Pre-natal brain development as a target for urban air pollution
Abstract
Air pollution is the main urban-related environmental hazard and one of the major contributors to the global burden of disease based on its cardiovascular-respiratory impacts. In children, exposure to urban air pollution is associated, among others, with decelerated neurodevelopment early in life and increased risk of neurodevelopmental problems such as attention-deficit hyperactivity disorder, autism spectrum disorders, academic failure and the start of Alzheimer's pathogenesis. However, the evidence of the effects of air pollution on brain development is still inadequate, mainly due to the limitations in (a) characterizing brain development (most studies were based on subjective tools such as questionnaires or neuropsychological tests) and (b) air pollution exposure (most studies only used residential levels based on geographical modelling and also overlooking the variation in the mixture of air pollutants as well as the composition and hence toxicity of particulate pollutants in different settings), (c) the lack of studies during the most vulnerable stages of brain development (foetal and early life (first two years post-natally)) and (d) the lack of structural and functional imaging data underlying these effects. In mice, in utero exposure to fine particles was linked to structural brain changes and there is a need to establish the generalizability of these findings in human beings. Though scarce, current evidence in children supports the importance of the pre-natal period as a susceptible window of exposure. Two studies in schoolchildren found that pre-natal air pollution exposure might damage brain structure while exposure during childhood was not linked to any structural alteration. Another study showed that children with higher traffic-related air pollution at school had lower functional integration in key brain networks, but no changes in brain structure, possibly partly because of the time window of air pollution exposure (in utero versus childhood exposure). A key development is to discover the windows of greatest sensitivity of structural brain changes to air pollution exposure by incorporating the recent advances in non-invasive imaging to characterize natal and post-natal brain development and exploring whether and to what extend placental dysfunction could mediate such an association. Studying pre-natal life is important because effects at this time are of a potentially irreversible nature and because the largest preventive opportunities occur during these periods.
1 WHY TO INVESTIGATE AIR POLLUTION?
Over 80% of the world's population lives in urban areas where air pollution levels exceed the limits set by the WHO.1 Particulate air pollution is the main environmental contributor to the global burden of disease and is one of the top preventable causes of disease over time.1 More than 20% of stroke, ischaemic heart diseases and chronic respiratory diseases could be prevented by reducing particulate pollution to the WHO limits. The direct health costs associated with air pollution exceed 5% of Europe's GDP. So far, because of the inadequacy of the available evidence, the potential effects of air pollution on brain development (and cognitive decline) have not been considered when estimating the burden associated with air pollution. Pioneering studies on brain tissue from autopsies in dogs and children living in highly polluted areas of Mexico City showed inflammation in several brain areas.2 This work led to a series of experimental studies in mice exposed to fine, ultrafine and diesel particles.3 In mice, the central nervous system could be a direct or indirect target (via the olfactory or lung pathway, respectively) of particles that elicit a neuroinflammatory response in various brain regions.3 In human beings, our group4 and others5 have shown that exposure to air pollution in utero is associated with increased risk of neurodevelopmental delay and autism.6 We also found that air pollution exposure during childhood is inversely associated with brain executive trajectories,7 and others have found an inverse association with academic achievement8 and higher risk of pathological changes associated with Alzheimer's disease.9 However, the evidence is still inadequate, given the limitations in (a) characterizing brain development (most studies were based on subjective tools) and (b) air pollution exposure (most studies only used residential levels based on geographical modelling and also overlooking the variation in the mixture of air pollutants as well as the composition and hence toxicity of particulate pollutants in different settings), (c) the lack of studies during the most vulnerable stages of brain development (foetal and early life (first two years post-natally10)) and (d) the lack of structural and functional imaging data underlying these effects.
Of all air pollutants, particles are thought to be the most important inhaled toxicants in urban air,11 particularly in relation to brain damage.12 Among the various particle types, we found airborne copper to be associated with tissue modifications and functional connectivity in the caudate nucleus in children.13 Other metals, such as iron, have been linked to structural changes in mice brain,14 and environmental Fe nanoparticles were found in brain tissue.15 Furthermore, two recent studies in rats exposed to NO2 (an urban gas) reported clear evidence of brain damage,16, 17 suggesting a specific neurotoxicity of NO2. The specific constituents of the air pollution that causes these brain effects are unknown, mainly because studies in children have focused on individual pollutants, overlooking the variation in the mixture of air pollutants as well as the composition and hence toxicity of particulate pollutants in different settings.
2 ARE CHILDREN MORE VULNERABLE?
A growing body of evidence is indicative on how brain development is very vulnerable in early life compared to later stages of life.18 In Minamata, for example, pre-natal exposure to mercury resulted in a cerebral palsy epidemic in the offspring, while mothers and siblings, who had similar exposure levels but were exposed post-natally, were unaffected. Similarly, intake of alcohol during pregnancy could result in the foetal-alcohol syndrome including chronic mental health disorders in the offspring at levels that mothers are asymptomatic.
Strong evidence suggests that environmental toxicants contribute to a global, silent pandemic of brain developmental problems.18 Optimal brain development involves multiple complex stages that must be completed sequentially, principally during foetal and early life.10 These processes are uniquely vulnerable to adverse environment interferences.19 Impairment of this process could lead to: (a) neurobehavioural disorders (ie autism spectrum disorder and attention-deficit hyperactivity disorder (ADHD)20), the prevalence of which appears to be increasing; (b) dysfunctional cognitive development; and (c) long-term deleterious effects on well-being and mental health.21 Living in, or having been brought up in urban areas, is associated with a detrimental impact on brain function22 and higher risk of neurobehavioural disorders.22 Together, rapid urbanization and a higher prevalence of neurobehavioural disorders in urban areas are expected to accelerate the already considerable global burden associated with these conditions in the coming years.23, 24
Of particular interest is the pre-natal period, when brain structures are forming and growing, and when the effect of in utero exposure to environmental factors may cause permanent brain injury.19 In mice, exposure to fine particles in utero25 and during the period corresponding to the third trimester of pregnancy in human beings14 was linked to structural changes in white matter. Other studies of in utero exposure in mice showed delayed cognitive development,26 increased anxiety27 and disrupted methylation of genes related to neurogenesis.28 In human beings, the available evidence on the effect of in utero exposure to air pollution on foetal and early brain structure and function is very scarce.
Still, current evidence29-31 in children supports the importance of the pre-natal period. Children in NY showed structural brain alterations at school age, using brain magnetic resonance imaging (MRI), related to air pollution levels during pregnancy, while post-natal exposure was not linked to structural alterations.29 Similar brain structural lesions were found in children from a highly polluted area in Mexico City.30 We recently conducted a study in a large sample of schoolchildren using direct measurement of traffic particles at schools that showed that children from schools with higher traffic-related pollution had lower functional integration in key brain networks, measured using functional MRI.31 The same study showed no changes in brain structure related to air pollution exposure, possibly because of the window of exposure (in utero vs childhood exposure) in that study. Another recent study with a large sample of schoolchildren with brain MRI showed a relationship between the residential exposure to air pollution during pregnancy and a decrease in pre-frontal white matter in areas related with attention.32 Consistent with these findings, recent retrospective studies in children reported that specific time windows during gestation are crucial for several cognitive functions33 and autism.34 Hence, the windows of sensitivity to air pollution exposure in utero in human beings are yet to be defined, for each specific environment, profile of pollutants, and socio-economic and ethnic groups. The limited available evidence suggests that exposure to particulate pollution from week 20 onwards could affect brain development.33
The few published studies using neuroimaging suggest that pre-natal air pollution exposure affects myelinisation (ie smaller white matter volume,29 white matter lesions30 and decrease o pre-frontal cortical thickness32). These results are consistent with the observed effects in mice exposed in utero14, 25 and in elderly human beings,35-37 although cumulative exposure and the mechanisms of action could be different in later life. The studies in mice suggest that the effect of air pollution is principally mediated through chronic stimulation of microglial cells.14, 25 Microglia support myelin protection and induce synapse formation. Accordingly, experiments in mice exposed to diesel particles pre- and neo-natally showed a reduction in the area of the corpus callosum (the largest white matter tract) and dilatation of the ventricles (indicating white matter damage). The area of the corpus callosum during weeks 28-32 of gestation has been reported to predict post-natal neurobehavioural impairment in children.38 Similarly, white matter tracts and microstructure at birth are suggested to predict general cognition in infancy.39 The myelinisation process generally overlaps with the formation and function of synapses, which starts around week 20 of gestation, reaches at its peak around week 34 and continues actively during post-natal life into adulthood.10 Though insufficient, current evidence on time windows of susceptibility and the type of structural changes in human beings and mice generally suggests that myelinisation is probably one of the main brain development processes that is most susceptible to damage from particulate pollution. Other processes, such as changes in basal ganglia, may also be involved, though to a lesser extent.40
3 WHAT ARE THE POTENTIAL MECHANISMS UNDERLYING THE EFFECTS OF AIR POLLUTION ON FOETAL BRAIN DEVELOPMENT?
It is increasingly recognized that the protection that placenta can provide for foetus against environmental hazard to which the mother is exposed could be limited. For example, studies have shown cotinine and polycyclic aromatic hydrocarbon (PAH) adducts in cord blood, suggesting that tobacco smoke and air pollution could effectively cross the placenta barrier.29 In this context, it is important to highlight that foetus has higher exposure than mothers in relation to their body size. Moreover, foetal life exposure results in maternal effects such as plasma viscosity, or systemic low-grade inflammation, hormonal disruption or epigenetic changes in mother that could impair placenta function which in turn could lead to cardiovascular or neurological disruption.40-46
Impairing placental function and decreasing transplacental oxygen and nutrient transport could be among potential mechanisms by which air pollution could affect foetal brain development during pregnancy. Evidence from birth cohorts suggests that air pollution affects placental function measured by Doppler ultrasound (negative effects on umbilical artery resistance in one study,41 but not in another42 during the second and third trimester; and negative associations on flow indices in the first trimester47). There is consistent evidence that impaired placental function disrupts foetal neurodevelopment in animals and neurobehavioural development in children. Altered placental function is associated with the development of the corpus callosum in the foetal brain38 and with brain function in neonates48 and brain connectivity at one year of age.49 Experimentally reducing oxygen and nutrients during the last third of gestation in rabbits could result in poorer neurobehavioural performance and network diffusion early in life.50
Mice exposed to airborne particles during gestation were found to have an increased number of metal particles in the corpus callosum.25 In human beings, there is evidence of metal-based airborne nanoparticles in blood and plaques of the carotid artery.51 Moreover, metal nanoparticles resembling combustion nanoparticles abundant in urban air have recently been found in brain tissue in human beings.15 These magnetic particles have been linked to disrupted myelin integrity, but it is not yet known whether these particles could be translocated to the placenta and foetus. In this context, penetration of inhaled ultrafine air-suspended particles into the placenta could be another potential mechanism underlying effects of air pollution on foetus, including foetal brain development.
Oxidative stress has been proposed to mediate the adverse health effects of air pollution. In a study including several birth cohorts, we found that exposure to air pollution during pregnancy was associated with differential DNA methylation in mitochondria-related genes, as well as differential methylation and expression of genes involved in antioxidant defence pathways in the cord blood.43 Moreover, mitochondrial 8-hydroxy-2'-deoxyguanosine (8-OHdG) in maternal peripheral blood and cord blood, a biomarker related to oxidative stress, has been related to gestational particulate air pollution.44 Mitochondria play a key role in the responses to reactive oxygen species. Hence, we have recently shown that placental mitochondrial DNA (mtDNA) content can be one of the potential mediators of the association between gestational air pollution exposure and foetal growth.45 In turn, fine particles were related with global methylation of the mitochondrial genome which is associated with the mtDNA content.46 In addition, placental 3-nitrotyrosine (3-NTp), another biomarker related to oxidative stress, was related to pre-natal particulate air pollution.52
Since DNA methylation profiles are tissue-specific to a large extent, to elucidate the tissue-specific epigenetic variation in response to pre-natal exposures is crucial to understand mechanisms leading to health effects. Placental methylome may act as a functional record of in utero exposures; however, only few small studies have assessed the effect of maternal smoking during pregnancy on DNA methylation patterns in human placenta. We identified novel loci related to foetal growth that were differentially methylated in placenta in relation to maternal smoking during pregnancy which did not overlap with previous findings in cord blood.53
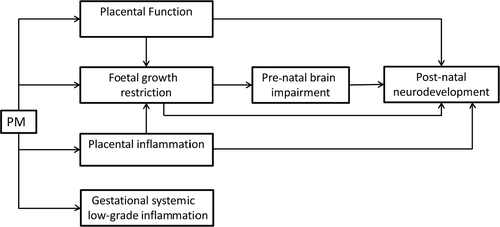
Overall (Figure 1), there is preliminary evidence of the important role of placenta in the pathways to brain development. Particulate matter (PM) could be related with mental development though impairing placental function, restricting foetal growth and inducting placental as well as systemic inflammation and oxidative stress. Finally, there is the door open to investigate presence of pollutants in placenta and its role on the above interrelationships.
4 IS NEUROIMAGING A NOVEL TOOL IN AIR POLLUTION EPIDEMIOLOGY?
During the past years, considerable advances have been made with regard to neuroimaging techniques such ultrasound and magnetic resonance imaging (MRI). They provide an unprecedented opportunity to investigate pre-natal and early post-natal brain development and the myelinisation process in particular.29-32 Advanced transabdominal and transvaginal neurosonography allows us to measure the depth and degree of maturation of specific cortical fissures and sulci, and the development of specific structures such as the corpus callosum and lateral ventricles with notable precision. Observations in mice exposed to diesel particles indicate that the area of the corpus callosum and the size of the lateral ventricles are good parameters for studying the effects of air pollution.25 In human beings, maternal exposures, such as to alcohol,54 tobacco55 and mercury,56 have been linked to several brain ultrasound measures. Abnormal pre-natal brain neurosonographic findings, in particular in the corpus callosum area, were predictive of post-natal neurobehavioural impairment. While foetal ultrasonography could reliably characterize the pre-natal brain development, it has only rarely been used to explore the effects of air pollution on foetal brain development. Moreover, the few available studies on placental function and air pollution did not measure the final impact on brain development.
Neonatal MRI has recently become a key technique in population-based studies since the Development and Human Connectome Project (DHCP), which is studying brain MRI in 1000 neonates.57 Neonatal brain MRI is practical because it can be done while the infant is asleep (and therefore has minimal movements of head) to avoid the need for anaesthesia. Furthermore, recent developments have significantly improved the efficiency of image capture in neonates.57 Thus, in addition to capturing the anatomical brain structure, MRI can now be used to visualise, delineate and quantitatively analyse regional connectivity (ie location, orientation and anisotropy of the brain's white matter sensorimotor tracts) using diffusion tensor imaging (DTI) and functional MRI (fMRI).
5 WHAT ARE THE RECOMMENDATIONS FOR THE FUTURE RESEARCH?
We propose to focus research on pre-natal life (Table 1) because, as elaborated above, it can be considered as a critical window of exposure and provide a great opportunity for prevention and there have been reports on transgenerational brain effects in mice.14, 25-27 Disruptions in the embryological process during pre-natal period could impair or make more fragile the brain for the rest of the life.19 Moreover, pregnancy offers the opportunity of a more feasible and valid assessment of the cumulative exposure. Additionally, the emergence of new techniques for imaging assessment of brain during the end of pregnancy and the neonatal life has provided an unprecedented opportunity to evaluate the aforementioned association of pre-natal exposure to air pollution and foetal brain development. Finally, placenta is a sensitive organ that is accessible for the tissue-specific study of epigenetics, inflammation and also biomarkers of exposure which could provide mechanistic evidence for this association.
| Critical periods of development |
| Higher opportunities for prevention |
| Studies in mice models |
| Placental dysfunction a mechanism |
| Placenta a pertinent tissue to assess epigenetics, inflammation and particles deposition |
| Opportunities for early brain imaging |
| Better assessment of exposure |
| Origin of developmental diseases |
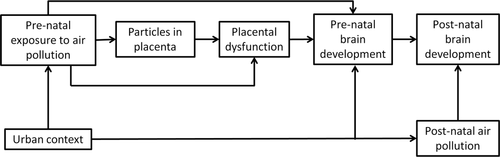
Overall, we suggest (Figure 2) that pre-natal exposure to urban air pollution during pregnancy impairs foetal and post-natal brain development (mainly by affecting the myelinisation process). We also postulate that the effects of air pollutants on pre-natal brain development are at least partially mediated by translocation of airborne particulate matter to the placenta and by placental dysfunction. We also suggest that pre-natal exposure to air pollution impairs foetal brain development independently of pre-natal urban context, and post-natal brain development independently of post-natal urban context and post-natal exposure to air pollution.
To date, a vast majority of epidemiological studies of the impacts of air pollution on pregnancy outcomes have: (a) relied on assessment of exposure in only one microenvironment (mainly the home), overlooking the contribution of other microenvironments (eg workplace and commuting) to personal exposure; (b) not characterized and accounted for exposure misclassification; and (c) have relied on exposure levels (level of pollution to which the individual is exposed) instead of dose (level of pollution inhaled by the individual). Development of a new generation of mobile air pollution monitors and economical air pollution sensors/samplers has provided an unprecedented opportunity to evaluate personal exposure and/or indoor/outdoor air pollution levels at homes and workplace in large sample sizes. At the same time, personal monitors and smartphone applications are increasingly being used in large-scale epidemiological studies to obtain personal real-time data on location and physical activity to characterize time-activity patterns for each study participant. This objective characterization of time-activity pattern can be integrated with the state-of-the-art modelling approaches and objective measures of personal, home and workplace air pollution levels to personalize exposure to air pollution at main microenvironments (home, work and commuting) and transform exposure levels in each microenvironment to inhaled dose.
Moreover, the urban context (including early life environmental stressors) could confound and/or interact with the effect of air pollution on brain development. The social environment and pre-natal stress, in particular, have been found to enhance the impairment of brain function by air pollution in both mice58 and children,59 for which various underlying mechanisms have been proposed. In addition to air pollution, urban residents are often exposed to higher noise levels, which we and others have shown to detrimentally affect brain development, and to increase the risk of various disorders, such as ADHD.60 However, the only currently available evidence on the effect of pre-natal noise exposure comes from experiments in rodents.61 Additionally, we found that growing up in urban non-natural built-up environments with less green areas is associated with higher levels of air pollution,62 higher risk of behavioural problems,63 poorer cognitive function64 and impaired brain structure.65 Another component that could modulate exposure to ambient air pollution is physical activity. These findings demand any study of the impact of air pollution on brain development to address comprehensively the urban context, including early life stressors.
6 CONCLUSIONS
Research of the air pollution effects on brain development is important, pertinent and challenging. Air pollution is the main urban-related environmental hazard and the main environmental contributor to the global burden of disease.1 Studying the structural and functional brain effects of air pollution exposure during foetal and early life is important, given the potential irreversible nature of these effects10 and the largest preventive opportunities during these periods.19 Detecting these critical periods is fundamental for preventive purposes during pregnancy. These studies could also benefit from better characterization of the mixture of air pollutants as well as constitutes of particulate pollutants and evaluating their impacts on developing brain. Interventions have been shown to be more efficient when carried out during earlier vulnerability windows. These pre-natal and early life effects could have long-lasting consequences and an accompanying societal burden, which is important both for the general public and for policymakers. To date, interventions to reduce air pollution have generally been modest and air pollution continues to grow globally. Demonstrating its impact on children's brains could create an impulse to definitively implement policy interventions that genuinely protect the health of urban citizens globally, as had occurred for lead in petrol.18 Furthermore, by understanding the role of the placenta in how air pollution leads to impaired brain development, we could be able to discover part of the causal nature of this relationship, which, among others benefits, will provide solid evidence to reinforce arguments for obstetricians and policymakers in implementing feasible and achievable individual- and community-level interventions.




