Turmeric essential oil improves intestinal integrity, immunological parameters, and performance of broiler chickens under cyclic heat stress
Abstract
This study aimed to examine whether dietary supplementation of broiler chickens with turmeric essential could mitigate the effects of cyclic heat stress conditions. Intestinal and immunological parameters and gene expression were evaluated during the grower phase. A total of 320 21-day-old male Cobb 500 broilers were distributed according to a completely randomized design with a 4 (diet) × 2 (environment) factorial arrangement and eight replications of five birds each. Dietary treatments consisted of a basal diet without essential oil (EO, negative control) and three diets containing low (100 mg kg−1), intermediate (200 mg kg−1), or high (300 mg kg−1) levels of turmeric EO. In the heat stress group, dietary supplementation with turmeric EO at 100 and 200 mg kg−1 improved body weight, feed conversion, breast yield, and relative liver weight. These supplementation levels reduced villus width, increased villus/crypt ratio, reduced the H/L ratio, and improved hepatic (HSP70 and SREBP1) and intestinal (OCLN) gene expression in birds under heat stress. These findings support the hypothesis that turmeric EO can be used to improve or restore intestinal integrity, modulate inflammation parameters, and, consequently, enhance the performance of broilers challenged by cyclic heat stress.
1 INTRODUCTION
Heat stress remains a major environmental stressor in contemporary poultry farming. Factors such as high metabolic rate, high production density, and global warming make birds more likely to experience heat stress. Furthermore, some unique physiological characteristics of birds exacerbate this problem by hindering their ability to dissipate heat. Heat stress impairs intestinal and immune functions and induces oxidative damage, culminating in reduced feed intake, feed use efficiency, and animal performance (Farag & Alagawany, 2018; Gonzalez-Rivas et al., 2020; Wasti et al., 2020; Kikusato et al., 2021; Park et al., 2022). It is noteworthy that modern broiler lines have high growth rates, high metabolic oxygen requirements, and high heat production, which results in an expressive generation of reactive oxygen species (Baghbanzadeh & Decuypere, 2008; Kalmar et al., 2013). Dietary factors and metabolic compensation continually trigger inflammatory responses, especially in the bird intestine. These characteristics produce a permanent state of oxidation and inflammation, rendering birds more susceptible to the deleterious effects of heat stress (Emami et al., 2020).
According to Goel (2021), chronic heat stress is induced when the ambient temperature remains well above the comfort zone for prolonged periods, such as between 27 and 38°C for more than 7 days. Chronic heat stress can be either constant or cyclic. The latter is fairly common under field conditions. One solution to mitigate the negative effects of heat stress on poultry metabolism is dietary supplementation with plant extracts. This strategy has attracted much attention for its simplicity and effectiveness. For instance, members of the family Zingiberaceae, in particular Curcuma longa L., commonly known as turmeric, have been extensively studied and used in medicinal applications. Research has shown that turmeric has antioxidant, anti-inflammatory, antibacterial, anti-apoptotic, and anticancer properties, reducing the negative effects of stress (He et al., 2015). The major bioactive component of the powder extract is curcumin, which inhibits multiple pro-inflammatory mediators, such as nuclear factor kappa B (NF-κB) and tumor necrosis factor-alpha (TNF-α) (Grasso et al., 2017). Despite some good results of turmeric supplementation in broilers (Baghban et al., 2016; Kanani et al., 2016; Ramadan et al., 2021; Swathi et al., 2012; Zhang et al., 2015), the limited solubility and absorption of curcumin represent a challenge to its effective utilization. Volatile extracts are a viable alternative to curcumin, given that the terpenes (rather than curcuminoids) found in the essential oil (EO) have major anti-inflammatory and antioxidant power, producing highly effective responses comparable to those obtained with curcumin complexes (Shishu & Maheshwari, 2010).
This study aimed to examine whether dietary supplementation of broiler chickens with turmeric EO could mitigate the effects of cyclic heat stress conditions. For this, intestinal and immunological parameters and gene expression were evaluated in broilers during the grower phase.
2 MATERIAL AND METHODS
2.1 Bird management and experimental design
The experiment was conducted at the Multifunctional Laboratory of Poultry Metabolism and Production (LAMEPA) of the Department of Animal Science, Federal University of Sergipe (UFS), Brazil. Experimental procedures were approved by the Animal Research Ethics Committee at UFS (CEPAP protocol No. 01/2021).
A total of 320 male Cobb 500 broilers were used. Birds were housed in 3000 cm2 metabolic cages (60 cm width × 50 cm length × 40 cm height) placed at three levels on metal supports in a shed with a ceiling height of 2.8 m. In the starter phase (1 to 21 days of age, before the experimental period), birds were reared under optimal conditions and had ad libitum access to water and feed. At 21 days, birds were weighed and distributed in a completely randomized design with a 4 (diet) × 2 (environment) factorial arrangement and eight replications of five birds each. Dietary treatments consisted of a basal diet without EO (negative control) and three diets containing low (EO1, 100 mg kg−1), intermediate (EO2, 200 mg kg−1), or high (EO3, 300 mg kg−1) levels of turmeric EO, as shown in Table 1. Birds in the thermal comfort group were maintained at the optimum temperature and humidity conditions (22 to 24°C, 70% relative humidity). Temperature and humidity were monitored daily. Birds in the heat stress group were subjected to cyclic high temperatures from 21 to 42 days to simulate field conditions, as follows: 33 to 35°C from 08:00 to 18:00 h and 21 to 22°C from 18:00 to 08:00 h at 70% relative humidity.
| Treatment | Environment | Diet |
|---|---|---|
| TC-BD | Thermal comfort | Basal diet |
| TC-EO1 | Basal diet supplemented with 100 mg kg−1 TEO (low dose) | |
| TC-EO2 | Basal diet supplemented with 200 mg kg−1 TEO (intermediate dose) | |
| TC-EO3 | Basal diet supplemented with 300 mg kg−1 TEO (high dose) | |
| HS-BD | Heat stress | Basal diet |
| HS-EO1 | Basal diet supplemented with 100 mg kg−1 TEO (low dose) | |
| HS-EO2 | Basal diet supplemented with 200 mg kg−1 TEO (intermediate dose) | |
| HS-EO3 | Basal diet supplemented with 300 mg kg−1 TEO (high dose) |
2.2 Experimental diets and turmeric EO
The birds were fed diets based on corn and soybean meal. Diets were formulated to meet nutritional requirements in the grower and finisher phases according to the recommendations of Rostagno et al. (2017) (Table 2). Turmeric EO was kindly donated by Delacon Biotechnik Ges.m.b.H. (Steyregg, Austria). The oil was obtained from plant rhizomes by conventional water vapor distillation. Gas chromatographic analysis of the oil revealed the major compounds to be α-turmerone (30–32%), ar-turmerone (17–26%), and β-turmerone (15–18%). EO aliquots were first incorporated into 240 g of vegetable oil and then added to 500 g of basal diet as a premix. Subsequently, this portion was mixed with the rest of the diet and fed to the animals. This procedure was performed daily.
| Composition | Grower diet1 | Finisher diet1 | ||||||
|---|---|---|---|---|---|---|---|---|
| BD2 | EO1 | EO2 | EO3 | BD | EO1 | EO2 | EO3 | |
| Ingredient composition (%) | ||||||||
| Corn (7.88% protein) | 62.992 | 62.992 | 62.992 | 62.992 | 69.626 | 69.626 | 69.626 | 69.626 |
| Soybean meal (45% protein) | 31.687 | 31.687 | 31.687 | 31.687 | 26.086 | 26.086 | 26.086 | 26.086 |
| Soybean oil | 2.602 | 2.602 | 2.602 | 2.602 | 2.057 | 2.057 | 2.057 | 2.057 |
| Turmeric essential oil | - | 0.010 | 0.020 | 0.030 | - | 0.010 | 0.020 | 0.030 |
| Salt | 0.481 | 0.481 | 0.481 | 0.481 | 0.430 | 0.430 | 0.430 | 0.430 |
| Dicalcium phosphate | 0.620 | 0.620 | 0.620 | 0.620 | 0.337 | 0.337 | 0.337 | 0.337 |
| Calcitic lime | 0.917 | 0.917 | 0.917 | 0.917 | 0.787 | 0.787 | 0.787 | 0.787 |
| Lysine | 0.167 | 0.167 | 0.167 | 0.167 | 0.179 | 0.179 | 0.179 | 0.179 |
| dl-Methionine | 0.273 | 0.273 | 0.273 | 0.273 | 0.246 | 0.246 | 0.246 | 0.246 |
| Threonine | 0.041 | 0.041 | 0.041 | 0.041 | 0.061 | 0.061 | 0.061 | 0.061 |
| Vitamin premix3 | 0.120 | 0.120 | 0.120 | 0.120 | 0.080 | 0.080 | 0.080 | 0.080 |
| Mineral premix4 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Vaccfit 0.5 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutrient composition (%) | ||||||||
| Metabolizable energy (kcal kg−1) | 3.150 | 3.200 | ||||||
| Crude protein | 20.00 | 18.00 | ||||||
| Ether extract | 5.426 | 5.031 | ||||||
| Crude fiber | 2.769 | 2.587 | ||||||
| Ash | 2.664 | 2.422 | ||||||
| Calcium | 0.770 | 0.640 | ||||||
| Total phosphorus | 0.568 | 0.501 | ||||||
| Available phosphorus | 0.341 | 0.280 | ||||||
| Digestible phosphorus | 0.222 | 0.178 | ||||||
| Digestible arginine | 1.219 | 1.064 | ||||||
| Digestible isoleucine | 0.760 | 0.668 | ||||||
| Digestible lysine | 1.120 | 1.000 | ||||||
| Digestible methionine | 0.542 | 0.495 | ||||||
| Digestible methionine + cysteine | 0.820 | 0.750 | ||||||
| Digestible threonine | 0.720 | 0.670 | ||||||
| Digestible tryptophane | 0.217 | 0.188 | ||||||
| Digestible leucine | 1.578 | 1.459 | ||||||
| Digestible histidine | 0.487 | 0.438 | ||||||
| Digestible valine | 0.832 | 0.744 | ||||||
| Digestible glycine + serine | 1.636 | 1.453 | ||||||
| Digestible phenylalanine | 0.214 | 0.237 | ||||||
| Potassium | 0.763 | 0.563 | ||||||
| Sodium | 0.210 | 0.190 | ||||||
- 1 Grower phase, 22 to 35 days of age; finisher phase, 36 to 42 days of age.
- 2 BD, basal diet; EO1, basal diet supplemented with 100 mg kg−1 turmeric essential oil (low dose); EO2, basal diet supplemented with 200 mg kg−1 turmeric essential oil (intermediate dose); EO3, basal diet supplemented with 300 mg kg−1 turmeric essential oil (high dose).
- 3 Provided per kg of product: vitamin A, 7000 IU; vitamin D3, 2500 IU; vitamin E, 18 IU; vitamin K3, 1 mg; vitamin B1, 1.5 mg; vitamin B2, 5.5 mg; vitamin B6, 1.6 mg; vitamin B12, 12 μg; pantothenic acid, 10 mg; biotin, 0.05 mg; folic acid, 0.9 mg; nicotinic acid, 32.5 mg.
- 4 Provided per kg of product: iron, 30 mg; manganese, 60 mg; zinc, 50 mg; iodine, 2.5 mg; selenium, 0.25 mg.
2.3 Animal performance and organ weights
Bird performance was evaluated from 21 to 42 days of age. Feed intake was determined by subtracting the amount of leftover feed from the total amount of feed provided weekly. Body weight was calculated by dividing the total body weight of the cage by the number of live birds per cage. Feed conversion ratio was determined weekly by calculating the amount of feed consumed (g) divided by body weight (g), corrected for the total weight of dead birds.
At 42 days of age, five birds per treatment were randomly selected, weighed, desensitized by asphyxiation with carbon dioxide, and euthanized. The thymus, spleen, bursa of Fabricius, liver, heart, entire intestine (duodenum, jejunum, ileum, cecum and colon; without digesta, empty), and breast were dissected and weighed. Relative organ weights were calculated by dividing the organ weight by body weight and multiplying by 100.
2.4 Jejunal histomorphometry
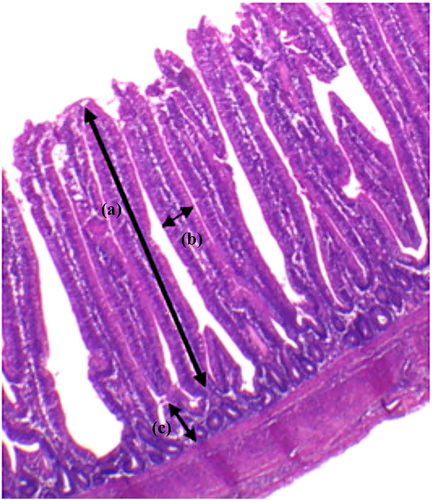
For histological analysis (%), jejunum specimens were collected from five birds per treatment immediately after slaughter. Samples were cut lengthwise, thoroughly washed with cold sterile saline, fixed in Bouin's solution for 24 h, and then incubated in 70% ethanol to remove the fixative. After fixation, samples were dehydrated through a graded ethanol series, cleared with xylol, and immersed in paraffin. Longitudinal sections (3 μm thick) were cut and stained with hematoxylin and eosin. Images were captured using an Olympus BX 50 P1 optical microscope (4 × objective lenses) coupled to an Olympus PMC 35B digital camera.
Morphometric measurements were performed using the ImageJ® software (Schneider et al., 2012). Only epithelial sections with intact lamina propria were selected. For each specimen, 10 villi and 10 crypts were measured to determine the average villus height, villus width, and crypt depth, as shown in Figure 1 (Assis et al., 2021). The relationship between villus height and crypt depth was calculated from these parameters (Assis et al., 2021). Villus height was measured from the basal region coinciding with the upper part of the crypt to the apex of the villus. Crypt depth was measured from the basement membrane to the mouth of the crypt.
2.5 Heterophil/lymphocyte ratio
At 42 days of age, five birds per treatment were selected based on the average weight of the group, desensitized by asphyxiation with carbon dioxide, and euthanized by bloodletting. For analysis of heterophils, lymphocytes, and heterophil/lymphocyte (H/L) ratio, the right jugular vein was incised with a scalpel, and blood samples (5 mL) were collected into heparinized tubes. Samples were gently homogenized, and 10 μL aliquots were mounted, in duplicate, onto microscope slides. The slides were air dried, stained with Wright–Giemsa stain, and examined at 100× magnification under an optical microscope (Olympus BX50 P1) coupled to a digital camera (Olympus PCM 35B). Heterophil percentages, lymphocyte percentages, and H/L ratios were determined as described by Zhang et al. (2009).
2.6 mRNA quantification
Liver and jejunum samples (n = 5) were collected at the end of the experimental period and stored in RNA Holder solution (BioAgency Biotechnology, São Paulo, SP, Brazil) at −20°C until RNA extraction. Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's recommendations. RNA integrity was assessed by 1% agarose gel electrophoresis, followed by ethidium bromide (10 mg mL−1) staining and visualization under ultraviolet light. RNA samples were treated with DNase I (Invitrogen, Carlsbad, CA, USA) as per the manufacturer's instructions to eliminate DNA contamination. Complementary DNA (cDNA) synthesis was performed using the GoScript Reverse Transcription System (Promega, Madison, WI, USA) and 4 μL of DNase-treated RNA, according to the manufacturer's instructions. Total RNA and cDNA concentrations were determined by using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Pittsburg, PA, USA) at 260 nm.
Real-time PCR reactions were performed using SYBR™ Green PCR Master Mix (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's recommendations. Specific oligonucleotide primers were designed using the NCBI Primer-Blast tool. The following genes were analyzed: heat shock protein 70 kDa (HSP70), interleukin-1 beta (IL1B), occludin (OCLN), carnitine palmitoyltransferase I (CPT1), sterol regulatory element-binding protein 1 (SREBP1), and beta-actin (endogenous control) (Table 3).
| Gene | Amplicon2 (bp) | Primer sequence (5′-3′) | No. de acesso |
|---|---|---|---|
| β-actin gene | 136 | F-ACCCCAAAGCCAACAGA | L08165.1 |
| R-CCAGAGTCCATCACAATACC | |||
| HSP701 | 64 | F-CGGGCAAGTTTGACCTAA | NM_001006685.1 |
| R-TTGGCTCCCACCCTATCTCT | |||
| IL1B | 90 | F-GTCAACATCGCCACCTACAA | HM179638.1 |
| R- GGTTTCCATCTCGTATGTACCG | |||
| OCLN | 123 | F-ACGGCAGCACCTACCTCAA | D21837.1 |
| R-GGGCGAAGAAGCAGATGAG | |||
| CPT1 | 60 | F-GCCCTGATGCCTTCATTCAA | AY675193 |
| R-ATTTTCCCATGTCTCGGTAGTGA | |||
| SREBP1 | 82 | F-CATCCATCAACGACAAGATCGT | AY029224 |
| R-CTCAGGATCGCCGACTTGTT |
- Abbreviation: PCR, polymerase chain reaction.
- 1 CPT1, carnitine palmitoyltransferase I gene; HSP70, heat shock protein 70 kDa gene; IL1B, interleukin-1 beta gene; OCLN, occludin gene; SREBP1, sterol regulatory element-binding protein 1 gene.
- 2 Amplicon size (base pairs, bp).
HSP70, CPT1, and SREBP1 expression levels were assessed in the liver, and IL1B and OCLN expression was assessed in the small intestine (jejunum). The efficiency of each primer/gene set was evaluated by using a series of 25 μL reactions containing 5 μL of a serial dilution of pooled cDNA (80, 40, 20, and 10 ng μL−1). The thermocycler program for all genes was as follows: 95°C for 10 min, followed by 40 cycles of denaturation and annealing/extension at 95°C for 15 s and 60°C for 1 min. Dissociation curves were obtained to analyze the specificity of each primer.
The β-actin gene was used as the reference gene. The expression data of this gene were subjected to analysis of variance (ANOVA), and no significant effects of diet or environment were observed, confirming that the use of β-actin as an endogenous gene was adequate. All analyses were performed in duplicate in a total volume of 25 μL. Amplification efficiencies were similar between the endogenous gene and genes of interest, ranging from 90% to 100%. The 2−ΔCt method was used for gene expression analysis, according to Livak and Schmittgen (2001). Gene expression results are expressed in arbitrary units.
2.7 Statistical analysis
The data were subjected to one-way ANOVA. EO levels, heat stress, and comfort environments, as well as their interactions, were included in the mathematical model. When the effect of diet, environment, or their interaction was significant, means were compared by Tukey's test (p < 0.05). All data were analyzed using the GLM procedure of the SAS software (SAS Institute Inc., Cary, NC, USA). Results are expressed as mean and standard error.
3 RESULTS
3.1 Performance and relative organ weights
There was an interaction effect of diet and environment on body weight (p = 0.0486), feed conversion (p = 0.0013), and relative breast weight (p = 0.0459) (Table 4). For birds reared under thermal comfort conditions, there were no differences in body weight or relative breast weight between dietary treatments. However, birds fed the control diet showed better feed conversion than birds supplemented with turmeric EO, regardless of the inclusion level. For birds reared under heat stress conditions, the EO2 diet resulted in higher body weight and improved feed conversion than the unsupplemented diet. All EO treatments resulted in high relative breast weight compared with the control. There was an effect of the environment on feed intake. Reduced feed intake was observed in birds reared under heat stress compared with birds reared under thermal comfort conditions (p < 0.05) (Table 4).
| BW2 (g) | FI (g) | FCR | RBW (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM3 | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Thermal comfort | BD1 | 2657.11a | 13.52 | 3711.50 | 34.14 | 1.35c | 0.049 | 43.00a | 0.67 |
| EO1 | 2553.40ab | 31.84 | 3687.21 | 44.48 | 1.47ab | 0.011 | 41.72a | 0.77 | |
| EO2 | 2514.70ab | 39.55 | 3715.17 | 48.25 | 1.49ab | 0.021 | 41.27a | 0.91 | |
| EO3 | 2649.23a | 61.89 | 3776.33 | 33.47 | 1.47ab | 0.014 | 41.68a | 1.81 | |
| Heat stress | BD | 2237.66c | 31.89 | 3493.90 | 38.01 | 1.56a | 0.015 | 34.56b | 0.38 |
| EO1 | 2410.25bc | 15.65 | 3560.92 | 23.63 | 1.48ab | 0.012 | 41.72a | 1.76 | |
| EO2 | 2461.16b | 61.70 | 3613.22 | 72.83 | 1.44b | 0.015 | 39.16a | 0.95 | |
| EO3 | 2418.75bc | 51.78 | 3493.90 | 52.60 | 1.49ab | 0.014 | 38.90a | 0.88 | |
| Main effects | |||||||||
| Environment | Thermal comfort | 2582.77 | 22.48 | 3721.97a | 20.25 | 1.45 | 0.017 | 41.90 | 0.61 |
| Heat stress | 2386.60 | 22.64 | 3551.60b | 24.71 | 1.50 | 0.009 | 38.53 | 0.81 | |
| Diet | BD | 2447.38 | 59.47 | 3590.61 | 37.45 | 1.45 | 0.041 | 37.72 | 1.41 |
| EO1 | 2489.77 | 25.56 | 3628.92 | 29.66 | 1.47 | 0.008 | 41.72 | 0.99 | |
| EO2 | 2496.86 | 28.65 | 3664.19 | 43.40 | 1.48 | 0.014 | 40.06 | 0.70 | |
| EO3 | 2546.79 | 48.77 | 3652.54 | 43.88 | 1.45 | 0.010 | 40.29 | 1.16 | |
| Probabilities | |||||||||
| Environment | <0.0001 | <0.0001 | 0.0251 | 0.0034 | |||||
| Diet | 0.5631 | 0.7005 | 0.7709 | 0.2710 | |||||
| Interaction | 0.0486 | 0.4782 | 0.0013 | 0.0459 | |||||
- 1 BD, basal diet; EO1, basal diet supplemented with 100 mg kg−1 turmeric essential oil (low dose); EO2, basal diet supplemented with 200 mg kg−1 turmeric essential oil (intermediate dose); EO3, basal diet supplemented with 300 mg kg−1 turmeric essential oil (high dose).
- 2 BW, body weight; FI, feed intake; FCR, feed conversion ratio; RBW, relative breast weight.
- 3 SEM, standard error of mean.
- a,b,c,d Means followed by different superscript letters are significantly different from each other (p < 0.05).
Diet × environment effects were observed on relative spleen (p = 0.0017) and liver (p = 0.0105) weights (Table 5). In the thermal comfort group, EO2 resulted in the highest spleen weight, differing from the other diets. In the heat stress group, there were no significant differences in spleen weight between diets. By contrast, birds under thermal comfort conditions did not differ in liver weight, regardless of the diet. Among birds reared under heat stress, EO1 and EO3 diets resulted in the lowest liver weight.
| Thymus (%) | Bursa (%) | Spleen (%) | Heart (%) | Liver (%) | Intestine (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM2 | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Thermal comfort | BD1 | 0.721 | 0.03 | 0.072 | 0.010 | 0.119b | 0.003 | 0.085 | 0.01 | 2.309bc | 0.099 | 3.693 | 0.243 |
| EO1 | 0.749 | 0.05 | 0.088 | 0.003 | 0.108b | 0.009 | 0.089 | 0.003 | 2.306c | 0.147 | 3.399 | 0.032 | |
| EO2 | 0.693 | 0.04 | 0.061 | 0.014 | 0.175a | 0.015 | 0.094 | 0.03 | 2.288c | 0.084 | 3.673 | 0.170 | |
| EO3 | 0.619 | 0.16 | 0.103 | 0.004 | 0.123b | 0.008 | 0.093 | 0.01 | 2.348bc | 0.082 | 3.383 | 0.184 | |
| Heat stress | BD | 0.391 | 0.16 | 0.068 | 0.005 | 0.108b | 0.011 | 0.086 | 0.01 | 2.976a | 0.153 | 3.292 | 0.098 |
| EO1 | 0.632 | 0.02 | 0.102 | 0.007 | 0.126b | 0.007 | 0.102 | 0.007 | 2.175c | 0.062 | 3.014 | 0.226 | |
| EO2 | 0.624 | 0.03 | 0.087 | 0.008 | 0.103b | 0.007 | 0.077 | 0.01 | 2.669ab | 0.129 | 3.436 | 0.140 | |
| EO3 | 0.533 | 0.13 | 0.119 | 0.007 | 0.115b | 0.051 | 0.119 | 0.007 | 2.270c | 0.062 | 3.160 | 0.145 | |
| Main effects | |||||||||||||
| Environment | Thermal comfort | 0.659 | 0.04 | 0.081b | 0.02 | 0.131 | 0.007 | 0.090b | 0.009 | 2.492 | 0.12 | 3.512a | 0.010 |
| Heat stress | 0.545 | 0.05 | 0.097a | 0.03 | 0.113 | 0.005 | 0.096a | 0.005 | 2.315 | 0.09 | 3.237b | 0.020 | |
| Diet | BD | 0.556 | 0.09 | 0.067c | 0.02 | 0.115 | 0.008 | 0.085 | 0.008 | 2.641 | 0.21 | 3.443 | 0.138 |
| EO1 | 0.690 | 0.03 | 0.095ab | 0.03 | 0.117 | 0.007 | 0.095 | 0.004 | 2.249 | 0.08 | 3.227 | 0.130 | |
| EO2 | 0.659 | 0.02 | 0.074bc | 0.03 | 0.139 | 0.013 | 0.085 | 0.01 | 2.478 | 0.14 | 3.541 | 0.129 | |
| EO3 | 0.576 | 0.10 | 0.112a | 0.03 | 0.119 | 0.005 | 0.106 | 0.007 | 2.314 | 0.06 | 3.271 | 0.130 | |
| Probabilities | |||||||||||||
| Environment | 0.5475 | 0.0368 | 0.0196 | 0.0381 | 0.0226 | 0.0368 | |||||||
| Diet | 0.4209 | 0.0002 | 0.2909 | 0.4722 | 0.0190 | 0.1154 | |||||||
| Interaction | 0.6940 | 0.9759 | 0.0017 | 0.7384 | 0.0105 | 0.7242 | |||||||
- 1 BD, basal diet; EO1, basal diet supplemented with 100 mg kg−1 turmeric essential oil (low dose); EO2, basal diet supplemented with 200 mg kg−1 turmeric essential oil (intermediate dose); EO3, basal diet supplemented with 300 mg kg−1 turmeric essential oil (high dose).
- 2 SEM, standard error of mean.
- a,b,c,d Means within columns followed by different superscript letters are significantly different from each other (p < 0.05).
The environment significantly influenced relative weights of the bursa of Fabricius (p = 0.0368), heart (p = 0.0381), and intestine (p = 0.0368) (Table 5). Bursa and heart weights were higher in heat-stressed birds, whereas intestine weight was higher in unstressed birds. Diet influenced the relative weight of the bursa of Fabricius (p = 0.0002) (Table 5), which was higher in birds fed the EO3 diet. There was no effect of the environment, diet, or their interaction on the relative weight of the thymus.
3.2 Jejunal histomorphometry
Diet × environment had a significant effect on villus width (p = 0.0487) and villus/crypt ratio (p = 0.0449) (Table 6). Among unstressed birds, those fed the EO2 diet had lower villus width than unsupplemented birds. In the heat stress group, villus width was lower in birds supplemented with turmeric EO, regardless of the level. Villus/crypt ratio did not differ between diets among birds reared under thermal comfort conditions. However, among heat-stressed birds, turmeric EO supplementation increased the villus/crypt ratio. The environment had an effect on villus height, which was lower in heat-stressed birds than in unstressed birds (p = 0.0010) (Table 6).
| Villus height (μm) | Villus width (μm) |
Crypt depth (μm) | villus/crypt ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM2 | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Thermal comfort | BD1 | 1315.87 | 7.440 | 112.89b | 2.81 | 175.44 | 12,54 | 6.22bc | 0.76 |
| EO1 | 1435.56 | 5.214 | 88.28bc | 3.86 | 193.79 | 12.80 | 6.04bc | 0.81 | |
| EO2 | 1351.55 | 7.500 | 79.45c | 5.19 | 379.94 | 19.08 | 6.65bc | 0.97 | |
| EO3 | 1324.77 | 0.946 | 84.06bc | 7.19 | 161.35 | 8.20 | 5.94bc | 0.08 | |
| Heat stress | BD | 825.77 | 4.591 | 174.11a | 14.30 | 185.92 | 10.30 | 4.78c | 0.51 |
| EO1 | 855.27 | 2.194 | 95.75bc | 11.70 | 116.61 | 8.59 | 9.40a | 1.14 | |
| EO2 | 1148.64 | 5.353 | 102.25bc | 8.70 | 173.82 | 23.83 | 8.16ab | 0.80 | |
| EO3 | 1103.13 | 3.828 | 104.74bc | 8.40 | 161.70 | 26.08 | 7.88ab | 0.91 | |
| Main effects | |||||||||
| Environment | Thermal comfort | 1359.86a | 45.65 | 91.43 | 3.63 | 231.12 | 49.54 | 6.19 | 0.34 |
| Heat Stress | 992.93b | 60.14 | 122.82 | 9.15 | 163.84 | 10.20 | 7.42 | 0.55 | |
| Diet | BD | 1035.81 | 91.45 | 146.91 | 12.51 | 180.68 | 7.75 | 5.50 | 0.49 |
| EO1 | 1103.96 | 148.41 | 91.08 | 5.02 | 164.85 | 14.71 | 7.53 | 0.85 | |
| EO2 | 1224.73 | 79.11 | 92.12 | 6.19 | 288.33 | 101.73 | 7.40 | 0.64 | |
| EO3 | 1177.01 | 41.77 | 95.88 | 6.20 | 161.52 | 12.49 | 6.91 | 0.56 | |
| Probabilities | |||||||||
| Environment | 0.0010 | 0.0004 | 0.2444 | 0.0314 | |||||
| Diet | 0.5577 | <0.0001 | 0.4133 | 0.0554 | |||||
| Interaction | 0.4169 | 0.0487 | 0.5017 | 0.0449 | |||||
- 1 BD, basal diet; EO1, basal diet supplemented with 100 mg kg−1 turmeric essential oil (low dose); EO2, basal diet supplemented with 200 mg kg−1 turmeric essential oil (intermediate dose); EO3, basal diet supplemented with 300 mg kg−1 turmeric essential oil (high dose).
- 2 SEM, standard error of mean.
- a,b,c,d Means followed by different superscript letters are significantly different from each other (p < 0.05).
3.3 Heterophil and lymphocyte parameters
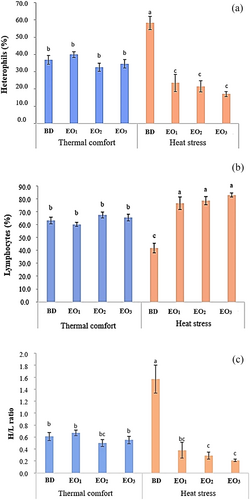
There were interaction effects on heterophil percentage (Figure 2(a); p < 0.0001), lymphocyte percentage (Figure 2(b); p < 0.0001), and H/L ratio (Figure 2(c); p < 0.0001). Among unstressed birds, diet did not influence heterophil percentage, lymphocyte percentage, or H/L ratio. Among heat-stressed birds, EO-supplemented diets (EO1, EO2, and EO3) reduced heterophil percentage and H/L ratio and increased lymphocyte percentage.
3.4 Gene expression
HSP70 (p = 0.0011), OCLN (p = 0.0174), and SREBP1 (p = 0.0174) expressions were influenced by the interaction effect of diet and environment (Table 7). Among birds reared under thermal comfort conditions, HSP70 expression was lower in animals fed EO2 and control diets. Under heat stress conditions, all supplemented diets resulted in reduced HSP70 expression compared with the control diet. In the heat stress group, OCLN expression was higher in birds fed EO1 and EO2 diets. In the thermal comfort group, SREBP1 expression did not differ according to diet. However, under heat stress, birds fed EO1 and EO2 diets had reduced SREBP1 expression. The environment had a significant effect on IL1B (p = 0.0372) and CPT1 (p = 0.0047) expressions, which were higher in birds subjected to heat stress (Table 7).
| HSP702 | IL1B | OCLN | CPT1 | SREBP1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM3 | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Thermal comfort | BD1 | 0.860c | 0.16 | 1.176 | 0.30 | 0.908a | 0.13 | 0.917 | 0.25 | 1.048c | 0.19 |
| EO1 | 1.263b | 0.16 | 1.048 | 0.31 | 0.605b | 0.10 | 0.717 | 0.23 | 2.186bc | 0.39 | |
| EO2 | 0.815c | 0.16 | 1.066 | 0.11 | 0.664b | 0.11 | 0.640 | 0.12 | 1.344bc | 0.39 | |
| EO3 | 1.467b | 0.23 | 1.232 | 0.30 | 1.004a | 0.15 | 0.886 | 0.25 | 2.234bc | 0.67 | |
| Heat stress | BD | 5.030a | 0.84 | 2.054 | 0.47 | 0.706b | 0.11 | 2.687 | 0.54 | 3.910a | 0.65 |
| EO1 | 2.292b | 0.39 | 1.276 | 0.18 | 1.254a | 0.14 | 0.956 | 0.37 | 1.742bc | 0.30 | |
| EO2 | 2.010b | 0.71 | 1.500 | 0.27 | 1.017a | 0.14 | 1.253 | 0.26 | 2.356bc | 0.55 | |
| EO3 | 1.220b | 0.57 | 1.537 | 0.07 | 0.774b | 0.25 | 1.230 | 0.37 | 2.564ab | 0.45 | |
| Main effects | |||||||||||
| Environment | Thermal comfort | 1.063 | 0.11 | 1.125b | 0.12 | 0.815 | 0.07 | 0.787b | 0.10 | 1.703 | 0.24 |
| Heat stress | 2.518 | 0.34 | 1.600a | 0.15 | 0.957 | 0.09 | 1.422a | 0.21 | 2.622 | 0.29 | |
| Diet | BD | 2.250 | 0.63 | 1.615 | 0.30 | 0.932 | 0.08 | 1.675 | 0.33 | 2.320 | 0.56 |
| EO1 | 1.851 | 0.24 | 1.162 | 0.17 | 0.965 | 0.13 | 0.850 | 0.22 | 1.989 | 0.25 | |
| EO2 | 1.327 | 0.44 | 1.258 | 0.15 | 0.792 | 0.09 | 0.870 | 0.16 | 1.850 | 0.36 | |
| EO3 | 1.368 | 0.29 | 1.385 | 0.15 | 0.909 | 0.15 | 1.058 | 0.22 | 2.399 | 0.39 | |
| Probabilities | |||||||||||
| Environment | <.0001 | 0.0372 | 0.6408 | 0.0047 | 0.0110 | ||||||
| Diet | 0.0072 | 0.3991 | 0.5456 | 0.9087 | 0.4676 | ||||||
| Interaction | 0.0011 | 0.9923 | 0.0174 | 0.9519 | 0.0174 | ||||||
- 1 BD, basal diet; EO1, basal diet supplemented with 100 mg kg−1 turmeric essential oil (low dose); EO2, basal diet supplemented with 200 mg kg−1 turmeric essential oil (intermediate dose); EO3, basal diet supplemented with 300 mg kg−1 turmeric essential oil (high dose).
- 2 CPT1, carnitine palmitoyltransferase I gene; HSP70, heat shock protein 70 kDa gene; IL1B, interleukin-1 beta gene; OCLN, occludin gene; SREBP1, sterol regulatory element-binding protein 1 gene.
- 3 SEM, standard error of mean.
- a,b,c,d Means followed by different superscript letters are significantly different from each other (p < 0.05).
4 DISCUSSION
The results of interaction effects between factors (diet × environment) provide insight into the effect of turmeric EO on regulation of lipid metabolism, as well as on intestinal integrity and immune response. These effects are closely related to the recovery of performance observed in supplemented birds subjected to heat stress. The next sections present the results of parameters influenced by interaction effects. Of note, birds reared under thermal comfort conditions did not show improvements stemming from turmeric EO supplementation. Therefore, the discussion is focused on the results of heat-stressed birds.
4.1 Performance parameters
It is well known that heat stress alters broiler physiology, consequently affecting productive performance, growth performance, breast muscle mass, and meat yield (Nawaz et al., 2021). Interestingly, in the current study, supplementation of heat-stressed birds with an intermediate level of turmeric EO led to improvements in body weight and feed conversion, as compared with non-supplementation. These results are in agreement with those of previous studies using turmeric extract on heat-stressed broilers (Akhavan-Salamat & Ghasemi, 2016; Baghban et al., 2016; El-Maaty et al., 2014; Sadeghi & Moghaddam, 2018). In the cited studies, turmeric extract was used in powdered form at higher doses (0.5%). It is possible to infer, therefore, that turmeric EO has greater biological power than turmeric extract powder. This result can be explained by the fact that, overall, dietary supplementation with herb EOs can enhance digestive enzyme secretion, thereby improving feed digestibility and broiler performance (Al-Kassie et al., 2011). Furthermore, this improvement can be attributed to the active components of EOs, which have antimicrobial, antifungal, and antioxidant properties, contributing to nutrient utilization (Al-Sultan, 2003; Osawa et al., 1995; Radwan et al., 2008).
The negative effect of heat stress on breast weight was minimized by supplementation with turmeric EO, in agreement with the improvements in body weight and feed conversion. These results are important considering that skeletal muscles account for 40–60% of the total body weight and play a crucial role in movement, respiration, and homeostasis (Yin et al., 2014), in addition to having significant economic importance in the food industry.
Combined with these results, the increased HSP70 expression underscored the potential of turmeric EO in attenuating the response to thermal stress and protecting cells against damage. It is well known that under various stress conditions, the adaptive synthesis of stress-inducible HSP70 increases the ability of stressed cells to maintain proteostasis when faced with high concentrations of unfolded or denatured proteins (Balogi et al., 2019; Clerico et al., 2019; Fernández-Fernández & Valpuesta, 2018; Mayer & Gierasch, 2019). In birds under heat stress, turmeric EO supplementation led to downregulation of the gene. It is likely that EO supplementation, even at the lowest level, caused a reduction in protein damage in heat-stressed birds, allowing them to more efficiently direct metabolic resources toward growth and production. Surai (2013) found similar results when supplementing broiler chickens subjected to cyclic heat stress with Curcuma xanthorrhiza EO: HSP70 mRNA levels in the heart were lower in supplemented birds (400 mg kg−1) than in control birds (heat stress, basal diet).
4.2 Lipid metabolism and inflammatory response
In our study, the relative liver weight of broilers not supplemented with turmeric EO was higher in the heat stress group than in the thermal comfort group. This phenomenon was reported by researchers who assessed broilers under continuous heat stress (Chen et al., 2021; Hosseini-Vashan et al., 2019; Ma et al., 2022). As discussed by Lu et al. (2019), the abnormal liver weight caused by heat stress can be attributed to accelerated fat accumulation and hypertrophy of the liver, as a means to compensate for the decline in organ function.
CPT1 and SREBP1 expressions were evaluated to gain a deeper understanding of the effects of diet and environment on lipid metabolism. CPT1 expression is related to regulation of lipid oxidation, and SREBP expression is associated with the anti-inflammatory response to lipid metabolism. Previous studies indicated that SREBP1 activation can directly promote the transcription of lipogenic enzyme genes (Abu-Elheiga et al., 2001; Ishii et al., 2004). It is well known that broiler exposure to heat stress increases fat synthesis in the liver and abdominal fat deposition (Liu et al., 2019; Yin et al., 2021). Some researchers also observed that under heat stress, the fat content of intramuscular, subcutaneous, and abdominal adipose tissues increased in broilers (De Antonio et al., 2017; Lu et al., 2019; Zhang et al., 2012). The findings suggest induction of lipogenesis in animals under heat stress, as there was a significant increase in SREBP1 expression, which is associated with lipid synthesis. The reduction in SREBP1 expression in heat-stressed broilers supplemented with EO1 or EO2 reveals the potential of turmeric EO to promote a negative regulation of lipid metabolism. In other words, turmeric EO compounds play a role in optimizing energy use, which would contribute to improved feed conversion and increased breast weight. Furthermore, the regulation of lipid metabolism may be associated with the reduction of hepatic stress, contributing to metabolic balance.
4.3 Intestinal barrier integrity
In addition to the effects on lipid and hepatic metabolism, turmeric EO showed potential in restoring the integrity of the intestinal barrier. The analysis was performed by taking measurements of the jejunal portion of the intestine. The jejunum is the site of maximum intestinal nutrient absorption and digestion (Aderibigbe et al., 2020). The intestinal mucosa develops via the increase in villus height and density, and villi are constantly renewed by intestinal crypts. If a nutrient halts or decreases cell proliferation, the villus height is reduced and, consequently, nutrient digestion and absorption are hampered (Macari, 1998). Heat stress affects the intestinal morphology by reducing villus height and increasing villus width and crypt depth. In the present study, the effect of heat stress on villus width and villus/crypt ratio was mitigated by supplementation with turmeric EO, even at the lowest level. Rajput et al. (2013) observed that supplementation with curcumin reduced villus width and increased villus height and absorption area in broilers. Here, turmeric EO probably caused an increase in epithelial cell renewal in this segment of the small intestine and might even have played a role in the activation of cell mitosis (Samanya & Yamauchi, 2002).
Villus/crypt ratio is an indicator of the digestive capacity of the small intestine, with a higher ratio indicating increased digestion and absorption capacity (Montagne et al., 2003). Under heat stress, a decrease in villus/crypt ratio may indicate higher migration rate of enterocytes (crypt cells) to villi (Silva et al., 2009). In the current study, the villus/crypt ratio in heat-stressed EO-supplemented birds was higher than that of birds reared under thermal comfort conditions. This may demonstrate the ability of turmeric EO to heal intestinal injuries resulting from continuous thermal stress.
The increase in villus/crypt ratio agrees with the improvement in OCLN gene expression, which is also indicative of intestinal architecture restoration. Occludin is a tight junction protein that acts in the intestinal barrier, separating the host tissue from luminal components to maintain homeostasis. Heat stress was shown to impair the structure of the small intestine and decrease the expression of genes related to tight junctions in broilers (He et al., 2019). In agreement with literature data, it was found that OCLN expression was lower in heat-stressed birds as compared with birds reared under thermal comfort conditions. In heat-stressed birds, supplementation with EO1 and EO2 led to an increase in OCLN expression, suggesting a recovery in the integrity of the intestinal barrier. Specific components of turmeric EO may have positively influenced gene expression related to the maintenance of the intestinal mucosa, favoring the efficient absorption of nutrients, even under heat stress.
4.4 Immune response
The ability of turmeric EO to preserve the integrity of the intestinal mucosa may be related to the modulation of inflammation associated with heat stress. In this study, heterophil and lymphocyte levels were determined to assess the immune response of broilers. It is known that energy stress can suppress broiler immunity by elevating heterophils while reducing lymphocytes, leading to an increase in H/L ratio (Cheng et al., 2001). The H/L ratio is used as an indicator of stress in several animal species, including birds (Felver-Gant et al., 2012). Here, in heat-stresses birds, supplementation with turmeric EO, even at the lowest level, restored lymphocyte and heterophil levels and H/L ratio to levels comparable to those in non-stressed birds. It was previously reported that turmeric prevents the rise of corticosterone in the bloodstream of heat-stressed broiler chickens (Swathi et al., 2016) while increasing the activity of antioxidant enzymes and anti-inflammatory molecules (El-Maaty et al., 2014). Akhavan-Salamat and Ghasemi (2016) and Swathi et al. (2016) supplemented heat-stressed chickens with 0.4% turmeric powder and observed an improvement in humoral immune response, H/L ratio, and immunoglobulin antibody titers.
Our findings support the hypothesis that turmeric EO is efficient in reestablishing or improving organic homeostasis in broilers reared under heat stress conditions. The results for intestinal integrity/function could explain the enhanced performance of EO-supplemented broilers under heat stress. The exact mechanisms by which EO exerts these effects under heat stress conditions deserve further investigation, but the results provide insight into its potential to improve broiler resilience and performance under challenging conditions.
ACKNOWLEDGMENTS
The authors would like to thank the Departments of Animal Science and Agronomy and the Graduate Program in Agriculture and Biodiversity (PPGAGRI) of the Federal University of Sergipe (UFS), Brazil, for the technical support; Delacon Biotechnik Ges.m.b.H. (Steyregg, Austria) for donating the EO; the Brazilian National Council for Scientific and Technological Development (CNPq, grant no. 403205/2021-2) for the financial support; and the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES, Finance Code 001) for the scholarship awarded to Maise dos Santos Macário.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




