Liver lipids ↓Body weight |
[93] |
| HFD-induced male C57BL/6J mice | 500 mg/kg for 10 weeks |
↓FAS ↓ABCA1 ↓MicroRNA-33 ↓MicroRNA −122 |
↓Fatty acid ↑Hypolipidaemic activity |
[94] |
|
HepG2 cells Male golden Syrian hamsters feed HFD-diet |
3–200 μM for 24 h 2.5 g/kg for 8 weeks |
↑SREBP2 ↑LDLR ↑LXRα |
↓Cholesterol | [95] |
| OA-induced HepG2 cells | 100/150/200 μM for 24 h |
↑SOD ↑GPX ↓FAS |
↓Lipid accumulation ↓Lipogenesis ↑Antioxidant |
[96] |
|
Male C57BLKs/J-db/db OA-induced Hela cell PA-induced HepG2 cell |
100/200 mg/kg for 8 weeks 150 μM for 24 h 20 μM for 24 h |
↓MDA ↑SOD ↓TNF-α ↑p-ACC |
↓Oxidative injuries ↓Inflammation ↓Fat synthesis |
[97] |
|
3T3-L1 cells Male imprinting control region mice feed HFD diet |
100 μg/mL for 24 h 500/1000 mg/kg for 50 days |
↓LDL ↓TC ↓TG |
↓Fat accumulation ↓Blood glucose ↑Adiponectin |
[98] |
| Tetracyclines-induced male Wistar Albino rats | 100 mg/kg for 28 days |
↑HDL-C ↓VLDL-C ↓TG |
↑Histoarchitecture ↓Lipid profile |
[99] |
| HFD-induced male Sprague–Dawley rats | 0.2% for 12 weeks |
↓TNF-α ↓LDL-C ↓insulin |
↓Fat accumulation ↓Inflammation |
[100] |
| Ethanol-induced HepG2 cells and zebrafish | 0.01/0.1/1 μM for 24 h |
↓ATP ↓DRP1 ↓ACC |
↓Hepatic steatosis ↑Cell viability |
[101] |
- Note: ↓ indicates inhibition ↑ indicates promotion.
- Abbreviations: ABCA1: ATP-binding cassette transporter A1; ACC: acetyl-CoA carboxylase; Akt: protein kinase B; ALT: alanine aminotransferase; AMPK: adenosine 5′-monophosphate (AMP)-activated protein kinase; AST: aspartate transaminase; ATP: adenosine triphosphate; CPT-1: carnitine palmitoyltransferase1; DRP1: dynamin-related protein 1; FAS: fatty acid synthase; FFA: free fatty acid; FOXO1: forkhead box protein O1; FXR: farnesoid X receptor; G6Pase: glucose-6-phosphatase; GPX: glutathione peroxidase; GSH: glutathione; GSK-3β: glycogen synthase kinase-3β; HDL-c: high-density lipoprotein-cholesterol; HMG-CoA: hydroxy methylglutaryl coenzyme A reductase inhibitor; IRS-2: human insulin receptor substrate 2; JNK1: c-Jun N-terminal kinase 1; LDL: low-density lipoprotein; LDL-c: low-density lipoprotein-cholesterol; MDA: malonaldehyde; PI3K: phosphatidylinositol-3 kinase; PKC1: phosphoenolpyruvate carboxy kinase1; SOD: superoxide dismutase; SREBP-1c: sterol regulatory element-binding protein 1c; T-AOC: total antioxidant capacity; TC: total cholesterol; TG: triglycerides; TNF-α: tumour necrosis factor α; VLDL: very-low-density lipoprotein.
Experimental evidence demonstrates that rutin enhances glucose uptake in 3T3-L1 cells through concurrent activation of Akt and AMP-activated protein kinase signalling pathways [83], while improving atherogenic indices by reducing low-density lipoprotein cholesterol (LDL-C)/high-density lipoprotein cholesterol (HDL-C) and TC/HDL-C ratios [102], in HFD-induced diabetic obesity models [84]. These findings position rutin as a promising therapeutic candidate for type 2 diabetes mellitus (T2DM) management, particularly in attenuating the progression of diabetes-associated hepatic complications [103]. In diet-induced obesity, rutin exhibits protective effects through enhanced antioxidant capacity (increased SOD and GSH levels) and hypolipidaemic actions (reduced serum TG, TC and LDL levels) [85]. Notably, in diabetes-induced hepatic dysfunction, rutin attenuates oxidative stress markers (plasma MDA and NO) [86], modulates short-chain fatty acid profiles and ameliorates hepatic steatosis, thereby optimizing glucose-lipid metabolic homeostasis [104].
Rutin exerts hepatoprotective effects through activation of the insulin receptor substrate-2 (IRS-2)/phosphatidylinositol 3-kinase (PI3K)/AKT/glycogen synthase kinase-3β (GSK-3β) signalling cascade, upregulating IRS-2 and GSK-3β protein expression while attenuating advanced glycation end products (AGEs) formation [87]. In combination therapy studies [88], rutin demonstrates synergistic effects with metformin in STZ-induced diabetic models and enhanced anti-hypercholesterolaemic properties when co-administered with lovastatin [89]. In STZ-induced diabetic rats, rutin significantly improves glycaemic control, optimizes lipid profiles and attenuates hepatic enzyme activities, including AST, ALT and lactate dehydrogenase (LDH), while providing protection against STZ-induced hepatotoxicity and cardiotoxicity [90, 91, 105]. These comprehensive therapeutic effects establish rutin as a promising agent for diabetes management and its associated complications.
Mechanistic investigations have elucidated that rutin modulates glucose metabolism through activation of the AKT signalling pathway by upregulating phosphorylated AKT and Forkhead box protein O1 (FOXO1) while downregulating glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxylase 1 (PCK1) expression, demonstrating therapeutic potential in T2DM management [92]. Its lipid-regulating effects manifest through multiple mechanisms: enhancement of adenosine triphosphate-binding cassette transporter A1 (ABCA1) and carnitine palmitoyl transferase 1 alpha (CPT1 alpha) expression [93], downregulation of fatty acid synthase (FAS) networks [94] and modulation of key cholesterol regulators, for example, sterol regulatory element-binding protein 2 (SREBP2), low-density lipoprotein receptor (LDLR), liver X receptor α (LXRα). In HFD-induced models, rutin attenuates hepatic lipid accumulation while modulating intestinal microbiota [95]. Additionally, rutin augments lecithin–cholesterol acyltransferase expression and suppresses farnesyl diphosphate farnesyltransferase 1, effectively reducing cellular cholesterol content in HepG2 cells [106].
In oleic acid–induced hepatocellular models, rutin attenuates lipogenesis through suppression of SREBP-1 expression, enhancement of AMPK activity and inhibition of key lipogenic enzymes, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA), FAS, 1-aminocyclopropane-carboxylic acid (ACC) and glycerol-3-phosphate acyltransferase (GPAT) [96]. In glucose and palmitic acid-induced diabetic NAFLD models, rutin ameliorates lipid accumulation via AMPK/SREBP1 signalling modulation and enhancement of antioxidant enzyme activities [97]. Rutin demonstrates comprehensive metabolic effects, including optimization of LDL/HDL ratios [98], attenuation of hepatic steatosis and regulation of cholesterol homeostasis [93]. Additionally, rutin modulates serum lipid profiles by reducing very low-density lipoprotein cholesterol (VLDL-C) and elevating HDL-C levels, effectively ameliorating tetracycline-induced hepatorenal toxicity [99].
In conclusion, rutin exhibits pleiotropic effects on hepatic lipid metabolism: attenuation of hepatic triglyceride and total cholesterol levels, activation of AMPK signalling, suppression of hepatic lipogenesis and lipid accumulation, modulation of hepatic cholesterol metabolism and regulation of lipid metabolism-associated gene expression, collectively promoting metabolic homeostasis. These comprehensive hepatoprotective effects, particularly in ameliorating hepatic lipid metabolic perturbations, position rutin as a promising therapeutic agent for hepatic metabolic disorders. Therapeutic effects of rutin on hepatic lipid metabolism are comprehensively presented in Table 3.
3.4 Pharmacokinetics and Clinical Applications of Rutin
The bioavailability of rutin is predominantly dependent on hydrolytic metabolism by cecal microflora. Following oral administration, rutin undergoes biotransformation into various metabolites, notably 3-hydroxyphenylacetic acid, which has been identified in human urinary excretion [107].
Pharmacokinetic investigations of rutin remain relatively limited, with the majority of studies conducted in preclinical models. Pharmacokinetic studies of rutin reveal significant lymphatic absorption, with lymphatic fluid peak plasma concentration (Cmax) and area under the curve (AUC) exceeding plasma levels two-fold following intraduodenal administration (300 mg/kg) in rodent models [108]. Subsequent investigations by Maciej et al. [109] and Gohlke et al. [110] elucidated the bioavailability profile in bovine models, demonstrating dose-dependent increases in Cmax after 2 h of administration, with substantial interindividual variability ranging from 368.8 to 983.9 nmol/L. Clinical pharmacokinetic studies, including a double-blind, two-period crossover investigation in healthy volunteers (n = 16), demonstrated dose-proportional increases in plasma concentrations [111]. Clinical studies in healthy volunteers demonstrate dose-proportional plasma concentration increases, with peak levels at 6–7 h post-administration (500-mg dose) and complete clearance within 24 h [112]. Novel electrochemical analysis using graphene oxide-modified sensors yields comparable pharmacokinetic parameters (T1/2: 3.345 ± 0.647 min; AUC: 5750 ± 656.0 min μg/mL) to conventional HPLC methods, establishing an efficient analytical approach for rutin pharmacokinetic evaluation [113].
Rutin's therapeutic application is limited by poor aqueous solubility and suboptimal absorption kinetics.
Advanced nanomaterial platforms, including cyclodextrin-MOF complexes [114], ultra-small manganese oxide and metal-magnetic graphene composites [115, 116] and corn starch–derived nanoparticles [117], demonstrate significant enhancement in rutin's bioavailability (4.24-fold increase) [118]. These pharmaceutical modifications optimize rutin's physicochemical properties, including solubility, stability and biocompatibility, substantially expanding its therapeutic potential. Extensive investigations have focused on rutin-nanomaterial co-delivery systems and their pharmacological implications. Novel delivery systems, such as rutin–zinc complexes [119], rutin-loaded lipid nanoparticles [120] and GPIIb/IIIa receptor-targeted rutin-loaded liposomes [121], demonstrate enhanced pharmacokinetic profiles with enhanced Cmax and accelerated time to peak concentration (Tmax). Furthermore, innovative platforms incorporating DNA nanoflower–modified MicroRNA-124 and selenized rutin derivatives show promising therapeutic potential, particularly in neurodegenerative disorders, expanding rutin's clinical applications [122-124].
Rutin undergoes absorption through glycosidic ligand binding followed by conjugation, exhibiting a T1/2 of 12-19 hours in humans [125]. Clinical evidence demonstrates rutin's diverse therapeutic applications: enhanced wound healing in perineal sutures (16.3 mg/g topical application) [126], amelioration of oxidative stress in haemodialysis patients (16-week administration) [127] and significant reduction in intraocular pressure in glaucoma patients [128]. In type 2 diabetes mellitus patients, 3-month rutin supplementation (500 mg daily) significantly improved metabolic parameters [129], including reduced LDL-C, TG, IL-6 and MDA levels, with concurrent elevation of HDL-C [130]. Additionally, clinical trials demonstrate rutin's efficacy in haemorrhoidal disorders [131], with significant improvement in anorectal symptomatology following 130 mg weekly administration, establishing the safety and efficacy of rutin interventions in haemorrhoidal disease management [132, 133]. However, a study in athletes showed no significant effect on post-marathon inflammatory responses, highlighting the context-dependent nature of rutin's therapeutic effects [134].
3.5 Safety of Rutin
Safety assessment is essential for the clinical use of natural compounds like rutin. Experimental studies involving rutin extracts administered at a single dose of 2000 mg/kg during acute oral toxicity tests in animals revealed no observed side effects [135]. Research conducted on ACI rats examined the carcinogenic potential of rutin, finding that even at high concentrations and with prolonged administration [136], rutin exhibited no evidence of carcinogenicity. Furthermore, rutin treatment did not cause DNA damage in mouse bone marrow cells, nor was any mutagenic activity detected [137]. Clinical trials indicate that the safe dosage of rutin for humans is 500 mg per day [13]. However, treatment with high concentrations of rutin in vitro on normal human lung fibroblasts and human umbilical vein endothelial cells has been shown to increase intracellular levels of ROS, potentially leading to cytotoxic effects [138]. In conclusion, rutin has the potential to be developed as an effective candidate drug for the treatment of liver diseases.
4 Summary and Discussion
Hepatic disorders represent a substantial global public health burden, with conventional therapeutic approaches predominantly encompassing pharmacological interventions and surgical protocols. However, these traditional methodologies frequently entail significant adverse effects and elevated therapeutic risks. In this context, rutin, a naturally occurring flavonoid compound characterized by multi-targeted efficacy and minimal toxicity, has emerged as a promising therapeutic candidate in hepatological research.
The hepatoprotective mechanisms of rutin demonstrate remarkable mechanistic complexity and pathway diversity. Primarily, it attenuates hepatic inflammatory responses through modulation of the NF-κB signalling cascade, resulting in diminished expression of proinflammatory mediators (IL-1β, IL-6, TNF-α). Additionally, rutin augments antioxidant enzyme activity, including SOD, CAT and GSH-Px, via activation of the Nrf2/HO-1 signalling axis, thereby ameliorating hepatic oxidative stress. Notably, rutin exhibits regulatory effects on the AMPK/SREBP-1C pathway, inhibiting hepatic lipogenesis and accumulation while ameliorating hepatic lipid metabolic dysregulation, thus mitigating associated metabolic complications. Nevertheless, the clinical implementation of rutin faces substantial challenges, primarily attributed to its high molecular mass and limited aqueous solubility, resulting in suboptimal bioavailability.
To address these limitations, innovative delivery systems incorporating advanced nanomaterials and liposomal formulations have been developed, demonstrating significant enhancement in bioavailability (fourfold to fivefold increase) and hepatic targeting efficiency.
Current research limitations encompass several critical aspects. First, the human pharmacokinetic profile of rutin remains incompletely elucidated, particularly regarding the mechanistic actions of its primary bioactive metabolites. Second, existing evidence predominantly derives from preclinical models and in vitro investigations; clinical trials are characterized by limited sample sizes, insufficient special population data and relatively brief intervention periods, potentially undermining comprehensive safety and efficacy assessments. Furthermore, crucial parameters including optimal formulation selection, dosing regimen optimization and potential drug combination strategies require thorough investigation. Future research directions may benefit from emerging technologies, including multiomics approaches and artificial intelligence, facilitating deeper mechanistic insights into rutin's molecular actions. Moreover, the evolution of precision medicine paradigms and individualized therapeutic strategies may enhance the standardization and precision of rutin's clinical applications.
In conclusion, while rutin demonstrates considerable therapeutic potential in hepatic disorders, its clinical translation necessitates substantial support from comprehensive basic research and robust clinical data. The continued advancement of scientific understanding and technological capabilities presents opportunities for realizing rutin's therapeutic potential in hepatological applications.
Author Contributions
Yanting Feng and Lanchun Peng performed the literature search and wrote the manuscript. Xiaohui Liu, Qingzhu Zheng, Min Qian and Meiling Deng provided writing methods and data supervision. Jiangli Peng, Yamei Li, Limei Lin and Qiuxain Peng reviewed and edited the manuscript before submission. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.