Comparative transcriptome sequencing of the hepatopancreas reveals differentially expressed genes in the precocious juvenile Chinese mitten crab, Eriocheir sinensis (Crustacea: Decapoda)
Abstract
Overnutrition was considered to be the main cause of the precocity in the Chinese mitten crab. Hepatopancreas plays a key role in nutrition metabolism of the crustaceans. However, gene regulation and expression profiles of the hepatopancreas for the precocious crabs still remain ill-defined. Here through de novo sequencing coupled with RNA-Seq (Quantification) analysis, a number of differentially expressed genes (DEGs) in the precocious juvenile crabs were identified. The pooled total RNA from hepatopancreas of the precocious juvenile crabs, normal juvenile crabs and normal matured crabs was used to obtain a reference transcriptome. De novo assembly of the transcriptome revealed 55 561 unigenes with an average length of 1153 bp. Another three cDNA libraries were constructed with the same samples and sequenced separately with the RNA-Seq analysis. A total of 996 DEGs between the precocious and normal juvenile crabs were identified, of which 508 DEGs were upregulated in the precocious juvenile crabs. KEGG enrichment analysis revealed that most enriched DEGs (162, 23.28%) were involved in the metabolic pathways. The expression profiles of 15 DEGs, which includes chitinase, alkaline phosphatase, insulin receptor, juvenile hormone esterase and signal transducer and activator of transcription, were determined by qRT-PCR to confirm the accuracy of the RNA-Seq. These DEGs had already been suggested to be potentially related to the growth and development in crustaceans. Our results, including the assembled transcriptome and the identified DEGs in the precocious juvenile crabs, provide us new resources in the understanding of the functions of the hepatopancreas in the precocity of the Chinese mitten crab.
Introduction
Chinese mitten crab Eriocheir sinensis (Henri Milne Edwards 1854), an important economic crustacean, is naturally distributed in the northern and central coastal region of China (Wang, Li & Li 2008). Chinese mitten crab has also been introduced to Europe and North America, where it has been officially listed as one of the worst invasive species. Because of its abundant nutrition and great taste, Chinese mitten crab has long been considered to be one of the most flavourful and prevalent delicacy in China for thousands of years. During the past two decades, the aquaculture of the Chinese mitten crab has expanded rapidly and become a vigorous industry in China. In 1993, the cultured production of mitten crab in China was only 17 500 tons, while this figure had risen to more than 720 000 tons in 2013, with a commercial value over 40 billion yuan of the total production (Fishery bureau of Ministry of Agriculture PRC 2014). With the development of intensive culture, various problems have appeared in cultured populations, and the sexual precocity is one of the important problems to be solved. It is reported that the sexual precocity rate of 1-year-old crabs in the natural Yangtze River is about 5–10%, but the rate could be up to 20–98% under culture conditions (Zhang & Xu 2001). Precocious crabs mature one year earlier and die off at a small size, where this occurs it usually leads to great losses for the crab farmers, and this problem has already seriously hindered the development of crab aquaculture (He, Jiang, Cao, Liu, Hu & Wang 2013).
Up to now, little is known about the exact mechanism controlling the precocity of the Chinese mitten crab, although researchers have speculated that it may be highly related to the external environmental factors and internal genetic factors (Jin, Xie & Li 2002; Li, Li, Liu & De Silva 2011; Li, Li, Liu, Zhang & Zhang 2011; Wu, Wang, Cheng, Zeng, Yang & Lu 2011; Xu, Tang, Ge & Pan 2016). The main external reasons for the precocity of the Chinese mitten crab were thought to be overnutrition and high cumulative temperature; however, the internal regulation mechanisms of these external factors controlling the process of the precocity are still unknown. Hepatopancreas is a key organ playing important roles in nutritional status, energy storage, moulting, reproduction and other life activities in crustaceans (Huang, Wang, Yue, Chen, Gaughan, Lu, Lu & Wang 2015). Du, Lai, Chen, Cheng and Nan (1999) found that the lipids stored in the hepatopancreas of female were mainly used for the egg development in yolk formation process, and deduced that hepatopancreas could be the primary site of exogenous yolk synthesis in the Chinese mitten crab. In addition, they have also observed that the weight of the ovary was lighter than hepatopancreas when the females were immature, and found that the size of the hepatopancreas decreased to one-third of the ovary after reaching sexual maturity (Du et al. 1999). Vitellogenin gene expression profiles of the Chinese mitten crab have also showed that hepatopancreas was the primary site of exogenous yolk synthesis, which was highly correlated with the development of crab gonad (Li, Chen, Zhou, Li, Zhao & Guo 2006). Considering the close relationship between precocity and nutritional factors, researchers have focused on the comparison of the structure and chemical composition variations of hepatopancreas of the precocious crabs, with emphasis placed on the role of hepatopancreas in nutrition metabolism during the process of the precocity of the Chinese mitten crab (Wu & Jiang 2000; Chen, Cheng & Wang 2003; Chen, Wang & Cheng 2005). Wu and Jiang (2000), Chen et al. (2003) investigated the variation of the chemical compositions (total protein, liver lipid) of the hepatopancreas (and hepatopancreas index) of the precocious juvenile crab and suggested that nutrition were transferred from hepatopancreas to gonad during the process of the precocity in the Chinese mitten crab. Besides, the alkaline phosphatase activity in hepatopancreas of the precocious crabs was also found to be significantly changed compared with the normal juvenile Chinese mitten crabs (Chen et al. 2005). Therefore, we speculated that the abnormal physiological activities in the hepatopancreas might result in the precocity of the juvenile Chinese mitten crab. Consequently, the gene expression patterns of the hepatopancreas should also significantly changed in the precocious juvenile crabs. However, up to now, little information is known about the gene regulation and expression profiles of the hepatopancreas of the precocious juvenile Chinese mitten crab. Sequencing the hepatopancreas transcriptome of the precocious juvenile crabs may greatly facilitate the discovery of functional genes and abnormal gene regulations being related to the precocity of the Chinese mitten crab.
In this study, we first generated a reference transcriptome of the hepatopancreas with pooled total RNA from the precocious juvenile crabs, normal juvenile crabs and normal matured crabs using an Illumina HiSeq 2000 massively parallel sequencing method. A large number of genes were identified from the transcriptase sequencing data, and these genes were mainly enriched in the metabolism pathways. On this basis, three cDNA libraries with total RNA from hepatopancreas of the same three samples (precocious juvenile crabs, normally developed juvenile crabs and mature) were constructed and sequenced separately with RNA-Seq (Quantification) analysis. Differentially expressed genes (DEGs) between the precocious and normal juvenile crabs were determined and analysed. The result provides us a comprehensive hepatopancreas transcriptome of the precocious juvenile crabs, which may help us to understand the molecular mechanisms controlling the precocity of the Chinese mitten crab.
Materials and methods
Preparation of tissue samples
All the experiment samples were obtained from the National Research Center for Chinese Mitten Crab Breeding, China. Precocious and normal juvenile crabs were randomly sampled in a pond in March 2014. The normal mature crabs were randomly sampled in the same pond in December 2014. The mean body weight of the precocious juvenile crabs was 9.03 ± 2.28 g, which was roughly the same size as that of the normal juvenile crabs (about 8.63 ± 2.19 g). The mean body weight of the normal mature crabs was 158.38 ± 6.89 g. Precocious and normal juvenile crabs were determined according to the descriptions in the related reference (Li, Li, Liu & De Silva 2011). All samples from each group were anaesthetized on ice and dissected separately to collect the hepatopancreas (six crabs for each group). All the samples were immediately frozen in liquid nitrogen and stored at −80°C. All crabs used in this study were females.
RNA extraction, cDNA synthesis and sequencing
Total RNA were extracted from the hepatopancreas tissues of each crab in each group with TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer's instructions. RNA integrity and quantity were determined using an Agilent 2100 Bioanalyser (Agilent, Shanghai, China). Equal amounts of RNA from every crab within each group were pooled together for the de novo sequencing to generate a reference transcriptome. To identify the differentially expressed genes between the precocious and normal juvenile crabs, another three cDNA libraries (precocious juvenile crabs, normal juvenile crabs and normal mature crabs) were constructed separately with the same samples above and sequenced separately with the RNA-Seq (Quantification) analysis method. Poly (A) mRNA was isolated using Oligo (dT) beads from the total RNA (Illumina; San Diego, CA, USA) and sheared into small strands with Fragmentation Buffer (Illumina). The fragmented mRNAs were used as templates to construct the cDNA libraries using a TruSeq RNA Sample Prep Kit following the manufacturer's instructions (Illumina). All the cDNA libraries were sequenced on an Illumina HiSeq 2000 platform at Beijing Genomics Institute China (BGI).
Sequence assembly and annotation of the transcriptome
Raw sequence data generated from Illumina HiSeq 2000 platform were treated with the following steps. First, remove adapters that were added for reverse transcription and sequencing, the sequences containing too many ambiguous bases (>5%) and low-quality reads (the rate of reads which quality value ≤10 is more than 20%) were also removed. Then, trinity software was used for the De novo transcriptome assembly with three independent software modules: Inchworm, Chrysalis and butterfly (http://trinitynaseq.sourceforge.net/). Parameters with k-mer length of 25 were used referring to the strategy of Grabherr, Haas, Yassour, Levin, Thompson, Amit, Adiconis, Fan, Raychowdhury, Zeng, Chen, Mauceli, Hacohen, Gnirke, Rhind, Palma, Birren, Nusbaum, Kerstin, Friedman and Regev (2011). Gene functional annotations were performed by BLAST (Version 2.2.25) against the databases of NR, NT, Swiss-Prot, KEGG, COG and GO with an E-value of <1E−05. blast2go (http://www.blast2go.com/b2ghome) was used to perform the functional enrichment analysis for the uniquely assembled transcripts.
Identification and validation of differentially expressed genes
The transcriptome generated through de novo assembly was used as a reference transcriptome for RNA-Seq (Quantification) expression analysis. Raw reads generated from the three cDNA libraries (precocious juvenile crabs, normal juvenile crabs and normal mature crabs) were mapped to the reference transcriptome, and FPKM values were calculated by RSEM software (Li & Dewey 2011). We used ‘FDR ≤0.001 and the absolute value of log2Ratio ≥1 (fold change values ≥2)’ as the threshold to judge the significance of gene expression difference (Audic & Claverie 1997). Besides that, the pathway enrichment analysis was performed for these DEGs using KEGG databases (http://www.genome.jp/kegg/).
To confirm the RNA-Seq (Quantification) results, quantitative real-time reverse transcription PCR (qRT-PCR) was performed on the 15 selected unigenes that were potentially related to the growth and development of the crustaceans in previous studies. qRT-PCR was implemented with the same RNAs as used in the RNA-Seq (Quantification) expression analysis. Fold change of the gene expression relative to the controls was determined by the 2−ΔΔCt method (Livak & Schmittgen 2001), and β-actin was used as a reference gene. The primers were designed based on the assembled transcriptome sequences with Primer 3 (http://frodo.wi.mit.edu/primer3) (Table S1). The qRT-PCR procedure used was as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. All qRT-PCR was carried out on an ABI 7300 Real-Time PCR System (Applied Biosystems Inc.; Waltham, MA, USA) using the SYBR Green PCR Master Mix (Takara, Dalian, China) and performed in triplicate. The specificity of the amplification for each transcript was confirmed by melting curve analysis with SDS software (Applied Biosystems Inc.)
Results
De novo sequencing of the reference transcriptome
Transcriptome sequencing and reads assembly
A total of 56 722 744 raw reads were obtained from the library constructed with pooled total RNA from hepatopancreas of the normal developed juvenile crabs, precocious juvenile crabs and normal matured crabs. After trimming out the low-quality reads, 52 379 428 clean reads were used for the de novo transcriptome assembly. Of these clean reads, the Q20 percentage (proportion of nucleotides with quality value larger than 20) was 97.61% and GC percentages obtained from the mixed sample library was 51.07%. These results demonstrate that the sequencing data have sufficient quantity and high quality to ensure accurate sequence assembly and adequate transcriptome coverage. After de novo assembly and quantitative assessment, 89 374 contigs with mean length of 383 bp and N50 of 715 bp were generated. The contigs were assembled into 55 561 unigenes with an average length of 1153 bp. The summary of the transcriptome sequencing was shown in Table 1. All the assembled unigenes have been deposited in the Transcriptome Shotgun Assembly (TSA) database under the accession GEFT00000000. The version described in this manuscript is the first version, GEFT01000000.
| De novo sequencing and assembly statistics | |
|---|---|
| Sequencing data | |
| Total raw reads | 56 722 744 |
| Total clean reads | 52 379 428 |
| Total clean nucleotides (nt) | 4 714 148 520 |
| Q20 percentage | 97.61% |
| GC percentage | 51.07% |
| Contig | |
| Total number of contigs | 89 374 |
| Average length (bp) | 383 |
| Contig N50 (bp) | 715 |
| Unigene | |
| Total number of unigenes | 55 561 |
| Average length (bp) | 612 |
| Unigene N50 (bp) | 1153 |
Functional annotation and classification of the unigenes
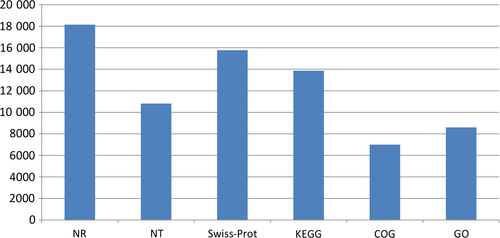
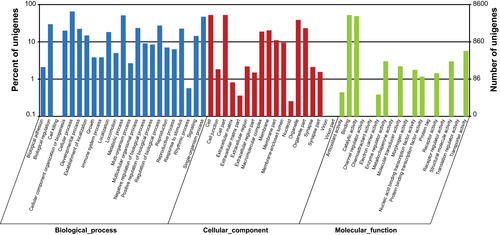
A total of 55 561 unigenes were assembled and selected for the functional annotations. There were 21 833 (39.3%) unigenes with homologous sequences in at least one of the protein databases (with NR, NT, Swiss-Prot, KEGG, COG and GO database). The numbers of the unigenes annotated in the six different databases were shown in Fig. 1. Information from proteins with the highest similarity to the given unigenes was used to annotate the unigene functions (Table S2). Based on the NR annotations, Gene Ontology (GO) gene functional classification was performed to classify the possible functions of all the unigenes. A total number of 8600 unigenes were annotated to at least one GO term annotation by GO classification system. Based on the GO annotation, the unigenes were classified into 59 different groups belonging to three main categories: biological process, cellular component and molecular function. In the categories of biological processes, the dominant subcategories were involved in ‘cellular process’ (5597 unigenes). In the categories of cellular components, the dominant subcategories were ‘cell’ (4511 unigenes). For the categories of the molecular functions, most of the genes were assigned to the subcategories of ‘binding’ (4493 unigenes) and ‘catalytic activity’ (4216 unigenes) (Fig. 2).
Differentially expressed gene (DEG) analysis
Identification and KEGG pathway analysis of the DEGs
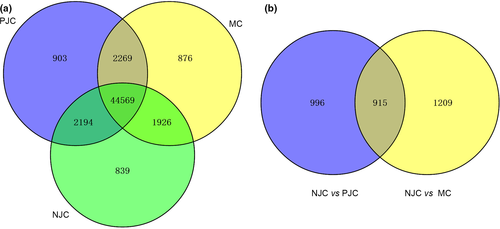
To find the potential precocity-related genes in the Chinese mitten crab, the gene expression patterns and the DEGs among the three libraries were identified by using a RNA-Seq (Quantification) expression analysis. The summary of the RNA-Seq (Quantification) expression analysis was shown in Table S3. A total of 44 569 unigenes were detected to be co-expressed in all the three libraries, which accounted for about 83.2% of all the expressed genes (Fig. 3a). There were 1911 DEGs with significant difference (P < 0.05) between the precocious juvenile crabs (PJC) and normal juvenile crabs (NJC), which include 842 upregulated genes in precocious juvenile crabs and 1069 upregulated genes in normal juvenile crabs. Meanwhile, 2124 DEGs between the normal mature crabs (MC) and the normal juvenile crabs have also been identified. To reduce the risk of selecting the genes probably just being related to the normal sexual maturation, the DEGs (between NJC and MC) were eliminated from the DEGs between the precocious and normal juvenile crabs. Then, 996 DEGs between the precocious and normal juvenile crabs were identified (Fig. 3b), of which 508 DEGs were upregulated in the precocious juvenile crabs. Among these differentially expressed genes, 444 unigenes could be functionally annotated based on the NR database (Table S4).
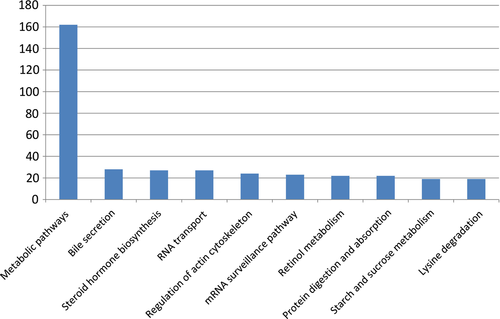
To understand the functional distribution of these DEGs, all the DEGs (NJC versus PJC) were further analysed by KEGG enrichment. KEGG enrichment analysis revealed that all these DEGs were involved in 225 predicated pathways. The most enriched sequences were involved in the metabolic pathways (162, 23.28%), which was followed by the Bile secretion, steroid hormone biosynthesis, RNA transport and regulation of actin cytoskeleton. The top 10 pathways with the largest number of annotated unigenes were shown in Fig. 4.
Validation of the differentially expressed genes
To verify the results of the RNA-Seq (Quantification) expression analysis, 15 differentially expressed genes, which were potentially related to the growth and development of the crustaceans in previous studies, were chosen to be assayed by qRT-PCR (Table 2). The selected genes included chitinase, alkaline phosphatase, insulin receptor, juvenile hormone esterase and signal transducer and activator of transcription, and these genes have been demonstrated to be related to the functions of growth and development in many crustaceans (Chen et al. 2005; Boucher, Ditlecadet & Dubé 2010; Lee, Jeon, Ohc, Kim, Kang, Lee, Mykles & Kim 2011; Okugawa, Mekata & Inada 2013; Li, Xu, Zhou, Lin, Zhou, Zeng, Mao & Gu 2015). As shown in Fig. 5, the expression patterns of the qRT-PCR showed general agreement with those from the RNA-Seq (Quantification) expression analysis. Five genes were upregulated, and 10 genes were downregulated in the precocious juvenile Chinese mitten crabs. Although the expression levels of these genes were not consistent completely, the tendency between the two methods was highly consistent.
| Gene ID | Gene annotation | Length (bp) | E-value |
|---|---|---|---|
| Unigene7853_B4_Live | K01363 cathepsin B [EC:3.4.22.1] | 1919 | 2.00E−37 |
| Unigene8658_B4_Live | NV16524; chitotriosidase-1-like; K01183 chitinase [EC:3.2.1.14] | 1390 | 1.00E−80 |
| Unigene8042_B4_Live | SGK3; serum/glucocorticoid-regulated kinase family, member 3; K13304 serum/glucocorticoid-regulated kinase 3 [EC:2.7.11.1] | 1826 | 1.00E−17 |
| Unigene7861_B4_Live | similar to ubiquitin; K08770 ubiquitin C | 961 | 4.00E−11 |
| Unigene10184_B4_Live | alkaline phosphatase, tissue-nonspecific isozyme-like; K01077 alkaline phosphatase [EC:3.1.3.1] | 2431 | 4E−125 |
| Unigene27318_B4_Live | similar to insulin receptor; K04527 insulin receptor [EC:2.7.10.1] | 515 | 2.00E−31 |
| Unigene6824_B4_Live | treh, si:ch211-147p17.2; trehalase (brush-border membrane glycoprotein); K01194 alpha,alpha-trehalase [EC:3.2.1.28] | 2876 | 3.00E−161 |
| CL71.Contig2_B4_Live | retinol dehydrogenase 12 (all-trans/9-cis/11-cis); K11153 retinol dehydrogenase 12 [EC:1.1.1.-] | 1810 | 7.00E−64 |
| Unigene16375_B4_Live | cadherin-23-like; K06813 cadherin 23 | 1661 | 3.00E−71 |
| CL801.Contig2_B4_Live | GA12945 gene product from transcript GA12945-RA; K15001 cytochrome P450, family 4 [EC:1.14.-.-] | 2607 | 2.00E−161 |
| Unigene8553_B4_Live | GI14754 gene product from transcript GI14754-RA; K00844 hexokinase [EC:2.7.1.1] | 1705 | 2.00E−99 |
| Unigene6917_B4_Live | GI18209 gene product from transcript GI18209-RA; K14843 pescadillo | 2045 | 1.00E−96 |
| CL137.Contig1_B4_Live | juvenile hormone esterase; K01063 juvenile hormone esterase [EC:3.1.1.59] | 2442 | 3.00E−65 |
| Unigene11963_B4_Live | NV13582; signal transducer and activator of transcription 5B-like; K11224 signal transducer and activator of transcription 5B | 3727 | 0 |
| CL2776.Contig3_B4_Live | MGC53924; similar to catecholamine-O-methyltransferase; K00545 catechol O-methyltransferase [EC:2.1.1.6] | 922 | 3.00E−07 |

Discussion
The mechanism for the precocity of the Chinese mitten crab is extremely complex, and it is considered to be highly related to both endogenous and environmental factors (Du, Zhang, Zhao & Zheng 2000). The main internal factors were thought to be involved of the genetic characteristics, while the external factors include ambient temperature, water depth, nutrition and stocking density. Among these external factors, it has been widely believed that overnutrition is the main reason for the precocity of the Chinese mitten crab. Hepatopancreas is an important organ that plays important roles in nutrition metabolism during various significant life activities, such as ovarian maturation, vitellogenin synthesis and moulting in crustaceans (Wang et al. 2008). Large amounts of energy, particularly lipids, are stored in the hepatopancreas in preparation for the energy expenditure in different physiological conditions. Furthermore, hepatopancreas has also been demonstrated to be an important site for the synthesis of vitellogenin and sex steroid hormone in crustaceans. Recently, the hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus were investigated with a comparative transcriptomics method. A plenty of differentially expressed genes being involved in the functions of food digestion, nutrition metabolism and ovarian development have been identified from different development stages (Wang, Wu, Liu, Zheng & Cheng 2014). In Chinese mitten crab, Huang et al. (2015) investigated the transcriptomic profiles of the hepatopancreas and identified some differentially expressed genes at four moulting stages. The differentially expressed genes enriched in post-moult stage were mostly linked to energy consumption whereas genes enriched in intermoult were related to carbohydrates, lipids metabolic and biosynthetic processes. The results further confirmed the critical roles of hepatopancreas in the energy metabolism and biological processes in crustaceans. Sun, Zhang, Liu, Xue, Geng, Hao and Sun investigated the transcriptome of the hepatopancreas of E. sinensis, and found that the expression profiles of the hepatopancreas were significantly changed under eyestalk ablation conditions (Sun, Zhang, Liu, Xue, Geng, Hao & Sun 2014). Transcriptome analysis of the hepatopancreas and discovery of genes involved in the immune pathways have also been well-documented in the Chinese mitten crab (Jiang, Cai, Chen, Zhang, Hu & Wang 2009; Jiang, Yin, Zhang, Hu & Wang 2009; Li, Cui, Liu, Song & Shi 2013). Considering the important roles of the hepatopancreas in crustacean nutrition metabolism, we hypothesized that the gene expression patterns in the hepatopancreas should also significantly changed in the precocious juvenile crabs. To address this hypothesis, the current study explored the transcriptomic profiles of the hepatopancreas from the precocious juvenile crabs by high-throughput sequencing technology. A total of 55 561 unigenes were assembled, and 21 833 (39.3%) unigenes were well annotated. Transcriptomic comparison revealed 996 differentially expressed genes between the precocious and normal juvenile crabs. As expected, we identified a plenty of differentially expressed genes from the RNA-Seq (Quantification) analysis. These differentially expressed genes, including chitinase, alkaline phosphatase, insulin receptor, juvenile hormone esterase (JHE) and signal transducer and activator of transcription (STAT), have long been reported to be related to the growth or development in many other crustaceans (Chen et al. 2005; Boucher et al. 2010; Lee et al. 2011; Okugawa et al. 2013; Li et al. 2015).
Chitinases have widely different functions, which are associated with cuticle turnover, nutrition digestion, disease resistance, metamorphosis, growth and reproduction in the crustaceans (Arakane & Muthukrishnan 2010). The functions of the chitinases and chitinase-like proteins have already been identified in many invertebrates, including Drosophila melanogaster (Zhu, Deng, Vanka, Brown, Muthukrishnan & Kramer 2004) and Bombyx mori (Pan, Lu, Wang, Yin, Ma, Ma, Chen & He 2012). Functional studies suggest that the chitinases have specific roles in insect growth and development (Zhu, Arakane, Beeman, Kramer & Muthukrishnan 2008). In crustaceans, nearly thirty chitinase genes have been reported in Marsupenaeus japonicas, Penaeus monodonl and Fenneropenaeus chinensis, which have been demonstrated to be involved in the degradation of exoskeletons, digestion of chitinous food or the defence against disease (Watanabe, Kono, Aida & Nagasawa 1996; Watanabe & Kono 1997; Priya, Li, Zhang, Wang, Zhao & Xiang 2009; Proespraiwong, Tassanakajon & Rimphanitchayakit 2010; Zhang, Sun, Li, Huang & Xiang 2010). In a recent study, five chitinase genes, including the EsCht1, EsCht2, EsCht3, EsCht4 and EsCht6, were identified in the Chinese mitten crab. The tissue-dependent, developmental stage-related and moulting stage-related differential expression patterns of these chitinase genes were also determined. These results suggest important roles of these chitinase genes for the metamorphosis, growth and reproduction of the Chinese mitten crabs (Li et al. 2015). Therefore, it can be speculated that the upregulated expression of chitinases in hepatopancreas of the precocious juvenile crabs might be of special physiological significance for the gonad development, and which might be associated with the precocity of the Chinese mitten crab.
Alkaline phosphatase, a hydrolase enzyme, is responsible for the removing phosphate groups from many types of molecules in biological metabolic processes. Alkaline phosphatase, which is widely distributed among invertebrates and vertebrates, such as mammals, fish, reptiles and crustaceans, indicates its essential functional roles in life activities. In crustaceans, the activity of alkaline phosphatase has already been considered to be an important immune parameter for many years (Vaseeharan, Ramasamy, Wesley & Chen 2013). The activity of alkaline phosphatase can be influenced by temperature, juvenile hormone and ecdysteroids, which have been considered to be the vital factors of precocity in Chinese mitten crab. Chen et al. (2005) found that the alkaline phosphatase activity in hepatopancreas of the precocious crabs was significantly changed compared with the normal juvenile Chinese mitten crabs. In the present study, we have also found that the expression of the alkaline phosphatase gene was significantly upregulated in the precocious juvenile crabs.
Juvenile hormone (JH) was best known for its pleiotropic functions in insect metamorphosis, development, moulting and reproduction (Nijhout 1994; Durand, Carot-Sans, Chertemps, Montagne, Jacquin-Joly, Debernard & Maibeche-Coisne 2010; Tsubota, Nakakura & Shiotsuki 2010). In the honeybee Apis mellifera, JH plays essential functions in caste determination during larval phases of development and controls the age-related transition, this is also discovered in other social insects (Bomtorin et al. 2014). Methyl farnesoate (MF), the juvenile hormone (JH) analog, plays important roles in the regulation of moulting, metamorphosis and reproduction in crustaceans. By injection of exogenous MF, the ovarian index of green crab Carcinus maenas was significantly enhanced compared to those control crabs (Nagaraju, Rajitha & Borst 2011). It has been demonstrated that the MF titre was negatively regulated by a kind of juvenile hormone esterase-like carboxylesterases in crustaceans. MF esterase activity has been characterized in the hepatopancreas and gonads in many crustaceans, which includes Libinia emarginata, Homarus americanus, Pandalopsis japonica and Procambarus clarkii (Homola & Chang 1997a,b; Tamone, Prestwich & Chang 1997; Lee et al. 2011). In the present study, the expression of a transcript encoding the juvenile hormone esterase-like carboxylesterase gene was found to be downregulated in the hepatopancreas of the precocious juvenile crabs. Previous studies have shown that the concentration of MF in crustaceans would be increased significantly when the female crabs approached an advanced reproductive stage (Nagaraju, Reddy & Reddy 2004). In our study, the downregulated expression of the juvenile hormone esterase-like carboxylesterase in the hepatopancreas may have reflected the increased MF concentration in the precocious juvenile crabs.
The Janus kinase/signal transducer and activator of transcription (JAK–STAT) pathway have been discovered in many multicellular organisms, such as mammals, fish and crustaceans. The JAK/STAT pathway is evolutionarily conserved from insects to mammals, with homologous transduction machinery in each (Chen, Giedt, Tang & Harrison 2014). JAK–STAT pathway is activated by several cytokines and growth factors and playing important roles in various biological processes, such as cell apoptosis, proliferation and differentiation, pathogen resistance, and immune response (Kisseleva, Bhattacharya, Braunstein & Schindler 2002; Harrison 2012). In previous studies, JAK/STAT has been reported to be necessary for the neuroendocrine control of female development and fertility in female mice (Wu, Divall, Hoffman, Le, Wagner & Wolfe 2011). In yellow catfish Pelteobagrus fulvidraco, JAK/STAT pathway was proved to be highly correlated with lipid metabolism (Wu, Tan, Xu, Chen & Pan 2016). The invertebrate JAK/STAT signal pathway was first investigated in embryonic segmentation in Drosophila (Binari & Perrimon 1994). JAK/STAT activation has recently also been demonstrated to have homoeostatic and developmental functions of regulating energy metabolism and organismal growth in Drosophila, (Chen et al. 2014). In our present study, STAT gene expression was differentially downregulated in the hepatopancreas of the precocious juvenile crab. Considering the important roles of JAK/STAT signally in the homoeostasis and development of metabolic system, we speculate that the JAK/STAT pathway might also play important roles in the precocity of the Chinese mitten crab, but the exact mechanisms still need to be further investigated. In the present study, several limitations need to be addressed. Firstly, owing to the financial restraints and the complexity of the development mechanism for the male crabs, only female samples were included in our study. Secondly, the mean size of the adult mature crabs was significantly larger than that of the normal juvenile crabs, some differentially expressed genes identified between the normal juvenile crabs and normal mature crabs may be generated just because of the individual size differences. In future studies, functional characterizations of the key genes (the identified DEGs between the normal and precocious juvenile crabs) or factors should be further investigated to reveal the mechanisms underlying the regulation of precocity in the Chinese mitten crab. In conclusion, this study not only provided us the hepatopancreas transcriptome of the precocious juvenile crabs, but also identified some differentially expressed genes in the precocious juvenile crabs. The results will facilitate further research on the mechanisms of energy metabolism and other biological processes during the process of precocity.
Availability of supporting data
Raw sequencing data are available through the NCBI Sequence Read Archive under Project Accession PRJNA310144.
Acknowledgements
We acknowledge Dr. Qingshun Zhao and Dr. Xiaohua Dong (Model Animal Research Center, MOE Key Laboratory of Model Animal for Disease Study, Nanjing University) for the professional advice and technical support to our manuscript. This work was supported in part by Natural Science Foundation of Jiangsu Province (No. BK20151597); the Fund for Independent Innovation on Agriculture Science and Technology of Jiangsu Province (No. CX(15)1011); the Science and Technology Support Programs of Jiangsu Province (Nos. BE2015369, BE2014412); and the Aquaculture Project of the Jiangsu Ocean and Fishery Bureau (No. Y2014-1, DY2016-1).
Conflict of interest
We declare that we have no conflict of interest other than that stated in the paper.




