Strain-specific effects of broodstock size on fecundity and offspring performance in bighead catfish (Clarias macrocephalus Günther, 1864)
Abstract
This study investigated the relationships among parental body weight, egg size and number, and offspring performance at the larval, juvenile and grow-out stages of bighead catfish (Clarias macrocephalus) in Mekong domesticated (Can Tho; CT) and wild (Ca Mau; CM) strains. One-year-old broodstock from each strain were separated into two size groups, with those in the large (L) group having, on average, approximately double the body weight of those in the small (S) group. Both strains exhibited a strong linear increase in absolute fecundity (slope = 58.8, p < 0.01 for CT and slope = 52.3, p < 0.01 for CM) and a slight increase in egg diameter associated with increasing female body weight (slope = 0.0002, p = 0.03 for CT and slope = 0.0003, p = 0.06 for CM). However, there were no linear correlations between female body weight and yolk-sac volume or larval body length at hatching (p > 0.05). Despite similar fecundities, the CM wild strain had larger eggs and more favourable larval properties than the CT domesticated strain (p < 0.01). However, these advantages did not result in better growth and survival of CM offspring at any stage. The CT strain showed improved growth in offspring with parents in the L group compared to those with parents in the S group across the three stages (35.7%, 50.0% and 43.9% respectively), while there was no significant increase in growth in the CM wild strain (p > 0.05). Parental body weight and strain had no effects on survival and feed conversion ratios (FCRs), with the exception that juveniles of the CM S group had the lowest SR (38.8 ± 6.0%) and FCR (1.09 ± 0.08) due to disease infection. The CT L group grew fastest and consumed the highest amount of food with similar feed efficiency to the other groups in juvenile and grow-out stages (p < 0.05). These results indicated that the pertinence of ‘the bigger the better’ paradigm varied between growth stages in the two bighead catfish strains.
1 INTRODUCTION
The paradigm of ‘the bigger the better’ has been applied in aquaculture and achieved remarkable improvements through selective breeding programmes because of the relatively high heritability of growth traits in many species (Dunham, 2011; Gjedrem et al., 2012; Gjedrem & Rye, 2018; Gjerde, 1986; Tave, 1993). Some examples of successful selection programmes have been reported for common carp (Bakos & Gorda, 1995; Hulata, 1995; Moav & Wohlfarth, 1976), the genetically improved farmed tilapia (GIFT) strain of Nile tilapia (Oreochromis niloticus) (Ponzoni et al., 2011), Atlantic salmon (Salmo salar) (Gjedrem et al., 2012), and rainbow trout (Oncorhynchus mykiss) (Leeds et al., 2016; Wiens et al., 2018). Additional species and selection programmes were reviewed by Gjedrem et al. (2012) and Janssen et al. (2017).
Offspring produced by fast-growing broodstock can not only inherit fast-growth traits from their parents but can also receive advantages from parental effects, especially maternal effects via egg size (Green, 2008; Kamler, 2005; Trippel et al., 1997). Larger eggs usually result in larger larvae and more nutrition in the yolk-sac, which is beneficial for fish growth and survival in early life stages (Johnston & Leggett, 2002; Kamler, 1992, 2005). However, the parent–egg–progeny relationships can vary among species, strains and populations, and depend on the interactions of different environmental factors such as temperature, pathogens and so on (Hendry & Day, 2003; Kamler, 2005). Understanding this relationship is crucial not only in wild fish recruitment but also in farmed fish production (Kamler, 2005; Trippel et al., 1997).
Bighead catfish (Clarias macrocephalus), an important aquaculture species in Southeast Asian countries, has been a target for some short-term selection programmes for growth traits (Chammankuruwet, 1996; Jarimopas et al., 1988) and disease resistance (Na-Nakorn et al., 1995; Srisapoome et al., 2019). However, the triangular relationship among parental size, egg size and number, and offspring performance is not yet fully understood. Ali (1993) reported only on fecundity and oocyte size at different stages of maturity in wild C. macrocephalus in the rice fields of Malaysia; this study found that fecundity increased with the body size of females, but relative fecundity (number of eggs per body weight unit) did not. In other studies, the effects of parental size on offspring have been reported only at the grow-out stage in selection experiments (Chammankuruwet, 1996; Jarimopas et al., 1988; Wagle & Na-Nakorn, 2007). Wagle and Na-Nakorn (2007) applied bidirectional selection to form high (larger size) and low (smaller size) broodstock lines; in the second generation, C. macrocephalus in the high line grew faster than those in the low line with weight differences of 8.3% and 6.8% at 29 and 33 weeks respectively. The published studies have not yet yielded a conclusive answer to the question of whether larger parents have superior offspring. In addition, it remains unknown how trade-offs in some reproductive traits, such as fecundity and egg size (Jonsson & Jonsson, 1999; Kamler, 2005; Morrongiello et al., 2012) affect offspring growth and survival. A recent study found that bighead catfish broodstock of 1 or 2 years old with similar body weight did not influence the growth and survival of offspring from the larval stage to commercially marketable size (Duong et al., in press).
The current study investigated whether larger parents produced offspring with better growth, survival and feed-utilization levels in domesticated and wild strains of bighead catfish across larval to grow-out stages. To disentangle the influence of parental age associated with body size, 1-year-old broodstock were used, as this is the most common age for artificial reproduction in bighead catfish aquaculture. The relationships of female size with egg diameter and larval properties were also investigated to understand the importance of the egg size–egg number trade-off and the role of the maternal effect on the growth process of offspring. The comparison of wild and domesticated strains was expected to reveal whether the effects were strain specific or consistent.
2 MATERIALS AND METHODS
2.1 Experimental breeders
The fish were handled properly and reared in the proper environment. The fish rearing procedure was approved by Can Tho University's Scientific Research Committee.
The study bighead catfish strains comprised 1-year-old broodstock that had been cultured in recirculating systems at Can Tho University, Vietnam. They were produced using artificial propagation in May 2019 from two strains originating from a wild population in Ca Mau (CM) province (latitude 9.324694 and longitude 104.889139) and a domesticated population in Can Tho (CT) city (latitude 10.148194 and longitude 105.601167). At 9 months of age, the fish reached marketable sizes of 136 ± 57 g (N = 252) for the CT strain and 94 ± 48 g (N = 210) for the CM strain. The body sizes of fish from both strains had a right-skewed distribution (Shapiro–Wilk test for normality p < 0.01). They were conditioned (cultured for maturity) for 3 months. In May 2020, mature (1-year-old) fish were selected for artificial propagation. Each strain was divided into two groups: on average, the large (L) group had about double the body weight of the small (S) group. The weights of breeders (5–9 families for each fish treatment) are reported in Table 1.
| Source | Treatments | Female weight (g) | Male weight (g) |
|---|---|---|---|
| Domesticated CT | Large size (7 pairs) | 182–296 (241 ± 23) | 163–244 (195 ± 27) |
| Small size (8 pairs) | 79–139 (114 ± 18) | 81–129 (102 ± 19) | |
| Wild CM | Large size (5 pairs) | 148–259 (218 ± 43) | 94–272 (156 ± 74) |
| Small size (8 pairs) | 66–132 (90 ± 26) | 53–89 (67 ± 11) | |
| Large/small | CT | 2.1 | 1.9 |
| CM | 2.4 | 2.3 |
2.2 Female fecundity, egg size, larval body length and yolk-sac volume
2.3 Larval rearing stage (day 1–40)
After yolk-sac absorption, larvae from the four groups were stocked in 50-L tanks at a density of 1000 per tank using a completely randomized study design with five replicates for each. The larvae were fed Moina sp. (Cladocera) twice per day (at 7 am and 4 pm) for 10 days. Two additional feeds using a commercial product containing 40% protein were then given during the weaning period from day 11 to 16. From day 17 to 40, the larvae were offered commercial feed four times per day. Uneaten food was siphoned 1 h after feeding and about 20%–40% of the water was exchanged daily. Aeration was used during the experiment.
Water temperatures in this experiment varied from 26 to 30°C in the morning and 27 to 32°C in the afternoon. Dissolved oxygen (DO) was from 4.3 to 6.4 mg/L. Values of pH were stable in the range 7.0–8.1. These water parameters were similar among fish tanks.
2.4 Juvenile rearing stage (day 41–100)
This study was conducted in four recirculating systems (corresponding to four replicates) each of which comprised four 500-L rearing tanks (one replicate for each treatment group), one 300-L sedimentation tank, and one 1000-L bio-filter tank containing 40% Kaldnes (RK BioElements). All of the systems were set up and managed in the same way. The use of four separate systems was intended to increase the effectiveness of the bio-filters; thus, the effect of different recirculating systems was assumed to be minor and was not treated as a block effect.
At the end of the larval rearing period, the fish in each group were pooled, and then 300 individuals with relatively uniform size were stocked randomly to each rearing tank. The total biomass was recorded to calculate the mean initial weight. A commercial floating feed containing 40% protein was given to the fish four times per day. The amount of feed was initially calculated as 8% of the total biomass and was then adjusted based on fish consumption (so that they were fed to satiation). The food that had not been consumed was removed and recorded. The water was drained regularly to remove any waste that had accumulated in the sedimentation and culture tanks and roughly 10%–15% of the volume was renewed. The study lasted for 2 months.
During this experiment, water temperatures were recorded, in the morning from 24.5 to 28.0°C and in the afternoon from 25.0 to 29.0°C. DO and pH were measured every three days, ranging from 5.1 to 6.9 mg/L and 7.0 to 8.5 respectively. Total ammonia nitrogen (TAN) and NO2− concentrations (measured every three days by Sera test kits) were low, less than 0.5 mg/L.
2.5 Grow-out stage (day 101–190)
The recirculating systems for juvenile rearing were continuously used for the grow-out experiment (which lasted for 3 months). Similar protocols were applied for the experimental design and management. Briefly, fish were pooled according to treatment groups and then introduced randomly into culture tanks (four replicates) with similar biomasses (1.2–1.4 kg/tank). The feeding rate was estimated as 4% of the total biomass initially and adjusted daily depending on the intake.
Water parameters were recorded similarly to the juvenile experiment, temperatures: 26.6–31°C; DO: 5.2–6.5 mg/L, pH: 7.0–8.5; TAN and NO2−: <0.5 mg/L.
2.6 Fish sampling and growth data calculations
In the larval rearing experiment, the body weights and lengths of the fish were measured at the start (day 0), middle (day 20) and end (day 40) of the experiment. The sample sizes were 30 individuals randomly collected from each treatment group before stocking, and 30 individuals from each tank for the later samplings. The survivors were counted in each tank.
In the juvenile and grow-out experiments, the fish were weighed in groups and then counted at stocking and harvest. Mid-point samplings were applied every 30 days by weighing 20–30 fish per tank. However, some fish were injured due to sampling, so the second middle-sampling of the grow-out stage (at day 160) was not conducted in these cases. At the end of each experiment, the individuals' weights were recorded for at least 30 randomly chosen fish from each tank to evaluate growth differentiation.
The measured growth parameters of bighead catfish included body weight (and body length in the larval experiment) at the sampling times and daily weight gain (DWG) calculated as follows: DWG = final weight–initial weight/experimental period (in days). Growth differentiation was indicated by the coefficients of variation (CVs) in individual weight, which were calculated as a percentage as follows: CV% = 100 × standard deviation/weight mean (on a tank basis). Food intake (FI; g/fish/day) was defined as the amount of feed given (g) per individual per day. The feed conversion ratio (FCR) was calculated as the ratio of the amount given (g)/weight gain on a wet basis (g). The FI and FCR were estimated for the juvenile and grow-out stages. The survival rate (SR) was defined as the percentage of survivors and calculated as follows: SR% = 100 × number of survivors/number of fish at stocking.
2.7 Data analysis
Data analyses were conducted using the spss 20 software. The SR and CV values were subjected to a natural logarithm transformation before statistical analyses (Warton and Hui, 2011). Data were initially checked for a normal distribution using the Shapiro–Wilk test. The growth and SR data were subjected to two-way analysis of variance (ANOVA) for two factors: broodstock strain and size. If the interaction between the two factors was significant (at p < 0.05), the four fish groups were independently compared using one-way ANOVA, followed by Duncan's multiple range tests. Absolute fecundity, egg diameter, larval length and yolk-sac volume were examined for linear relationships with the body weight of individual females for each strain. Differences in the slopes of these regressions between the CT domesticated and CM wild strains were compared using t-tests.
3 RESULTS
3.1 Female fecundity, egg size, larval body length and yolk-sac volume
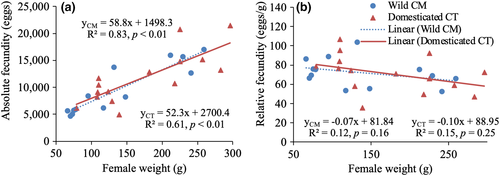
The absolute fecundity of individual females varied from 40,369 to 69,856 eggs. Larger females produced more eggs with a significant linear relationship in the CT (slope = 58.8, R2 = 0.83, p < 0.01) and CM (slope = 52.3, R2 = 0.61, p < 0.01) strains (Figure 1a). The difference in the slope of female body weight and absolute fecundity regression between the two strains was not significant (p = 0.64). Relative fecundity showed an opposite trend, in which larger females had fewer eggs per g body weight compared to smaller females (Figure 1b). However, this negative relationship was not significant in both strains (CT: slope = −0.10, R2 = 0.15, p = 0.25 and CM: slope = −0.07, R2 = 0.12, p = 0.16).
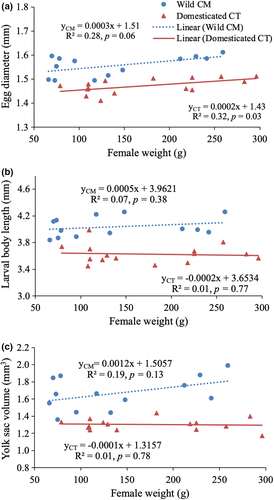
Female body size showed a similar positive linear relationship with egg diameter in both broodstock strains (slope = 0.0002, p = 0.03 for CT and slope = 0.0003, p = 0.06 for CM; Figure 2a). However, female size had no relationship with either the body length (Figure 2b) or the yolk-sac volume (Figure 2c) of the larvae at the time of hatching. Between the two strains, the CM strain had significantly larger egg size (1.56 ± 0.04 mm, n = 13), larval body length (4.04 ± 0.15 mm) and yolk-sac volume (1.63 ± 0.23 mm3) than those of the CT strain (1.47 ± 0.03 mm, n = 15, 3.67 ± 0.14 mm, 1.25 ± 0.23 mm3) respectively, all p < 0.01).
3.2 Growth rates of offspring at three culture stages
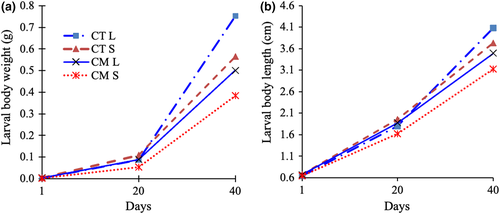
The growth lines (Figure 3) showed that the body weight and length of the four fish groups varied during the larval rearing period. The differences in growth among groups were more obvious for weight than for length. The highest growth was found in the offspring of the CT L group and the lowest was found in those of the CM S group. At day 40, the growth of the fish was significantly affected by the size and strain of the broodstock (p < 0.05) but not by the interaction of these two factors (p > 0.05; Table 2). Fingerlings of L group broodstock (627 ± 205 mg) were bigger than those of S group broodstock (464 ± 136 mg). Offspring produced by CM broodstock (442 ± 123 mg) grew more slowly than (p < 0.01) those of CT broodstock (668 ± 184 mg). DWG showed a similar trend to weight and length parameters. The DWG of fingerlings from L group broodstock was 35.7% and 30.0% higher compared to those from S group broodstock in the CT and CM strains respectively.
| Fish strain | Size | W1 (mg) | W40 (mg) | L40 (cm) | DWG (mg/day) | CVW (%) | CVL (%) | SR (%) |
|---|---|---|---|---|---|---|---|---|
| CT | Large | 2.1 | 752 ± 198 | 4.08 ± 0.43 | 19 ± 5 | 67.0 ± 11.6 | 21. 5 ± 1.1 | 31.1 ± 4.1 |
| Small | 3.2 | 564 ± 112 | 3.73 ± 0.30 | 14 ± 3 | 64.4 ± 14.8 | 20.7 ± 0.9 | 48.3 ± 11.9 | |
| CM | Large | 2.9 | 501 ± 127 | 3.49 ± 0.35 | 13 ± 3 | 72.3 ± 22.1 | 22.3 ± 3.2 | 36.4 ± 15.1 |
| Small | 2.0 | 383 ± 96 | 3.12 ± 0.27 | 10 ± 3 | 78.2 ± 17. 6 | 21.3 ± 3.2 | 36.0 ± 9.5 | |
| Mean by strain | CT | 2.6 | 668 ± 184B | 3.92 ± 0.40B | 17 ± 5B | 65.9 ± 12.3A | 21.1 ± 1.6A | 38.8 ± 12.0A |
| CM | 2.6 | 442 ± 123A | 3.31 ± 0.35A | 11 ± 3A | 75.3 ± 19.5A | 21.8 ± 3.0A | 36.2 ± 11.8A | |
| Mean by size | Large | 2.5 | 627 ± 205y | 3.79 ± 0.48y | 16 ± 5y | 69.7 ± 17.4x | 21.9 ± 2.6x | 33.8 ± 10.8x |
| Small | 2.3 | 464 ± 136x | 3.39 ± 0.41x | 12 ± 3x | 72.1 ± 17.0x | 21.0 ± 2.3x | 41.4 ± 11.8x | |
| p-Values indicate effects of broodstock strain and broodstock size | ||||||||
| Broodstock strain (BS) | 0.004 | 0.002 | 0.005 | 0.255 | 0.541 | 0.486 | ||
| Size (S) | 0.032 | 0.038 | 0.029 | 0.840 | 0.450 | 0.113 | ||
| BS*S | 0.598 | 0.959 | 0.554 | 0.603 | 0.924 | 0.099 | ||
- Note: Values in the same columns followed by the same superscripts (A, B for broodstock strain; and x, y for broodstock size) were not significantly different (p > 0.05).
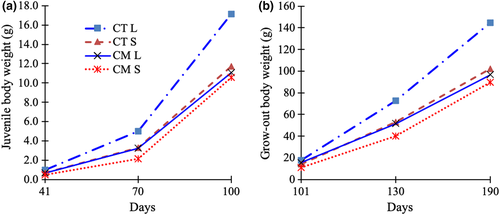
At the juvenile stage (100 days), bighead catfish obtained a weight of 10.42–17.01 g, and the CT L group offspring outperformed the other three groups (Figure 4a, Table 3). The growth parameters (i.e. weight and DWG) depended on the interaction between the size and strain of the broodstock (pBroodstock strain*size <0.05). Juveniles from L group broodstock grew faster than those from the S group in the CT strain (with a 50.0% difference in DWG), but not in the CM strain (DWG in both groups: 0.17 g/day).
| Fish strain | Size | W41 (g) | W100 (g) | DWG (g/day) | CVW (%) | FI (g/ind/day) | FCR | SR (%) |
|---|---|---|---|---|---|---|---|---|
| Can Tho | Large | 0.99 ± 0.01 | 17.14 ± 1.43b | 0.27 ± 0.02b | 53.5 ± 8.8 | 0.24 ± 0.04b | 0.91 ± 0.09a | 73.3 ± 8.2b |
| Small | 0.69 ± 0.01 | 11.72 ± 0.70a | 0.18 ± 0.01a | 74.9 ± 12.7 | 0.15 ± 0.01a | 0.85 ± 0.04a | 75.5 ± 3.6b | |
| Ca Mau | Large | 0.66 ± 0.04 | 11.05 ± 2.01a | 0.17 ± 0.03a | 61.5 ± 8.7 | 0.15 ± 0.02a | 0.90 ± 0.05a | 70.5 ± 1.4b |
| Small | 0.46 ± 0.01 | 10.56 ± 2.52a | 0.17 ± 0.04a | 67.1 ± 10.6 | 0.17 ± 0.04a | 1.09 ± 0.08b | 38.8 ± 6.0a | |
| Mean by strain | CT | 0.84 ± 0.16 | 14.43 ± 3.08 | 0.23 ± 0.05 | 64.2 ± 15.2A | 0.20 ± 0.05 | 0.88 ± 0.07 | 74.4 ± 6.0 |
| CM | 0.56 ± 0.11 | 10.81 ± 2.13 | 0.17 ± 0.04 | 64.3 ± 9.5A | 0.16 ± 0.03 | 0.99 ± 0.12 | 54.6 ± 17.4 | |
| Mean by size | Large | 0.83 ± 0.18 | 14.09 ± 3.84 | 0.22 ± 0.06 | 57.50 ± 9.2x | 0.19 ± 0.06 | 0.90 ± 0.07 | 71.9 ± 5.6 |
| Small | 0.58 ± 0.13 | 11.14 ± 1.43 | 0.18 ± 0.03 | 70.97 ± 11.6y | 0.16 ± 0.03 | 0.97 ± 0.14 | 57.1 ± 20.2 | |
| p-Values indicate effects of broodstock strain and broodstock size | ||||||||
| Broodstock strain (BS) | <0.01 | <0.01 | <0.01 | 0.986 | 0.036 | <0.01 | <0.01 | |
| Size (S) | <0.01 | <0.01 | 0.011 | 0.023 | 0.044 | 0.085 | <0.01 | |
| BS*S | <0.01 | 0.018 | 0.019 | 0.154 | 0.006 | <0.01 | <0.01 | |
- Note: Values in the same columns followed by the same superscripts (A, B for broodstock strain; x, y for broodstock size; and a, b for 4 independent treatments when the interaction between the two factors was significant (p < 0.05)) were not significantly different (p > 0.05).
At the grow-out stage, the growth lines were similar to those in the juvenile stage. The fish in the CT L group consistently showed the fastest growth. The weight and DWG of fish (Table 4) from the S group were lower than those from L group (p < 0.05), and the magnitude of difference between the two was larger in the CT group (DWG 1.41 g/day vs. 0.98 g/day, 43.9% difference, p < 0.05) than the CM group (DWG 0.90 g/day vs. 0.87 g/day, 3.4% difference, p > 0.05; pBroodstock strain*size <0.05).
| Fish strain | Size | W101 (g) | W190 (g) | DWG (g/day) | CVW (%) | FI (g/ind/day) | FCR | SR (%) |
|---|---|---|---|---|---|---|---|---|
| Can Tho | Large | 17.6 ± 1.3 | 144.7 ± 20.5 | 1.41 ± 0.22b | 35.7 ± 5.1 | 1.57 ± 0.26b | 1.20 ± 0.10 | 74.9 ± 5.3 |
| Small | 14.0 ± 1.1 | 102.0 ± 7.9 | 0.98 ± 0.09a | 40.9 ± 2.5 | 1.16 ± 1.30a | 1.24 ± 0.04 | 79.7 ± 5.8 | |
| Ca Mau | Large | 15.5 ± 2.3 | 96.8 ± 12.4 | 0.90 ± 0.13a | 43.8 ± 9.6 | 0.96 ± 0.19a | 1.22 ± 0.05 | 69.3 ± 3.7 |
| Small | 11.1 ± 0.5 | 89.7 ± 12.1 | 0.87 ± 0.14a | 43. 8 ± 4.6 | 1.07 ± 0.17a | 1.20 ± 0.04 | 74.9 ± 14.5 | |
| Mean by strain | CT | 15.8 ± 2.2B | 123.3 ± 27.0B | 1.20 ± 0.28 | 38.3 ± 4.6A | 1.37 ± 0.29 | 1.22 ± 0.07A | 77.3 ± 5.8A |
| CM | 13.3 ± 2.8A | 93.2 ± 11.9A | 0.89 ± 0.13 | 43.8 ± 7.0A | 1.02 ± 0.18 | 1.21 ± 0.04A | 72.1 ± 10.3A | |
| Mean by size | Large | 16.5 ± 2.1y | 120.7 ± 30.0y | 1.16 ± 0.32 | 39.7 ± 8.3x | 1.27 ± 0.39 | 1.21 ± 0.07x | 72.1 ± 5.2x |
| Small | 12.5 ± 1.7x | 95.8 ± 11.5x | 0.93 ± 0.12 | 42.3 ± 3.7x | 1.12 ± 0.15 | 1.22 ± 0.04x | 77.3 ± 10.6x | |
| p-Values indicate effects of broodstock strain and broodstock size | ||||||||
| Broodstock strain (BS) | <0.01 | <0.01 | <0.01 | 0.093 | 0.004 | 0.748 | 0.245 | |
| Size (S) | <0.01 | <0.01 | 0.011 | 0.406 | 0.140 | 0.752 | 0.245 | |
| BS*S | 0.571 | 0.226 | 0.023 | 0.408 | 0.020 | 0.326 | 0.931 | |
- Note: Values in the same columns followed by the same superscripts (A, B for broodstock strain; x, y for broodstock size; and a, b for 4 independent treatments when the interaction between the two factors was significant (p < 0.05)) were not significantly different (p > 0.05).
3.3 Growth differentiation
Within each group, the growth differentiation indicated by the CV showed a larger variation in weight than in length (by approximately three times) at the larval rearing stage (Table 2). At this timepoint, there were no effects of broodstock size and strain on CV. However, at the juvenile stage (Table 3), offspring from S group parents had a higher CV, indicating more variation among individuals than those within the L group (p < 0.05) from both CT and CM strains (pBroodstock strain*size > 0.05). In contrast, the size of the broodstock did not show an effect on CV at the grow-out stage (p > 0.05). Furthermore, fish from the CT strain generally had a lower CV compared to CM fish (p = 0.09; Table 4).
3.4 Food intake and FCRs
At the juvenile stage, the FI (range = 0.15–0.24 g/fish/day) and FCR (range = 0.85–1.09) varied among the groups depending on the interaction between broodstock strain and size (p < 0.05). Testing the four groups independently (Table 4) showed that the FI was highest in the CT L group and the FCR was highest in the in CM S group, which significantly differed from the other three groups (p < 0.05). At the grow-out stage, the FCRs (1.20–1.24) were similar among the four groups (p > 0.05) and were not affected by the broodstock size or strain (p > 0.05). However, the FI was significantly higher in the CT L group (1.57 g/fish/day) compared to the other treatments, which ranged from 0.96 to 1.16 g/fish/day (p < 0.05).
3.5 SRs
SRs varied from 31.1% to 48.3% at the fingerling stage (Table 2), from 38.8% to 75.5% at the juvenile stage (Table 3), and from 69.3% to 79.7% at the grow-out stage (Table 4). The effects of broodstock size on the SR differed among the three stages. The survival rates of the fingerling and grow-out experiments did not vary among the groups or show significant main effects of the broodstock strain and size. However, at the juvenile stage, the fish produced by the CM S group had the lowest SR (38.8 ± 6.0%), which significantly differed from the other groups (p < 0.05 and pBroodstock strain*size < 0.05).
4 DISCUSSION
4.1 Effects of broodstock size on egg diameter and larval properties
Both wild and domesticated strains of C. macrocephalus showed similar effects of female body weight on fecundity, egg size, larval length and yolk-sac volume at hatching. Absolute fecundity remarkably increased while relative fecundity slightly decreased with female body weight. Similar relationships were found in the same species in rice fields in Malaysia with lower values of relative fecundity (ranging from 17.6 to 53.7 eggs/g female body weight; Ali, 1993). Bigger females produced larger eggs in both of bighead catfish strains in the current study, but did not result in larger larval body length or yolk-sac volume at hatching. A positive female body weight–egg size relationship has been commonly reported in many fish species (Johnston & Leggett, 2002; Kamler, 1992, 2005), which is the consequence of the combination and interaction of different effects (Hendry & Day, 2003; Kamler, 2005). This finding is different from those of other studies, in which positive relationships between female size and/or egg size with larval body size and yolk-sac volume were found in freshwater fish such as Siberian sturgeon (Acipenser baerii) (Gisbert et al., 2000), and marine fish such as Atlantic cod (Gadus morhua) (Pepin et al., 1997; Roney et al., 2018) and Pacific herring (Clupea pallasii) (Takemura et al., 2020). However, in some species, egg size had a weak or no correlation with newly hatched larvae's body length and yolk-sac volume (Lochmann et al., 2009; Olin et al., 2012; Patterson et al., 2013). Because additional factors can influence embryogenesis, ‘the bigger the better’ paradigm can be discarded (Kamler, 2005), explaining inconsistent results across fish species.
Compared to the CT domesticated strain, the wild CM strain had slightly lower fecundity but significantly larger egg diameter, greater larval body length and bigger yolk-sac volume. A similar result was reported when comparing wild and domesticated strains of the silver perch (Bidyanus bidyanus) (Sulaeman & Fotedar, 2017). Both of the current breeding strains of bighead catfish had been cultured in the same recirculating system for 1 year; however, the results were similar to those found in the original stocks (Duong et al., 2020), indicating the effects of their origin (wild and domesticated strains) on the above mentioned parameters. A trade-off between egg size and egg number in fish has been well documented (Elgar, 1990; Jonsson & Jonsson, 1999; Kamler, 2005). Consistent with the predictions made under evolutionary theory (Rollinson & Hutchings, 2013), females of the domesticated strain bighead catfish have evolved towards greater fecundity, smaller egg size and lower yolk-sac reserves in captive environments that are less competitive and have lower risks compared to the wild.
4.2 Effects of broodstock size on growth, survival and feed utilization of offspring
The advantages of larger eggs, yolk-sacs and larval body length at hatching in the CM wild strain did not result in better growth and survival in offspring even at the early stages of 20 and 40 days post hatching. In contrast, the CT domesticated strain grew faster consistently across all stages. This result concerning the growth of the larval and fingerling stages of the two bighead catfish strains differed from observations in other fish species that showed parental (maternal) effects on offspring performance at early life stages (Einum, 2003; Green, 2008; Green & McCormick, 2005; Heath et al., 1999). The parental effects on offspring performance can depend on the environment. Theoretically, under severe environmental conditions, the effects of egg size on offspring growth can increase (Einum, 2003). In a hatchery with a benign environment, the offspring from the CM wild strain did not exhibit early advantages in terms of egg size and larval properties. Superior growth of the domesticated strain over the wild strain, regardless of smaller egg size and larval properties, indicated that the origin effect was stronger than these factors.
The effects of parental size on offspring growth differed between the two strains. The CT strain showed growth improvement in offspring from L group parents, while there was no significant increase in offspring of the CM wild strain. The two size groups of parents from each fish strain in this study were similar to a case of divergent selection or bidirectional selection. Although growth traits (e.g. body weight, body length and growth rate) are usually heritable, responses to selection can vary among species, strains, populations (Dunham, 2011; Dunham & Smitherman, 1983; Gjedrem & Rye, 2018; Rezk et al., 2003; Tave, 1993) and size-groups within a population (Abucayo & Mair, 2004; Chammankuruwet, 1996; Vandeputte et al., 2008; Wagle & Na-Nakorn, 2007). Previous studies on bighead catfish in Thailand reported asymmetric responses to the selection of high and low growth lines (Chammankuruwet, 1996; Wagle & Na-Nakorn, 2007). After two generations, the high line showed a significant response to selection, while the low line had no response (Chammankuruwet, 1996). The differences in body weight between the high and low lines observed at 29 weeks and 33 weeks were 8.3% (8.3 g) and 6.8% (6.38 g) respectively (Wagle & Na-Nakorn, 2007). In the present study, a high divergence in body weight and DWG was found only for the CT domesticated strain at the sampling times of 40, 100 and 190 days, with DWG differences of 35.7%, 50.0% and 43.9% respectively. If the bodyweight distribution of this bighead catfish strain were assumed to be normal (it was actually skewed), the corresponding responses to selection, estimated as half of the difference between the two size groups, would be 17.8%, 25% and 22.0% respectively. These differences were large compared to the findings in bighead catfish in Thailand mentioned above (Wagle & Na-Nakorn, 2007) and the overall average value of 12.7% from various studies reviewed by Gjedrem and Rye (2018).
In contrast, the two broodstock sizes of the CM wild strain showed no significant differences in offspring growth. This result indicated that S group broodstock did not result in slow growth of offspring, implying no responses to selection for small body size. Although this observation was at odds with the prediction of heritable growth traits, similar findings have been reported for bighead catfish in Thailand (Chammankuruwet, 1996), common carp (Cyprinus carpio) (Vandeputte et al., 2008) and medaka fish (Oryzias latipes) (Renneville et al., 2020). In medaka experiments, in which the fish originated from the wild and were subjected to six generations of divergent selection under hatchery conditions, the results showed that those in the small line grew faster with a higher standard body length compared to the controls (Renneville et al., 2020). However, the L group broodstock of wild bighead catfish might not transfer their superior growth to offspring. This could be explained by the low heritability of growth traits in the wild population; larger parents could be due to environmental effects (e.g. competition for food; Dunham, 2011; Tave, 1993). As evidenced by coefficient variations in weight, the individual growth of bighead catfish was highly differentiated. Notably, the growth differentiation was generally higher (with minor significance at the grow-out stage, p = 0.093) in the wild strain compared to the domesticated strain.
The SR is related to the responses of the growth traits (Gjedrem & Rye, 2018). The present study found that parental size had no effect on offspring survival across three culture stages in both bighead catfish strains, with the exception of the juvenile stage of the CM S broodstock. The juveniles in this treatment group had the lowest survival rate due to infectious diseases caused by Aeromonas bacteria, and Saprolegnia and Achlya fungi. Aside from the disease challenges (Srisapoome et al., 2019), the SR at the grow-out stage of bighead catfish is usually high (above 70%) and is not affected by broodstock size (Muiocha et al., 2017), broodstock age, broodstock strain (Duong et al., 2022) or differences in diet (Coniza et al., 2003). However, it is uncommon to see no impact of parental size, egg size and yolk-sac volume on the larval survival of bighead catfish, as compared to other species. Theoretical and empirical work has documented that larger females produced larger offspring with better survival (Green, 2008; Hendry & Day, 2003; Kamler, 2005; Sakai & Harada, 2001), mainly in the natural environment. Similar results were also reported for some freshwater fish species in captive conditions. Rahman et al. (2021) found that after 2 weeks of rearing, the larval survival of the Indian major carp (Labeo rohita) was positively correlated with female body weight but not with egg diameter. In contrast, Holtby and Healey (1986) reported no relationship between larval survival and female size in coho salmon, although larger females produced heavier eggs. Bighead catfish shows cannibalistic behaviour at young stages. Under rearing conditions, cannibalism is a major cause of mortality from the larval stage until the juveniles reach a body weight of about 10 g (Duong et al., 2022). Furthermore, mortality can occur during the several day-long transition period from live food to commercial feed, despite the use of weaning strategies, as bighead catfish larvae, like other fish species, lack the enzymes needed to digest non-live food (Lavens & Sorgeloos, 1996). These factors could dominate parental effects on offspring survival in bighead catfish. Similar observations were reported in another carnivore, climbing perch (Anabas testudineus) (Duong et al., 2014; Morioka et al., 2009) and snakehead (Channa striata) (Qin & Fast, 1996; War et al., 2011). This explanation is consistent with the suggestion that the SRs of young fish are strongly affected by environmental factors (Tave, 1993).
The FCR is another important trait in fish breeding programmes (Besson et al., 2020; Gjedrem & Rye, 2018). The FCR has been correlated to growth traits with different magnitudes, from negative to zero, depending on the species (Besson et al., 2020). In Atlantic salmon, Thodesen et al. (1999) reported that the selected line for five generations had higher growth rate and lower FCR (the inverse of feed efficiency ratio, which was the term used in that study) than the wild line. Similarly, rainbow trout showed a negative genetic correlation between FCR and growth rate in selection experiments (Kause et al., 2006) and wild–domesticated strain evaluation (Martens et al., 2014). In bighead catfish, however, faster growth rates of offspring produced from CT L broodstock did not correspond to lower FCR compared with the other fish treatments. Food intake and growth rate can explain these results. The fish in this group had a higher food intake and grew faster compared to the other fish groups, resulting in similar FCR. The exception was observed in the juveniles in the CM S group; the juveniles in this treatment had the lowest survival due to disease infection, which affected growth but not feed intake and thus increased FCR. Similar results for growth and feed utilization were reported for two strains of C. striata, in which the Vietnamese domesticated strain exhibited a higher growth rate and feed intake but a similar FCR compared to the Cambodian wild strain at both the juvenile and grow-out phases (Nen et al., 2018). The above mentioned results in C. macrocephalus and C. striata imply that fish that consume more food (with optimal nutrient levels) grow faster with similar feed efficiency. These findings could have applications for both individual- and group-based evaluations of carnivorous species with aggressive feeding habits.
ACKNOWLEDGEMENT
This study was funded by the Can Tho University Improvement Project VN14-P6, supported by a Japanese Official Development Assistance (ODA) loan. The authors thank the students who participated in fish rearing and samplings.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Thuy-Yen Duong: Conceptualization, Investigation, Formal analysis, Writing, Funding acquisition. Ngoc-Tran Thi Nguyen: Investigation, Data curation. Son Ngoc Le: Investigation.
ETHICAL APPROVAL
The fish were handled properly and reared in the proper environment. The fish rearing procedure was approved by Can Tho University's Scientific Research Committee.
Open Research
DATA AVAILABILITY STATEMENT
Data is not available for sharing.




