Transfer of cetirizine/levocetirizine into human breast milk and estimation of drug exposure to infants through breastfeeding: A human lactation study from the ConcePTION project
Abstract
Data on drug transfer into human breast milk are sparse. This study aimed to quantify concentrations of cetirizine and levocetirizine in breast milk and to estimate drug exposure to infants. Breastfeeding women at least 8 weeks postpartum and using cetirizine or its pure (R)-enantiomer levocetirizine were eligible to participate. Breast milk samples were collected at six predefined times during a dose interval (0, 2, 4, 8, 12 and 24 h after drug intake) at steady state. Infant drug exposure was estimated by calculating the absolute infant dose (AID) and the weight-adjusted relative infant dose (RID). In total, 32 women were eligible for final inclusion, 31 women using cetirizine and one woman using levocetirizine. Means of the individual maximum and average cetirizine milk concentrations were 41.0 and 16.8 μg/L, respectively. Maximum concentrations occurred on average 2.4 h after intake, and the mean half-life in milk was 7.0 h. Estimated AID and RID for cetirizine in a day were 2.5 μg/kg and 1.9%, respectively. The corresponding values for levocetirizine were 1.1 μg/kg and 1.9%. No severe adverse events were reported. Our findings demonstrate that the transfer of cetirizine and levocetirizine into breast milk is low and compatible with breastfeeding.
1 BACKGROUND
Allergic disorders including allergic rhinitis and urticaria affect up to 20%–30% of women of reproductive age1 and may require pharmacological treatment. Antihistamines are one of the most frequently used drugs for these conditions, including during pregnancy and lactation,2 and approximately 2%–3% of women are prescribed antihistamines during the first 3 months after delivery.3, 4 Second-generation antihistamines are first-line therapy as they are less sedative than the first-generation antihistamines. Despite their frequent use among breastfeeding women, data about the transfer of second-generation antihistamines into human milk are extremely sparse. A systematic literature review from 2022 identified only seven human lactation studies, including in total 25 mother–infant pairs, using cetirizine (three women), clemastine (one woman), ebastine (one woman), epinastine (seven women), loratadine (six women), terfenadine (four women) and triprolidine (three women).5 The calculated relative infant dose (RID) was below 5% for all these drugs, ranging from 0.3% for terfenadine to 4.5% for clemastine. For cetirizine, the mean and maximum concentration in breast milk in these women were 21 and 49 μg/L, respectively.6 The RID of cetirizine was 1.8%, and the absolute infant dose via breast milk was 3.1 μg/kg/day. The samples, however, were obtained after a single dose of 10 mg cetirizine and were thus not taken at steady-state conditions.6
Cetirizine is a racemic mixture of the pharmacologically active (R)-enantiomer and the inactive (S)-enantiomer, whereas levocetirizine consists of the pure (R)-enantiomer. Therefore, levocetirizine is given in half the dose compared to racemic cetirizine; the recommended doses are 5 and 10 mg/day, respectively.7, 8 No published data on excretion in breast milk are available for levocetirizine. Currently, most market authorization holders of these medications do not recommend use of cetirizine during breastfeeding due to the lack of data on breast milk transfer and safety during breastfeeding.5, 7, 8 Reference textbooks and databases,9, 10 however, state that use of cetirizine is compatible with breastfeeding, considering that the benefits of breastfeeding to the mother and child outweigh the potential risk of medication exposure to the breastfed child. The discrepancies between the manufacturers' product information (see drug label texts in Table S1) and clinical guidelines are challenging for patients and prescribers and may prevent optimal treatment of breastfeeding women with allergic conditions. Unfounded concerns about the risks to breastfed infants are unfortunately one of the common reasons for unnecessary cessation of breastfeeding.11 Human lactation studies, updated information and tailored evidence-based advice are imperative to counteract this.
This study is part of the Innovative Medicines Initiative (IMI)-funded ConcePTION project aiming to improve the knowledge about medication use and safety among pregnant and breastfeeding women (www.imi-conception.eu, 2019–2024). This includes establishing a European breast milk collection, demonstrating how human lactation studies can be conducted in Europe and developing the standards to do so. More information about this initiative is given in Data S1. The initiative is in line with guidelines from the European Medicines Agency (EMA)12 and US Food and Drug Administration (FDA).13
2 OBJECTIVE
The primary aim of this observational human lactation study was to quantify excretion of cetirizine and levocetirizine into human milk to determine the relative and absolute exposure to breastfed infants. An additional aim was to assess possible infant adverse drug reactions.
3 MATERIAL AND METHODS
3.1 Study design and recruitment
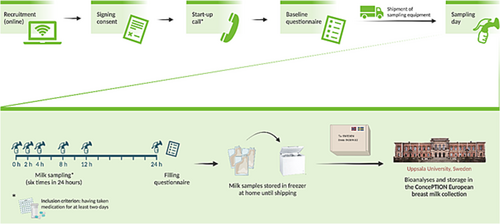
The study was designed as an observational human lactation study in line with European and US regulatory guidelines.12, 13 Individuals using either cetirizine or levocetirizine were eligible for inclusion. Further in this article, we use the term ‘(levo)cetirizine’ to describe cetirizine and levocetirizine together. Participants were recruited from July to December 2021 by means of online advertisements through patient organization websites (e.g. the Norwegian Asthma and Allergy Association and the Norwegian Women's Public Health Association) and social media (e.g. Facebook). These advertisements directed them to the study website to confirm their eligibility, sign online consent forms and fill an online baseline questionnaire. Then, a start-up call provided detailed information about the study procedures, and instructions were sent to the participants with the study equipment (Figure 1).
3.2 Ethics and approval
The study was approved by the Regional Committee for Medical and Health Research Ethics in South-East Norway (REK no. 232351, date: 1 June 2021) and the Data Protection Officer at the University of Oslo (date: 3 June 2021). All women gave their written informed consent prior to inclusion in the study. Moreover, both parents gave their consent for the collection of health information about the infant prior to the study inclusion.
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies14 and designed in line with the EMA and US FDA recommendations for clinical lactation studies.12, 13 The study is registered in the EU PAS Register (EUPAS46213)15 and at the Zenodo16 open science platform.
3.3 Inclusion criteria
Women were eligible to provide milk samples if they were over 18 years, had been using (levo)cetirizine for at least 2 days—to ensure steady state conditions—and were breastfeeding a healthy infant who was at least 8 weeks old. This latter criterion was set to characterize excretion in mature milk, to allow breastfeeding to be well established and to give the mother time to rest after delivery. Mothers were excluded if they were unable to provide an informed consent. Moreover, mothers with infants who were twins/multiples, were preterm or had malformations or severe illness were not included to avoid the extra burden of study participation.
We aimed to include at least 25 breastfeeding women with a full set of breast milk samples. To reach this sample size, we expected to have to include approximately 30 participants to allow for dropouts and for participants from whom meaningful data could not be provided.
3.4 Milk sampling procedure
The participants were supplied with a milk sampling kit including all necessary sampling equipment (i.e. plastic milk bags, an electric breast milk pump, ice packs and a polystyrene box for shipment of milk samples). They were given oral and written instructions on how to take the breast milk samples at home (approximately 20 ml by sample) and instructed to express a total of six such breast milk samples at predefined time intervals, 0, 2, 4, 8, 12 and 24 h after dose intake. They were instructed to record the exact time of drug intake and milk sampling. They were advised to express milk from one or both the breasts (i.e. as they usually would breastfeed) until the breast felt empty and gently turn the container upside down a few times to mix the full volume of pumped milk. Approximately 20 ml of the milk was then transferred into the milk bag and frozen in their home freezer. If someone was not able to express a full 20-ml milk sample, a lower volume was accepted.
They were then asked to store the samples in the provided milk bags in their home freezer until all samples had been collected. When sampling was completed, the samples were packed with ice packs in the polystyrene box and shipped with express delivery to the Uppsala Biobank, Uppsala, Sweden. A pictogram showing the study procedure is presented in Figure 1.
3.5 Questionnaire data collection
Participating mothers reported information about their background and health status at baseline and on the sampling day using online questionnaires. Specifically, they reported their age (years), body weight (kg), height (cm), the infant age (weeks), chronic and acute medical conditions and concomitant drug use. They also reported the birth weight and gestational age of their infant at delivery. On the sampling day, they reported the mode of breastfeeding (exclusively vs. partially breastfed infant), the milk production the previous 3 days (as before/less than before/more than before), time of (levo)cetirizine intake and time of each milk sampling.
3.6 Quantification of cetirizine concentrations in breast milk
Upon arrival, the samples were examined and aliquoted into maximum 16 × 1 ml aliquots. They were subsequently stored at −80°C until the day of analysis.
Prior to the study, we performed a stability analysis of human breast milk samples spiked with (levo)cetirizine to evaluate whether temperature impacted drug stability in milk. The stability testing showed that the temperature did not impact on drug stability in milk for up to 72 h at room temperature and 7 days at 4°C.17
Measurements of cetirizine levels in milk were carried out using a validated method consisting of liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) in the Department of Pharmacy, Uppsala University (UPPS), Sweden.17 The method was developed and validated according to the regulatory authorities' recommendations.13, 18 The lower limit of quantification (LLOQ) was set to 0.39 μg/L, and the method had a linear range from 0.39 to 194.5 μg/L. The intra-run and inter-run precision and accuracy were ≤9%.17
3.7 Pharmacokinetic analysis and estimation of infant drug exposure
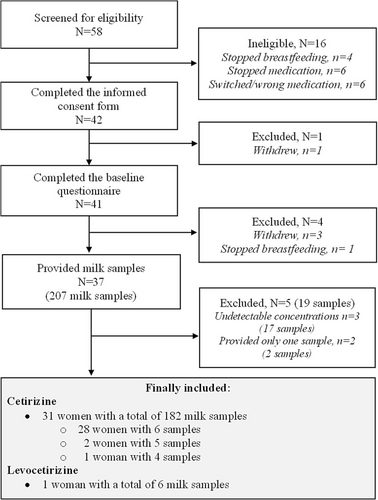
Maximum milk drug concentration (Cmax) and the corresponding time to maximum concentration (tmax) were extracted from the measured values. Other pharmacokinetic variables were calculated using the pharmacokinetic program package Kinetica, version 4.3 (Thermo Fisher Scientific, Waltham, MA, USA). The area under the time–milk concentration curve from 0 to 24 h (AUC) was calculated using a mixed log-linear model. Exact time intervals were used based on the times reported for drug intake and sampling. In a few cases where the 24-h sample either was lacking or the woman obviously had ingested the morning dose before sampling, we used the concentration at t = 0 also at 24 h, as all samplings took place at steady-state conditions. By applying a non-compartment model, the parameter estimate describing the decrease of the log concentration (λz) was calculated using the best-fit log-linear regression line of the samples representing the elimination phase. The elimination half-life (t1/2) was calculated as ln2/λz. Average concentration (Cavg) in milk throughout the 24-h sampling period was calculated as the AUC divided by 24 for every individual. No samples had concentrations below the LLOQ, with the exception that all the 17 samples from three women had unmeasurable concentrations. These three women were excluded from the study (Figure 2).
The absolute infant dose (AID) per kg body weight per day when fully breastfed was calculated by multiplying Cavg by a standard daily milk intake of 150 ml/kg. The RID in a day was calculated as the percentage of the AID (μg/kg/day) relative to the weight-adjusted maternal dose (μg/kg/day). Given the short elimination half-life of (levo)cetirizine, we considered it appropriate also to report the infant dose ingested in a feed. Therefore, we also calculated the AID and RID in a feed in the worst-case scenario of exposure, that is, when the infant was breastfed at the time of the peak concentration in milk. We then multiplied Cmax with a standard milk intake in a feed of 30 ml/kg.
To be able to compare the pharmacokinetic parameters and the drug exposure estimations among mothers and infants directly, the maternal cetirizine dose was standardized at 10 mg. Thus, for the two mothers with a cetirizine dose of 20 mg/day, all measured concentrations were given its linear (first-order) elimination kinetics8 divided by two before any pharmacokinetic calculations were made, and a maternal dose of 10 mg/day was used when calculating the RIDs.
3.8 Adverse events
At the day of sampling, mothers were asked to report whether their infant had shown any of the following symptoms in the previous 24 h: drowsiness, sleepiness/sedation, poor feeding/refusal of the breast, rash, bruising/bleeding, constipation, diarrhoea, stools with blood or unusual colour or fever. These symptoms were selected as they have previously been reported as adverse drug reactions in breastfed infants.19-21 The mothers were also asked to comment on reasons for the symptoms. For instance, they could report whether they considered it was more than usual or whether the symptoms could be caused by factors like fever, transitioning to solid foods or weaning. We summarized all reported symptoms irrespective of potential alternative explanations given by the mother to give a full overview of reported symptoms.
4 RESULTS
A total of 57 women were screened for eligibility, of whom 42 were initially included and completed the informed consent form. One woman withdrew before completing the baseline questionnaire. Of the 41 women who completed the baseline questionnaire, 37 provided milk samples. Five of these were excluded because they either had undetectable drug concentrations in all samples (n = 3) or provided only one milk sample (n = 2). The study thus finally included 32 breastfeeding women, 31 women using cetirizine and one woman using levocetirizine. These women provided either six (n = 29), five (n = 2) or four (n = 1) milk samples. A flow diagram presenting the details of inclusion and exclusion is shown in Figure 2. Table 1 summarizes the characteristics of the 32 mother–infant dyads. The mothers had a mean age of 30.3 years (range 22–40 years) and a mean body weight of 76.5 kg (range 53–110 kg). Their infants had a mean age of 7.8 months (range 8.3 weeks to 21 months), and a mean body weight of 8.3 kg (range 3.7–11.9 kg). Eleven mothers (34%) reported exclusive breastfeeding, whereas the remaining reported partial breastfeeding. The average pumping time to provide a full breast milk sample was 14 min.
| Maternal | Infant | Mode of breast-feeding | Maternal co-medication | |||
|---|---|---|---|---|---|---|
| Subject no. | Age (years) | Weight (kg) | Age (months) | Weight (kg) | ||
| 1 | 27 | 64 | 10.0 | 9.2 | Partial | Fluticasonea |
| 2 | 25 | 100 | 5.4 | 8.0 | Exclusive | — |
| 3 | 29 | 71 | 5.5 | 8.0 | Exclusive | Salmeterol/fluticasoneb; desogestrel |
| 4 | 33 | 76 | 10.0 | 8.9 | Partial | Desogestrel |
| 5 | 24 | 53 | 11.7 | 11.8 | Partial | — |
| 6 | 37 | 89 | 1.4 | 5.2 | Exclusive | — |
| 7 | 29 | 67 | 7.0 | 7.6 | Partial | — |
| 8 | 37 | 59 | 6.2 | 7.6 | Partial | Mometasonea |
| 9 | 28 | 83 | 13.5 | 9.5 | Partial | Desogestrel |
| 10 | 29 | 71 | 4.5 | 6.8 | Exclusive | Mometasonea |
| 11 | 33 | 110 | 11.5 | 7.7 | Partial | Levonorgestrel IUD; labetalol; levothyroxine; amlodipine |
| 12 | 40 | 88 | 21.2 | 10.8 | Partial | Fluticasonea |
| 13 | 23 | 58 | 6.0 | 7.0 | Partial | — |
| 14 | 27 | 63 | 3.7 | 7.9 | Exclusive | — |
| 15 | 35 | 98 | 8.2 | 10.2 | Partial | Levonorgestrel IUD |
| 16 | 31 | 72 | 6.6 | 7.9 | Partial | Fluticasonea; levonorgestrel IUD |
| 17 | 31 | 69 | 2.8 | 7.6 | Exclusive | — |
| 18 | 33 | 88 | 11.8 | 11.1 | Partial | Levothyroxine |
| 19 | 22 | 87 | 4.9 | 6.9 | Exclusive | Medroxyprogesterone |
| 20 | 32 | 93 | 6.5 | 6.7 | Partial | Desogestrel |
| 21 | 29 | 80 | 8.2 | 8.4 | Partial | — |
| 22 | 27 | 82 | 2.1 | 5.4 | Exclusive | Desogestrel |
| 23 | 25 | 64 | 11.2 | 9.5 | Partial | — |
| 24 | 33 | 59 | 15.7 | 11.9 | Partial | Dexchlorpheniramine |
| 25 | 32 | 75 | 9.1 | 9.1 | Partial | Escitalopram; pantoprazole |
| 26 | 27 | 100 | 5.5 | 6.9 | Exclusive | Allergen extract of house dust mites |
| 27 | 32 | 55 | 10.5 | 8.8 | Partial | — |
| 28 | 32 | 65 | 6.0 | 7.0 | Partial | — |
| 29 | 34 | 90 | 4.0 | 5.8 | Exclusive | — |
| 30 | 31 | 76 | 1.8 | 3.7 | Exclusive | — |
| 31 | 29 | 74 | 8.5 | 10.1 | Partial | — |
| 32 | 34 | 79 | 13.0 | 11.5 | Partial | — |
| Mean (SD) | 30.3 (4.2) | 76.5 (14.7) | 7.8 (4.5) | 8.3 (2.0) | ||
| Median | 31 | 74.5 | 6.8 | 8.0 | ||
| IQR | (27; 33) | (63; 88) | (5.3; 10.7) | (7.0; 9.5) | ||
- Abbreviations: BMI, body mass index; IUD, intrauterine device; IQR, interquartile range; SD, standard deviation.
- a For intranasal use.
- b For inhalation.
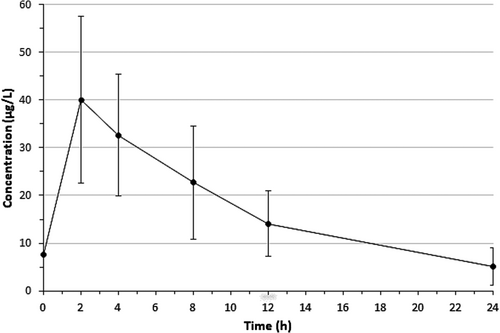
Figure 3 displays the cetirizine concentration–time profile in breast milk during the 24-h sampling period (182 milk samples). Table 2 presents pharmacokinetic data for cetirizine in milk. Mean Cmax was 41.0 μg/L (range 18.4–94.7 μg/L), mean Cavg was 16.8 μg/L (range 7.3–42.8 μg/L), mean tmax was 2.4 h (range 1.7–4.6 h), and mean t1/2 was 7.0 h (range 4.3–10.7 h).
| Subject no. | Cmax (μg/L) | tmax (h) | t1/2 (h) | AUC (μg·h/L) | Cavg (μg/L) |
|---|---|---|---|---|---|
| 1 | 33.6 | 2.0 | 6.1 | 289 | 12.0 |
| 2 | 35.8 | 1.9 | 8.0 | 317 | 13.2 |
| 3 | 44.7 | 1.7 | 7.4 | 447 | 18.6 |
| 4 | 37.9 | 2.6 | 6.3 | 387 | 16.1 |
| 5 | 39.7 | 2.2 | 6.7 | 314 | 13.1 |
| 6 | 33.8 | 4.1 | 8.8 | 470 | 19.6 |
| 7 | 30.2 | 2.0 | 5.2 | 250 | 10.4 |
| 8 | 64.7 | 1.9 | 5.1 | 492 | 20.5 |
| 9 | 24.5 | 4.6 | 8.3 | 312 | 13.0 |
| 10 | 48.9 | 1.8 | 6.2 | 459 | 19.1 |
| 11 | 42.8 | 2.9 | 7.5 | 461 | 19.2 |
| 12 | 18.4 | 4.5 | 5.0 | 174 | 7.3 |
| 13 | 42.1 | 2.0 | 7.9 | 433 | 18.0 |
| 14 | 45.4 | 1.9 | 7.2 | 480 | 20.0 |
| 15 | 24.3 | 2.4 | 7.1 | 259 | 10.8 |
| 16 | 33.2 | 3.8 | 9.6 | 398 | 16.6 |
| 17 | 31.7 | 2.1 | 6.4 | 364 | 15.2 |
| 18 | 31.2 | 2.5 | 7.9 | 314 | 13.1 |
| 19 | 27.5 | 2.0 | 6.5 | 228 | 9.5 |
| 20 | 61.3 | 2.6 | 7.2 | 609 | 25.4 |
| 21 | 42.9 | 2.3 | 9.5 | 480 | 20.0 |
| 22 | 28.4 | 2.0 | 4.3 | 214 | 8.9 |
| 23 | 32.6 | 2.2 | 6.6 | 327 | 13.6 |
| 24 | 49.8 | 1.9 | 5.1 | 360 | 15.0 |
| 25 | 23.9 | 1.9 | 5.8 | 212 | 8.8 |
| 26 | 42.1 | 1.7 | 6.2 | 302 | 12.6 |
| 27 | 37.5 | 2.1 | 5.4 | 315 | 13.1 |
| 28 | 63.7 | 1.9 | 6.2 | 639 | 26.6 |
| 29 | 72.1 | 2.0 | 10.7 | 827 | 34.5 |
| 30a | 94.7 | 1.8 | 8.1 | 1028 | 42.8 |
| 31a | 31.5 | 1.9 | 6.1 | 306 | 12.8 |
| Mean (SD) | 41.0 (16.2) | 2.4 (0.8) | 7.0 (1.5) | 404 (184) | 16.8 (7.7) |
| (Min; Max) | (18.4; 94.7) | (1.7; 4.6) | (4.3; 10.7) | (174; 1028) | (7.3; 42.8) |
- Abbreviations: AUC, area under the milk concentration–time curve from 0 to 24 h; Cavg, average concentration; Cmax, maximum concentration; Max, maximum; Min, minimum; SD, standard deviation; tmax, time to reach the maximum concentration; t1/2, elimination half-life.
- a Maternal dose was 20 mg/day. All concentrations were divided by 2 to adjust them to a dose to 10 mg/day. Pharmacokinetic calculations were based on these adjusted concentrations.
Table 3 shows the estimated theoretical AIDs and RIDs for cetirizine in a feed at Cmax and in a day. Assuming exclusive breastfeeding with an average infant milk intake of 150 ml/kg body weight, mean RID in 1 day was 1.9% (range 0.9–3.8%).
| Absolute infant dose (μg/kg) | Relative infant dose (%)a | |||
|---|---|---|---|---|
| Subject no. | In a feed at Cmaxb | In a dayc | In a feed at Cmaxb | In a dayc |
| 1 | 1.0 | 1.8 | 0.6 | 1.2 |
| 2 | 1.1 | 2.0 | 1.1 | 2.0 |
| 3 | 1.3 | 2.8 | 1.0 | 2.0 |
| 4 | 1.1 | 2.4 | 0.9 | 1.8 |
| 5 | 1.2 | 2.0 | 0.6 | 1.0 |
| 6 | 1.0 | 3.0 | 0.9 | 2.6 |
| 7 | 0.9 | 1.6 | 0.6 | 1.1 |
| 8 | 1.9 | 3.1 | 1.1 | 1.8 |
| 9 | 0.7 | 1.9 | 0.6 | 1.7 |
| 10 | 1.5 | 2.9 | 1.0 | 2.0 |
| 11 | 1.3 | 2.9 | 1.4 | 3.2 |
| 12 | 0.6 | 1.1 | 0.5 | 1.0 |
| 13 | 1.3 | 2.7 | 0.7 | 1.6 |
| 14 | 1.4 | 3.1 | 0.9 | 2.0 |
| 15 | 0.7 | 1.7 | 0.7 | 1.6 |
| 16 | 1.0 | 2.5 | 0.7 | 1.8 |
| 17 | 1.0 | 2.3 | 0.7 | 1.6 |
| 18 | 0.9 | 1.9 | 0.9 | 1.7 |
| 19 | 0.8 | 1.5 | 0.8 | 1.4 |
| 20 | 1.8 | 3.8 | 1.5 | 3.0 |
| 21 | 1.3 | 3.0 | 1.1 | 2.5 |
| 22 | 0.9 | 1.3 | 0.6 | 0.9 |
| 23 | 1.0 | 2.0 | 0.8 | 1.6 |
| 24 | 1.5 | 2.3 | 1.0 | 1.6 |
| 25 | 0.7 | 1.3 | 0.7 | 1.3 |
| 26 | 1.3 | 1.8 | 0.7 | 1.0 |
| 27 | 1.1 | 2.0 | 0.7 | 1.3 |
| 28 | 1.9 | 4.0 | 1.7 | 3.5 |
| 29 | 2.2 | 5.2 | 1.6 | 3.7 |
| 30 d | 2.8 | 6.4 | 0.8 | 3.8 |
| 31d | 0.9 | 1.9 | 0.4 | 1.4 |
| Mean (SD) | 1.2 (0.5) | 2.5 (1.2) | 0.9 (0.3) | 1.9 (0.8) |
| (Min; Max) | (0.6; 2.8) | (1.1; 6.4) | (0.4; 1.7) | (0.9; 3.8) |
- Abbreviations: Max, maximum; Min, minimum; SD, standard deviation.
- a Infant dose per kg body weight, expressed as a percentage of the maternal dose per kg body weight.
- b Assuming a milk intake of 30 ml/kg body weight, calculated at milk Cmax.
- c Assuming a milk intake of 150 ml/kg body weight, calculated by means of milk Cavg.
- d Maternal dose was 20 mg/day. All relevant values are adjusted to a maternal dose of 10 mg/day.
Only one mother was using levocetirizine, at a dose of 5 mg/day. For her, levocetirizine Cmax and Cavg were 19.3 and 7.2 μg/L, respectively, whereas tmax was 1.9 h and t1/2 was 6.2 h. AIDs were 0.6 μg/kg in a feed and 1.1 μg/kg in a day, and RIDs were 1.0% in a feed and 1.9% in a day.
Table 4 describes infant symptoms reported by the 31 mothers using cetirizine: Four had constipation, three rashes, three poor feeding or refusal of the breast, two bruising, two fever, two sedation, and one drowsiness. Sixty-one per cent of infants (n = 19) did not have any adverse events. In several cases, the mother contributed the symptom to other conditions as the infant having a cold (fever, sedation, rash), learning to crawl (bruising on knees), weaning (poor feeding/refusing of the breast), transitioning to solid foods (constipation) and other disease. On the sampling day, 32.3% (n = 10) of participating mothers estimated their milk production had decreased over the three previous days, whereas the remaining mothers declared a normal production. The mother using levocetirizine did not report any adverse events for her infant and described a normal milk production.
| Reported symptoms | n = 31 N (%) |
|---|---|
| Any symptoms | 12 (38.7%) |
| Constipation | 4 (12.9%) |
| Rash | 3 (9.7%) |
| Poor feeding or refusal of the breast | 3 (9.7%) |
| Bruising or bleeding | 2 (6.5%) |
| Fever | 2 (6.5%) |
| Sedation | 2 (6.5%) |
| Drowsiness | 1 (3.2%) |
| Diarrhoea | 0 (0%) |
| Stools with blood or abnormal colour | 0 (0%) |
- a In several cases, alternative explanations for the symptoms were present (i.e. cold, infection, weaning, learning to crawl, other disease). All reported symptoms are listed, irrespective of their origin, for full data transparency.
5 DISCUSSION
The principal finding in the present study is that the transfer of cetirizine and levocetirizine into breast milk is low (RID <2%). These results confirm current clinical practice of considering use of cetirizine to be compatible with breastfeeding and show that it is unlikely that breastfeeding while using cetirizine will pose any significant risk to the infant.
Our results align well with the results from the study in three women by Wilkerson et al, although that study was not performed at steady state.6 The RIDs were close to identical and Cmax in our study was 41 μg/L versus 49 μg/L in the study by Wilkerson et al. Relative to the peak plasma concentrations of approximately 300 μg/L in adults,8 the passage to breast milk is very low (milk/plasma [M/P] concentration ratio ~0.10). We found a milk half-life of 7 h (SD: 1.5 h), which is close to the plasma half-life of approximately 8 h.8 As expected, the tmax in milk was delayed in comparison to tmax in plasma in adults (mean 2.4 h vs. 1 h in plasma). Interestingly, in our milk study, we found a relatively large variability in AUC with a sixfold difference between the lowest and the highest value. In contrast, the variability for AID and RID was lower, about fourfold. Nevertheless, even the highest AID and RID values found were reassuringly low. In addition to estimating the clinically relevant RID and AID, we also surveyed the breastfed infants for potential adverse drug effects. Bertrand et al recently published baseline rates of common symptoms in breastfed infants without any medication exposure, finding rash (12.1%), irritability (9.4%), constipation (7.8%), poor sleep (7.1%) and fever (6.3%) to be most common ailments the past 2 weeks.21 In light of these findings, we consider that the reported symptoms in breastfed infants in our study reflect the normal, expected rates of symptoms in breastfed infants in the general population. As drowsiness and sedation previously have been reported after breast milk exposure to other antihistamines,21 this is especially reassuring.
The recommended paediatric doses for cetirizine in infants aged 6 months to 2 year is 0.5 mg/kg/day with a mean dose of 2.3 mg.8 For an infant weighting 10 kg, the fraction we detected in breast milk would constitute about 1/100 to 1/200 of a neonatal dose. Thus, (levo)cetirizine could clearly be considered a first-line antihistamine for breastfeeding women.
Given the prevalence of allergy and use of antihistamines among women of childbearing age, these results will be reassuring for many postpartum women and hopefully promote breastfeeding and optimal treatment of allergic disorders while breastfeeding. Equally important, this study, conducted according to European and US standards for human lactation studies, could provide a basis for updating cetirizine product information. Currently, the product information for cetirizine include unpublished data stating that it is excreted in human milk at concentrations representing 25%–90% of those measured in plasma (Appendix 1). Such information is not helpful for the estimation of the level of infant exposure or for assessments related to infant safety.22
This study has several strengths. It includes intensive sampling with six samples during a period of 24 h, allowing us to determine concentrations in breast milk at multiple time points throughout the dose interval and to make relatively robust AUC estimations. Moreover, the number of women included was high, thus increasing the reliability of the results. All procedures, including shipment, were piloted to ensure seamless logistics, and an accredited laboratory and biobank facility was utilized. Furthermore, we used a validated, sensitive and robust LC-MS/MS method for analysis.17 Designing the study to include home sampling and use online follow-up questionnaires enabled nationwide recruitment. Consequently, a rapid recruitment of the target number of participants was possible, and the sampling procedures including sampling at home had limited interference in the daily life for the breastfeeding mother and her infant.
As shown in Table 2, we detected a moderate variability in the mean and maximum concentrations in breast milk samples between the participants. This may be due to individual pharmacokinetic factors as well as differences in breast milk composition and/or milk sampling. Despite instructions to provide a full breast milk expression sample to obtain a representative sample of a feed, sampling/milk pumping time and milk volume could vary between the women. We did not analyse the milk samples with respect to pH and lipid and protein content and were thus unable to ascertain whether differences in milk composition impacted the drug concentrations.
When designing the study, we chose not to collect blood samples from the breastfeeding women, which prevented us from calculating M/P ratios. Although the M/P ratio is useful for assessing the extent to which a medication is transferred from blood into the breast milk, it is not a clinically useful parameter to evaluate the risk of infant drug exposure.22 As the amount of breast milk transfer is largely determined by the concentration of the medication in maternal plasma, even medications with a high M/P ratio may still result in low total drug milk concentrations. The EMA and FDA guidelines underline the importance of minimizing the burden of data collection for the mother while at the same time obtaining adequate data, and generally recommend milk-only studies unless there is a specific reason to conduct another type of study.12, 13
Although this study has several strengths, there are also some weaknesses that have to be addressed. Only one woman used levocetirizine. However, transfer of a drug from maternal plasma to breast milk is generally believed to be mainly a passive process where the key drug-specific factor determining its excretion is its physico-chemical properties. As the two enantiomers of a drug have identical physico-chemical properties, it is reasonable to assume that although we just were able to include one woman using levocetirizine, the cetirizine results will principally be applicable also for levocetirizine. This is further substantiated by the fact that the RID in a day for levocetirizine was exactly the same as the mean value in the cetirizine group, 1.9%. Thus, even though drug-specific studies are always wanted, we consider that in lack of more levocetirizine data, our cetirizine results could be considered valid also for levocetirizine. As the analytical method was not enantioselective, we cannot verify that all women used the drug stated. However, as all results were plausible, we have no reason to believe that any of the women had mixed up cetirizine with levocetirizine, or vice versa. Nevertheless, analysing the (R)- and (S)-enantiomers separately would have provided even more detailed information about the excretion of racemic cetirizine in breast milk.
Because we relied on participants' self-sampling and reporting at home, we were not able to observe and assess how well the women complied with the study procedures and instructions. Inaccurate recordings of timing of cetirizine intake and milk sampling or other deviations from instructions (e.g. lack of a full breast milk sample) could potentially influence the estimates presented and explain some of the variability. However, we excluded subjects with obvious protocol deviations or unreliable results (Figure 2), and the high number of samples included would counteract single reporting errors having a large impact on results. Our results must be interpreted with these limitations in mind.
6 CONCLUSION
This study shows that the transfer of cetirizine and levocetirizine into breast milk is low, with an average RID below 2% and an average absolute daily infant dose being less than 3 μg/kg/day. No severe adverse events were reported in the breastfed infants, and the reported symptoms reflected well the normal expected rates of minor ailments in healthy breastfed infants. These results confirm current clinical practice of considering cetirizine and levocetirizine to be compatible with breastfeeding and show that it is unlikely that breastfeeding while using cetirizine or levocetirizine will pose any significant risk to the infant.
ACKNOWLEDGEMENTS
The authors are grateful to the participating mothers for the milk donations. The research leading to these results was conducted as part of the ConcePTION consortium. The authors thank the IMI-ConcePTION WP4/3 members, in particular Mats Hansson (WP4 lead), Pieter Annaert (WP3 lead) for valuable and stimulating discussions, Jennifer Drevin (recruitment video) and especially to former master student Basma Kousa who performed her master thesis in pharmacy as part of this study and former PhD student Maria Bich-Thuy Truong and post doc Marion Taine at the University of Oslo. This paper only reflects the personal views of the stated authors.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data not available.




