Comparative analysis of the merino sheep and Iberian red deer abomasum during prenatal development
Abstract
The aim of this study is to describe differences in the ontogenesis of the abomasum in sheep (domestic ruminant) and deer (wild ruminant). Histomorphometric and immunohistochemical analysis were carried out on 50 embryos and fetuses of the sheep and 50 red deer from the first prenatal stages until birth. To compare similar periods of gestation in both species, we calculate the percentages of gestation. The appearance of the abomasum was earlier in the red deer (22% gestation) than in the sheep (25% gestation). Throughout development the epithelium happened sequentially, being of the types pseudostratified to simple cylindrical. This important modification was earlier in the red deer than the sheep. At 46% gestation in red deer and 50% in sheep, gastric pits were observed on the surface of abomasal folds. Our studies suggest a close link between the initial formation of these pseudoglandular structures and the clear separation of lamina propria and submucosa separated by de muscularis mucosae. At 54% gestation in red deer and at 60% in sheep, in the bottom of these pits the first outlines of glands were distinguishable. Finally, the presence of neuroendocrine and glial cells were detected in deer at earlier stages than in sheep.
Introduction
The ruminant stomach is particularly remarkable for its ability to transform low-quality forage into products of great nutritional value (García et al. 2013a,b,c, 2014a). Ruminants can be classified according to their feeding habits as grazers: concentrate selectors (red deer) and intermediate feeders (sheep) (Hofmann 1973). These different feeding types are reflected in distinct anatomical properties of the forestomach and stomach (Hofmann 1973). Sheep and red deer have a phylogenetic adaptation due to the difference in the acquisition of the nutrients (intermediate feeders vs. concentrate selector; Münnich et al. 2008), which has relevance for this study.
The stomach of sheep embryos has been subjected to numerous morphologic studies, including immunohistochemical and morphometric analyses (Franco et al. 1992, 1993a,b,c; Regodon et al. 1996). The hunting of red deer constitutes one of the main economic uses of Mediterranean forests and ‘dehesas’ (open oak forests) in the south-west of the Iberian Peninsula. The management of its population has undergone a notable evolution in recent years (Carranza et al. 2009). The traditional system of intensive farming methods is gradually being replaced by a mixed system of intensive/semi-intensive methods, in which new management methods require intervention in many areas, food being the area of most concern here. Food supplements, on the one hand, give rise to ecological and behavioral changes in the species, because the addition of food in winter can result in a large increase in the number of animals that an area can support. Moreover, the period of shortage in the southernmost areas coincides with the rutting season. On the other hand, added to the undesirable ecological effects from the spatial concentration of food, are the changes that result from such supplements on the histophysiology of the red deer stomach (Franco et al. 2004a,b; Redondo et al. 2005; Masot et al. 2007a,b).
The abomasum is the fourth and final gastric compartment of the stomach in ruminants, the glandular portion that is equivalent to the true stomach in monogastric animals (Nickel et al. 1973). Their main function is to prepare food for digestion by secreting gastric juice (Age et al. 2007, 2008). Like the stomach of other mammals, the abomasum contains gastric pits and glands (García et al. 2013a, 2014a). Several endocrine cell groups are found in the abomasal mucosa, including gastrin-producing cells. Gastrin is one of the major hormones in the gastrointestinal endocrine system. Among other things, it is essential for the normal growth of the gastrointestinal mucosa (Franco et al. 1993b,d), so that any disruption in gastrin production may give rise to digestive disorders (García et al. 2013a).
The objectives of this study were: (i) to provide a sequential description of the histochemical and histological structures of sheep and red deer abomasum, in order to analyze the differentiation of this compartment from the primitive gastric tube, and determine its histological evolution during intrauterine life; (ii) to analyze morphometrically the evolution of the integral layers of both abomasal walls during embryogenesis; (iii) to immunodetect the reaction of the abomasal wall of sheep and red deer in embryonic development toward neuroendocrine cell markers (synaptophysin (SYP)), glial cell markers (glial fibrillary acidic protein (GFAP) and vimentin (VIM)) and markers of peptidergic innervation (neuropeptide Y (NPY) and vasoactive intestinal peptide (VIP)); and (iv) to determine the distribution of gastrin cells in the abomasum, and the plasma gastrin levels, in both species during prenatal development.
Material and Methods
Animals
Embryos and fetuses of sheep (n = 50) and red deer (n = 50) from the first prenatal stages until birth were sampled. To compare similar periods of gestation in both species, we calculated the percentages of gestation. The specimens were divided into five groups, according to the most relevant histomorphogenic characteristics: Group I, sheep (0.4–2.6 cm crown-rump length (CRL), 23–37 days: 1–25% gestation), red deer (1.4–3.6 cm CRL, 30–60 days: 1–25% gestation); Group II, sheep (4–8 cm CRL, 39–52 days: 26–35% gestation), red deer (4.5–7.2 cm CRL, 63–85 days, 26–35% gestation); Group III, sheep (8.5–19 cm CRL, 54–75 days: 36–50% gestation), red deer (8–19 cm CRL, 86–120 days, 36–50% gestation); Group IV, sheep (20–3.5 cm CRL, 77–112 days: 51–75% gestation), red deer (21–33 cm C-R, 123–180 days, 51–75% gestation).; Group V, sheep (32–40 cm CRL, 114–150 days: 76–100% gestation), red deer (36–40 cm CRL, 183–240 days, 76–100% gestation). Each experimental group consisted of 10 specimens, divided into five females and five males. The merino breed ovine embryos and fetuses were all obtained at municipal slaughterhouse in Caceres (Spain) from pregnant females. These pregnant females were slaughtered by the usual process in the slaughterhouse, where the embryos and fetuses were obtained after opening the abdominal cavity and uterus. These actions were carried out in accordance with the regulation required for the protection of animals at the time of slaughter in slaughterhouses (Spanish Royal Decree 54/1195). Gestational age was estimated following Evans and Sack (1973) and Sivachelvan et al. (1996), as well as in the light of age classifications previously reported for sheep (Franco et al. 1992) and red deer (Franco et al. 2004a,b, 2012). The red deer females were hunted in legal shootings in 10 hunting grounds from extensive and non-enclosed estates from the Sierra de San Pedro (north-east of the Province of Cáceres, Spain). The estimation of gestation age was carried out according to the methodology proposed by Evans and Sack (1973).
Sampling and processing
All fetuses were processed for histological and immunohistochemical analyses. Once the abomasum had been separated, small pieces of tissue were dissected from the cardiac, fundic and pyloric regions of the abomasum of each animal. The tissue for histological study was fixed in 4% buffered formaldehyde for 24 h, processed by conventional paraffin-embedding methods, and sections 4 μm thick were cut in a transverse direction and treated with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS, pH 7.2) and PAS-Alcian blue (PAS-AB, pH 4.2), Mayer's mucicarmin (MM), Von Giesson (VG), Masson's Trichrome (MT) and reticuline of Gomori (RG).
Immunocytochemical analysis
The UltraVision One HRP polymer (polymer conjugated to horseradish peroxidase) was performed on tissue from the abomasum to detect the neuroendocrine cell marker with SYP, the glial cell marker with GFAP and VIM, markers of peptidergic innervation as NPY and VIP and gastrin. Tissues were deparaffinized and hydrated. Recovery of antigens was performed with citrate and microwave. Blocking of endogenous peroxidase activity was made with 0.5% hydrogen peroxide for 30 min. Non-specific tissue-binding sites were blocked by incubation in 1% normal goat serum for 30 min. Samples were incubated with the following primary antisera: 1:10 mouse monoclonal anti-SYP (MA1-35810; Thermo Scientific, Waltham, MA, USA); ready to use rabbit polyclonal anti-GFAP (RB-087-R7; Thermo Scientific), ready to use mouse monoclonal anti-VIM (MS-129-R7; Thermo Scientific), 1:50 rabbit polyclonal anti-NPY (PA1-41576; Thermo Scientific) and 1:50 rabbit polyclonal anti-VIP (AbD serotec, 9535-0204) and gastrin (PA1-36073) for 30 min at room temperature. Sections were finally incubated with polymer conjugated to HRP (UltraVision ONE HRP Polymer, TL-015-PHJ; Thermo Scientific) for 30 min at room temperature and without exposure to light. After that, diaminobenzidine was applied in the tissue (DAB Plus Chromogen TA-001-HCX and DAB Plus Substrate TA-015-HSX; Thermo Scientific) for 5–15 min, depending on the desired stain intensity. Finally, the reaction was contrasted with Mayer hematoxylin. The specificity of the staining reaction was determined in control experiments involving either substitution of the primary antibody by phosphate-buffered saline or normal goat serum 1:100, or omission of both primary and secondary antibodies. Absorption controls were obtained by incubating sections adjacent to those above with antiserum that contained 25 μg of Ag/mL of diluted antiserum. The antigens used were SYP (33R-6191; Fitzgerald, Acton, MA, USA), GFAP protein (30R-AG009; Fitzgerald), VIM (30R-2137; Fitzgerald), NPY (22465; AnaSpec, Seraing, Belgium) and VIP (22873; AnaSpec). No staining was found in structures on the sections which served as absorption controls. Immunolabeled sections were analyzed using the NIS-Elements 2.30 software package (Barcelona, Spain). The immunostaining surface was measured for various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis, serosa and myenteric plexus) and for the whole wall. Optimal intervals were performed statistically and four immunoreactivity density categories were established (García et al. 2014a,b): no immunoreactivity: no surface staining; low immunoreactivity: stained surface less than 200 μm2; moderate immunoreactivity: stained surface between 200 and 400 μm2; intense immunoreactivity: stained surface over 400 μm2.
Morphometric analysis
Small pieces of abomasum were embedded in paraffin, stained with H&E, and viewed through a microscope (NIKON Eclipse 80i, Barcelona, Spain) equipped with a digital video camera (NIKON DXMI200F). The computerized image was analyzed using the Nis-Element 2.30 software package. The variables studied were height of various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis and serosa) and total wall thickness. One hundred measurements were made in each tissue layer of each of the selected individuals from each group.
The results are shown as the mean ± SE (standard error). The data were analyzed using analysis of variance (anova). In cases where anova was significant, a post hoc (Tukey) analysis was carried out in order to study the significant differences among the distinct tissue strata in the two species and among the distinct groups. A value of P = 0.05 was considered significant.
Scanning electron microscopy
Small pieces of abomasum were fixed in 2.5% buffered glutaraldehyde for 24 h, dehydrated through graded ethanol and amyl acetate, and dried in a critical-point dryer. Sections were covered with coating materials including gold and examined and photographed with a Jeol JSM 6300 scanning electron microscope (Peabody, MA, USA) operating at 30 Kv at various tilt angles and at a magnification of 10–800×.
Radioimmunoassay
Forty blood samples, two per age group and specie, were obtained for measurement of plasma gastrin concentration to study the relationship between the gastrin concentration and gastrin cells appearance during abomasum development. The blood samples, obtained by venepuncture of the umbilical vein were centrifuged at 3000 × g for 6 min. The serum samples were stored at −20°C and analyzed using a Beckmann 1801 liquid scintillation counter following the method of Avila et al. (1989). The antibody used was 125I-gastrin (Human Synthetic Gastric; DAKO/AS, Madrid, Spain). This antibody recognizes the C-terminal end of gastrins larger than the pentapeptides (gastrins 17 and 34) and was used at a final concentration of 1:6 × 105 mol/L. Values (mean ± SE) are expressed in pg/mL. This assay is capable of detecting gastrin concentrations as low as 2 pg/mL and has inter- and intra-assay coefficients of variation of 7.8 and 1.2, respectively.
Results
Abomasal histomorphogenesis
Group I: sheep (0.4–2.6 cm CRL, 23–37 days: 1–25% gestation), red deer (1.4–3.6 cm CRL, 30–60 days: 1–25% gestation)
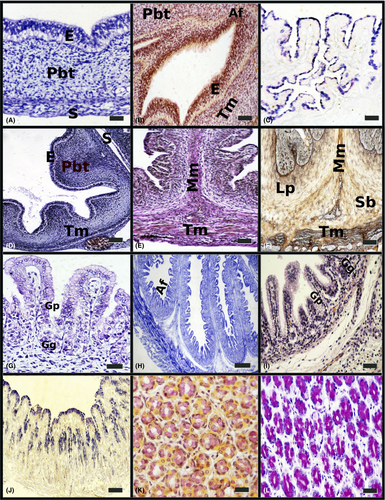
In the red deer at 53 days (22% gestation) the outline of the abomasal compartment appeared. This individualization in the sheep was observed later, at 37 days (25% gestation). In both species, the abomasal wall appeared as three well-defined layers (Fig. 1a). The internal epithelial layer was pseudostratified and showed a clear anuclear basal area and a dark apical area formed by the nuclei of the cells. The middle layer, or pluripotential blastemic tissue, barely increased its thickness, although morphologically fusiform myoblastic cells of longitudinal orientation began to be observed. It was more defined in the sheep, constituting the ontogenetical base for the future tunica muscularis. The external layer or serosa was formed by a mesothelium of flat cells and a lax connective tissue of cellular support.
Group II: sheep (4–8 cm CRL, 39–52 days: 26–35% gestation); red deer (4.5–7.2 cm CRL, 63–85 days, 26–35% gestation)
- The epithelium was of pseudostratified nature; so, although the nuclei were getting ready at different levels, all the cells were contacting with the lamina basal, for at 63 days (26% gestation) in red deer and to 53 days (35%) in sheep, to be constituted like cylindrical simple epithelia, with secretion capacity as for acid mucopolysacharides (Fig. 1c).
- The pluripotential blastemic tissue was separated from the epithelium by a clearly definided basal membrane. This tissue participated in the constitution of the primitive abomasal folds, in infiltrating toward the epithelium and putting pressure on the basal area. Its intense vascularization, accompanied with a large quantity of fibers of collagen, elastic and mainly reticulin fibers, marked the beginning of its differentiation in the lamina propria and submucous, that happened up to 63 days (26% of gestation) in red deer and to 53 days, 35% in sheep. At around 26% of gestation in both species, began the primitive differentiation of the tunica muscularis (Fig. 1d), consisting of the appearance of two layers of myoblasts with different orientations in the thickness of the mesenchymal connective tissue: an internal circular layer and an external and thinner, longitudinal layer. This primitive tunica muscularis showed a series of undulations in the base of implantation of the most developed abomasal folds and that will later form the future muscularis mucosae.
- The serosa was formed by an epithelium of flat cells and a highly cellular tissue or subserosa.
Group III: sheep (8.5–19 cm CRL, 54–75 days: 36–50% gestation); red deer (8–19 cm CRL, 86–120 days, 36–50% gestation)
In both species, the abomasal folds were more numerous and underwent a sharp increase in both length and width. They began to display a series of bumps on the surface: these were the primordial peak areas of the abomasal folds (Fig. 1e). Between the lamina propria and the submucosa the muscularis mucosae was much more defined, consisting of 2–3 layers of smooth muscular fibres originating from the internal fascicle of the circular internal bundle and protunding into the greater folds (Fig. 1e,f).
At around 50% of gestation, the space separating abomasal areas was much greater, giving rise to the formation of gastric pits; they appeared in the spaces between them. At the bottom of these pits the first outlines of glands, formed by several layers of cells irregularly tidy, could be distinguished (Fig. 1g). The epithelial layer was a simple mucous cubic to columnar in shape. Alcian blue staining revealed positive cells in superficial areas of the epithelium and in pseudoglandular structures of the lamina propria and submucosa.
Group IV: sheep (20–3.5 cm CRL, 77–112 days: 51–75% gestation); red deer (21–33 cm C-R, 123–180 days, 51–75% gestation)
In both species the fetuses showed a strong increase in length of the abomasal folds, simultaneously decreasing in thickness. These folds were very elongated and upholstered by abomasal areas on their entire surface (Fig. 1h). After continuous division of the folds, initiated in the previous stage, the whole surface was covered by a typical glandular epithelium (simple cylindrical mucous epithelium, formed by elongated cells with the nucleus placed in the normal area, Fig. 1i). This fact happened at 144 days (60% gestation) in red deer and at 81 days (54%) in sheep. In both species, the gastric glands appeared constituted by a cellular group about a central light. Alcian blue staining revealed positive cells in superficial areas of the epithelium and in glandular structures of the lamina propria and submucosa (Fig. 1j).
Group V: sheep (32–40 cm CRL, 114–150 days: 76–100% gestation); red deer (36–40 cm CRL, 183–240 days, 76–100% gestation)
In both species the mucosal surface of the abomasum showed small depressions of gastric pits, which appeared as direct continuation with the gastric glands to receive its secretion products. These gastric pits were lined by a high cylindrical epithelium of prismatic cells, already with the nucleus polarized in the base, or superficial mucous cells. No morphological differences were observed between these cells and the deep mucosal cells situated more deeply in the gastric pits. Histochemical reactions to acid mucopolisaccarides were being positive by the whole surface epithelial layer and for the first time (to 183 days, 76% in red deer, and 123 days, 82%, in sheep) shows a reaction positive to Mayer's mucicarmin (Fig. 1k), and PAS (Fig. 1l) and is indicative of the presence to neutral mucopolysacharides and mucins and mucinous compounds.
The lamina propria, composed of dense connective tissue, occupied the space between gastric glands. Several layers of smooth muscle fibers, separating the mucosa from submucosa, formed the muscularis mucosae. The submucosa was composed of highly vascularized connective tissue. The tunica muscularis was formed by two clearly defined bundles of smooth muscular fibres: an internal circular layer and an external and thinner, longitudinal layer. The serosa was comprised of a subserosa of connective tissue covered by a mesothelium.
Morphometric observations
Table 1 shows the tissue layer thickness in the abomasum of the sheep and red deer during prenatal development. A factorial anova indicated that the mean growth value of group I epithelium was significantly lower than in groups II-V in the sheep (P = 0.003) and in the red deer (P = 0.004). As indicated by main factor analysis in factorial anova, the lamina propia and submucosa of group III was significantly different from these layers of groups IV and V in the sheep (P = 0.002) and in the red deer (P = 0.002); and the tunica muscularis of group III was also significantly different from this tunica in groups IV and V in the sheep (P = 0.002) and red deer (P = 0.002). The mean growth value of group I serosa was significantly higher than in groups II-V in the sheep (P = 0.003) and red deer (P = 0.002), and the same for the wall in the sheep (P = 0.003) and in the red deer (P = 0.002). When comparing the thickness of each layer in two species, a factorial anova indicated that the mean growth value of epithelium of the sheep was significantly higher than red deer in all groups (P = 0.004) and the same for the wall (P = 0.003). The mean growth value of tunica muscularis of the red deer was significantly higher than sheep in groups IV and V (P = 0.004). There was no significant difference in the serosa.
| Group I Sheep (23–37 days: 1–25% gestation). Red deer (30–60 days:1–25% gestation) | Group II Sheep (39–52 days: 26–35% gestation) Red deer (63–85 days, 26–35% gestation) | Group III Sheep (54–75 days: 36–50% gestation). Red deer (86–120 days, 36–50% gestation) | Group IV Sheep (77–112 days: 51–75% gestation). Red deer (123–180 days, 51–75% gestation) | Group V Sheep (114–150 days: 76–100% gestation). Red deer (183–240 days, 76–100% gestation) | |
|---|---|---|---|---|---|
| Epithelium | |||||
| Sheep | 86 ± 7 | 76 ± 5a | 42 ± 4a | 39 ± 3a | 31 ± 3a |
| Red deer | 62 ± 5c | 49 ± 4ac | 41 ± 3ac | 33 ± 3ac | 21 ± 3ac |
| Lp + Sb | |||||
| Sheep | pbt | pbt | 87 ± 8 | 86 ± 7b | 84 ± 9b |
| Red deer | pbt | pbt | 96 ± 9 | 80 ± 7b | 79 ± 8b |
| Tm | |||||
| Sheep | 214 ± 8* | 201 ± 16* | 103 ± 9 | 98 ± 10b | 95 ± 9b |
| Red deer | 195 ± 8* | 164 ± 17* | 77 ± 7 | 80 ± 8bc | 82 ± 11bc |
| Serosa | |||||
| Sheep | 56 ± 3 | 44 ± 4a | 41 ± 6a | 28 ± 3a | 25 ± 4a |
| Red deer | 51 ± 5 | 32 ± 5a | 30 ± 6a | 25 ± 3a | 24 ± 3a |
| Wall | |||||
| Sheep | 356 ± 10 | 273 ± 10a | 273 ± 10a | 251 ± 10a | 235 ± 12a |
| Red deer | 308 ± 14c | 245 ± 10ac | 244 ± 11ac | 218 ± 11ac | 206 ± 35ac |
- *The pluripotential blastic tissue, which will later give rise to the lamina propia-submucosa and tunica muscularis, were not statistically compared owing to the fact that one structure will give rise to various others. aP < 0.005 vs Group I; bP < 0.005 vs Group III, cP < 0.005 vs sheep. Lp+Sb, lamina propria and submucosa; Tm, tunica muscularis; pbt, pluripotential blastic tissue.
Immunohistochemical observations
Immunohistochemical findings in the abomasum of the five groups of the sheep and red deer studied are summarized in Table 2 and Figure 2a–h. The neuropeptides present in the abomasum in both species during prenatal development were: SYP (integral membrane protein of small synaptic vesicles in brain and endocrine cells), gastrin (this hormone acts as a mitogenic factor for gastrointestinal epithelial cells, has high affinity for both gastrin and cholecystokinin and mediates secretory acid as well as the proliferative effects of gastrin), GFAP (one of the major intermediate filament proteins of mature astrocytes and is used as a marker to distinguish astrocytes from other glial cells during development), VIP (which stimulates myocardial contractility and relaxes the smooth muscle of trachea, stomach and gall bladder) and NPY (which is widely expressed in the central nervous system).
| Group I Sheep (23–37 days: 1–25% gestation). Red deer (30–60 days:1–25% gestation) | Group II Sheep (39–52 days: 26–35% gestation) Red deer (63–85 days: 26–35% gestation) | Group III Sheep (54–75 days: 36–50% gestation). Red deer (86–120 days: 36–50% gestation) | Group IV Sheep (81–112 days: 54–75% gestation). Red deer (142–191 days: 51–75% gestation) | Group V Sheep (114–150 days: 76–100% gestation). Red deer (183–240 days: 76–100% gestation) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | Lp+Sb | Tm | S | E | Lp+Sb | Tm | S | E | Lp+Sb | Tm | S | E | Lp+Sb | TM | S | E | Lp+Sb | TM | S | |
| SYP sheep | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | ++ | ++ | − |
| SYP red deer | − | − | − | − | − | + | + | − | − | ++ | ++ | − | − | +++ | +++ | − | − | +++ | +++ | − |
| Gastrin sheep | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − |
| Gastrin red deer | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | ++ | − | − | − |
| GFAP sheep | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | ++ | ++ | ++ |
| GFAP red deer | − | − | − | − | − | − | − | − | − | + | + | − | − | ++ | ++ | + | − | +++ | +++ | ++ |
| VIM sheep | − | − | − | − | − | − | − | − | − | + | + | + | − | ++ | ++ | ++ | − | +++ | +++ | +++ |
| VIM red deer | − | − | − | − | − | − | − | − | − | ++ | ++ | ++ | − | ++ | ++ | ++ | − | +++ | +++ | +++ |
| VIP sheep | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + |
| VIP red deer | − | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | + | − | ++ | ++ | ++ |
| NPY sheep | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + |
| NPY red deer | − | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | + | − | ++ | ++ | ++ |
- E, epithelium; Lp+Sb, lamina propria and submucosa; Tm, tunica muscularis; S, serosa; SYP, synaptophysin; GFAP, glial fibrillary acidic protein; VIM, vimentin; VIP, vasoactive intestinal peptide; NPY, neuropeptide Y; −, non-immunoreactivity (no surface staining); +, low immunoreactivity (stained surface less than 200 μm2); ++, moderate immunoreactivity cells (stained surface between 200 and 400 μm2); +++, intense immunoreactivity (stained surface between 200 and 400 μm2).
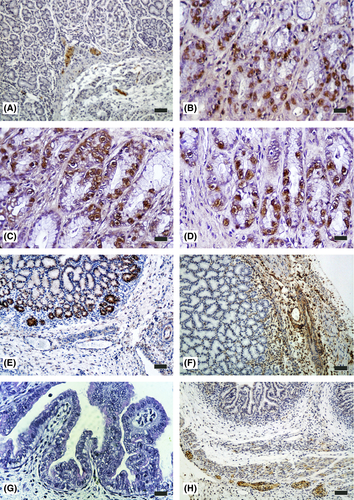
The presence of neuroendocrine cells (SYP-positive) in the abomasal wall was not detected until 63 days (26% gestation) in red deer and 81 days (54% gestation) in sheep. In both species, these cells were located in the lamina propria and submucosa (Fig. 2a), in gastric glands of the fundic area (Fig. 2b) and in the thickness of the intermuscular connective tissue in the myenteric plexus.
The presence of gastrin-positive cells in the abomasal wall was not detected until 120 days (50% gestation) in red deer and 114 days (76% gestation) in sheep. In both species these cells were located in the epithelial layer (stratum basal) and gastric glands in the pyloric zone (Fig. 2c,d); we could observe isolated gastrin cells in the fundic region next to the pyloric region in red deer fetuses from 180 days (75% gestation) and in sheep fetuses to term.
The presence of GFAP-positive cells occurred at around 120 days (50% gestation) in red deer and at 114 days (76% gestation) in the sheep (Fig. 2e). Immunoreactivity to VIM was detected in cells at 63 days (26% gestation) in red deer (Fig. 2f) and at 79 days (53% gestation) in the sheep. In both species, the localization of the glial cells in the abomasal wall during development affected the lamina propria, submucosa, tunica muscularis and serosa.
The detection of neuropeptides VIP (Fig. 2g) and NPY (Fig. 2h) in nerve fibers and in nerve cell bodies began at 123 days (51% gestation) in red deer and at 114 days (76% gestation) in sheep, with a moderate immunopositivity in the lamina propria, submucosa, tunica muscularis and serosa in both species.
Scanning electron microscopy observations
At 25% gestation (37 and 60 days for sheep and red deer, respectively) the mucosal surface of the abomasum appeared irregular. In both species at 33% gestation, primitive abomasal folds were visible as small elevations of the mucosal surface (Fig. 3a,b).
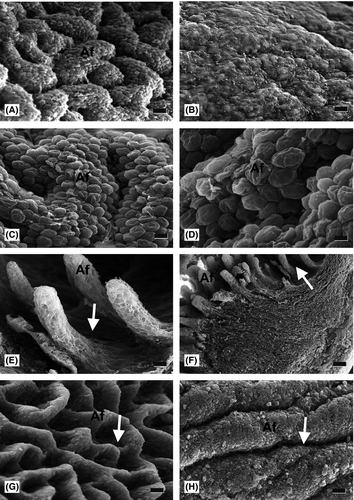
At 50% gestation (75 and 120 days for sheep and red deer, respectively), abomasal folds were larger, and were observed over the whole surface (Fig. 3c,d); thereafter, folds grew in both size and number throughout development.
By 112 and 191 days for sheep and red deer, respectively, incipient peak areas and gastric pits were evident as small irregularities on the surface of abomasal folds (Fig. 3e,f).
In the final stage of prenatal development in both species, abomasal folds were abundant, and gastric pits were clearly visible as furrow-like depressions on the fold surface, which also displayed mucous residue (Fig. 3g,h).
Radioimmunoassay observations
Figure 4 gives the plasma gastrin levels of red deer and sheep during development. Detection of gastrin began at 120 days, 50% gestation, in red deer and at 180 days, 75% gestation, in sheep. In both species plasma gastrin levels increased to the highest levels in perinatal stages.

Discussion
Differentiation of the sheep abomasum took place at 37 days (25% gestation) (Franco et al. 1993b). The individualization of red deer abomasum was observed at 53 days (22% gestation) (Masot et al. 2007a,b). Similar findings are reported in goat (Ramkrishna & Tiwari 1979; Molinari & Jorquera 1988; Mutoh & Wakuri 1989; García et al. 2013a). By contrast Asari et al. (1985) and Vivo et al. (1990) reported in cattle that differentiation occurs slightly earlier, at 30 days (11% gestation). In both species at 25% gestation, the abomasal surface displayed small undulations (primitive abomasal folds) that increased in size and number as the abomasum developed, giving rise to a substantial increase in epithelial surface area. Similar observations are reported in sheep by Del Rio Ortega (1973) and Franco et al. (1993b). However, Molinari and Jorquera (1988) and Fath El-Bab et al. (1983) reported abomasal folds at a later stage in goats and sheep (49% and 40% gestation, respectively), while in cattle it occurs earlier, at 14% gestation (Vivo et al. 1990). In both species at this early stage of development, the abomasal wall appeared as three well-defined layers: epithelial layer, pluripotential blastemic tissue and serosa, in agreement with Panchamukhi and Srivastava (1979) in buffalo, with Fath El-Bab et al. (1983); Franco et al. (1992, 1993c) in sheep; and Franco et al. (2004a,b) in red deer.
Throughout development the greatest morphological changes affected the epithelium, which from the first embryonic decreases in its thickness up to birth, being of type pseudostratified to simple cylindrical with secretion capacity to acid mucopolysaccharides. In both species, the presence of acid mucopolysaccharides in the epithelial layer could be related to the gradual adaptation of the abomasal mucosa to future digestive and metabolic functions after birth. This important modification was earlier in red deer (63 days, 26% of gestation) than sheep (53 days, 35%). This transition was observed in goats at 43% gestation (Lee et al. 1994; García et al. 2013a), and at an earlier stage (10% gestation) by Mutoh and Wakuri (1989). In cattle, a similar transformation has been reported at 32% gestation (Asari et al. 1985) and at 27% (Vivo et al. 1990). In a study by Fath El-Bab et al. (1983), epithelial transformation was not recorded in sheep until 63% gestation. We believe that the configuration of a simple epithelium with acidic secretions is enhanced at near birth by secretion of neutral glycoproteins which act as a buffer against acidic substances, and particularly against abomasal acidity during lactation (Franco et al. 1993b).
At about 50% of gestation in both species, gastric pits were observed on the surface of abomasal folds. Our studies suggest a close link between the initial formation of these psedoglandular structures and the clear separation of lamina propria and submucosa and its differentiation from pluripotential blastemic tissue as independent entities, separated by de muscularis mucosae. Finally, at around 50% gestation in both species, at the bottom of these gastric pits the first outlines of gastric glands were distinguishable, comprising cellular groupings around a central light. In sheep Del Rio Ortega (1973) reported gastric pits at 46% of gestation and gastric glands at 62%, while Fath El-Bab et al. (1983) observed them at 62 and 90%, respectively. In goat the presence of gastric pits and glands have been reported at 46% and 69% (Molinari & Jorquera 1988), and previously at 25 and 43% of gestation (García et al. 2013a). Differentiation of the lamina propria and the submucosa from pluripotential blastemic tissue took place at 63 days (26% of gestation) in red deer and to 53 days (35%) in sheep. Both layers were composed of fibroblasts and collagen fibers, as also indicated by Age et al. (2007, 2008) in cattle and sheep, respectively.
Differentiation of the tunica muscularis from pluripotential blastemic tissue also took place in both spcies at around 30% gestation, as noted in sheep by Franco et al. (1993b), in goat by García et al. (2013a,b,c) and red deer by Masot et al. (2007a,b). In the sheep (Del Rio Ortega 1973) and in the cow (Vivo et al. 1990) it was placed at earlier stages of development (around 18% gestation). At 50% gestation in both species from the inner circular layer of tunica muscularis the muscularis mucosae appeared, a finding also repoted for goat abomasum (García et al. 2013a,b,c). In other ruminants, the appearance was placed in perinatal stages in buffalo (Panchamukhi & Srivastava 1979) or during postnatal development (Wardrop 1961; Kitamura et al. 2003).
The serosa was composed of loose conective tissue underlying a layer of flat mesothelial cells and showed no distinct characteristic from those referenced for sheep and red deer comparative studies in the forestomach (Franco et al. 2011, 2012; Redondo et al. 2011) and for the goat forestomachs and abomasum (Al-Saffar 2012; García et al. 2012, 2013a,b,c, 2014b).
The SPY-immunoreactive cells were detected at 54% gestation in sheep in the lamina propria and submucosa, tunica muscularis, serosa and myenteric plexuses according to that reported by Kitamura et al. (1986) in cattle, by Groenewald (1994) in sheep and by García et al. (2012, 2013a,b,c) in goat. In red deer, Masot et al. (2007a,b) describe these neuroendocrine cells earliest (25% gestation).
The presence of gastrin-immunoreactive cells in the abomasal wall was not detected until 50% gestation in red deer, as also reported in goat by García et al. (2013a) and 76% gestation in the sheep in agreement with Franco et al. (1993d) and Redondo et al. (1997). In both species, these cells were located in the epithelial layer and gastric glands in the pyloric zone. The presence of gastrin cells in the abomasum has been reported by Calingasan et al. (1984) and Kitamura et al. (1985) in the cow, Dall′Aglio et al. (1999) in fallow deer, Adnyane et al. (2011) in barking deer and Agungpriyono et al. (2000) in babyrousa (suidae).
Plasma gastrin concentrations were first detected in red deer (at 120 days, 50% gestation) than in sheep (at 180 days, 75% gestation). These results are consistent with those reported by Shulkes et al. (1981), Avila et al. (1989), Franco et al. (1993d) in sheep and Masot et al. (2007a,b) in red deer. Our studies suggest that plasma gastrin concentrations increased throughout prenatal life, as in the case of the number of gastrin immunoreactive cells, the fetal stomach being the main source of gastrin (Shulkes et al. 1981; Franco et al. 1993d; García et al. 2013a).
In both species, the localization of the glial cells in the abomasal wall during development affected the lamina propria, submucosa, tunica muscularis and serosa and was particularly prominent in the myenteric plexus. The presence of GFAP-positive cells occurred later than VIM. The comparative study of immunostaining of both glial cell markers proved that VIM is a marker of more primitive glial cells (Franco et al.1993b). The observations of glial cells in the myenteric plexus and submucosa have been reported in sheep (Yamamoto et al. 1994), in goat (García et al. 2013a, 2014b) and cows (Kleinschmidt et al. 2011). Glial cells are similar in structure and function to astrocytes of the central nervous system, and play a key role in controlling gastrointestinal functions and protecting enteric neurons (Abdo et al. 2010). The immunoreactivity for glial cells in the abomasal wall suggested that some of the fuctions of abomasal mucosa could be intrinsically regulated by the submucosal plexus (Yamamoto et al. 1995; Teixeira et al. 1998).
Regarding the peptidergic innervation markers, positive staining for NPY and VIP had a similar pattern: in nerve fibers and in nerve cell bodies began at 51% and 76% gestation in red deer and sheep, respectively, with a moderate immunopositivity in the lamina propria, submucosa, tunica muscularis and serosa, and greatest staining intensity in myenteric plexus in both species. The presence of the neuropeptides VIP and NPY has been described in the forestomach and abomasum of adult sheep (Groenewald 1994; Yamamoto et al. 1995), in the forestomach of adult cattle (Kitamura et al. 1986), in the forestomach of fetal sheep (Franco et al. 1992, 1993a,b,c) and in the stomach of the fetal pig (Van Ginneken et al. 1996) and in goat abomasum (García et al. 2013a).
Under the scanning electron microscope, the abomasal mucosa appeared as an irregular surface, with small elevations which would subsequently give rise to the abomasal folds. As development progressed, small furrow-like depressions observed on the surface of these folds developed into gastric pits. Similar findings have been reported in goat abomasum (García et al. 2013a), and in the fetal (5 and 8 weeks of fetal life) human stomach (Otani et al. 1993).
We can deduce from our findings that the abomasum in both species during fetal life undergoes gradual adaptations prior to the intense changes that will be produced at the moment of birth. When we compare the two species, we can deduce that red deer are more precocious than sheep. It is earlier in terms of the appearance of a complete stratification of the abomasal wall, of the specific adaptations of its mucosa (configuration of a simple epithelium with acidic secretions, differentiation of the muscularis mucosae and the gastric pits and glands on the surface of abomasal folds) and its neuroendocrine nature, determined by the presence of neuroendocrine cells, glial cells and peptidergic innervations markers.




