Stimulatory effect of thyroid hormones improves larval development and reproductive performance in alligator gar (Atractosteus spatula) and spotted gar (Lepisosteus oculatus)
Abstract
Development of alligator gar (Atractosteus spatula) and spotted gar (Lepisosteus oculatus) larvae was evaluated after exposure of embryos to thyroid hormones (TH) by two different methods of administration. For the first method, alligator gar embryos were placed in a water bath with triiodothyronine (T3; 1 ppm for 2 h), and control treatment embryos were placed in a water bath without T3. For the second method, spotted gar adult males and females were injected with different TH during induced spawning: a group was injected with T3 [20 mg kg−1 body weight (BW)], a second group with thyroxine (T4; 20 mg kg−1 BW), a third group with thyroid-stimulating hormone (TSH; 4 IU kg−1BW) and a control group with dimethyl sulfoxide. Both methods resulted in increases in the concentrations of T3 and T4 in embryos of both species. An increase in the alkaline enzymatic activity of TH-treated larvae was observed as well as an increase in the RNA:DNA, RNA:Dry weight and DNA:Dry weight ratios at hatching. Some positive effects of TH application were: a significant increase in the hatching rate of spotted gar larvae from TH-injected parents and a higher percentage of successful spawns. Evenness of larval sizes and a higher survival rate were observed in alligator gar larvae. Snout development was accelerated by TH treatment in both species.
Introduction
Alligator gar, Atractosteus spatula (Lacépède) and spotted gar, Lepisosteus oculatus (Winchell) are members of the ancient Lepisosteidae family, which evolved approximately 180 million years ago (Grande 2010). Extant gar species in North and Central America and Cuba are scientifically and commercially valuable. As ancient fish, alligator gar and spotted gar are important to the study of evolutionary physiology; furthermore both species are appreciated as sport and edible fish (Mendoza, Aguilera, Rodríguez, González & Castro 2002; Mendoza, Aguilera & Ferrara 2008a).
Many populations of lepisosteids have declined in overall abundance, geographical range and average individual size because of overfishing, habitat loss and alteration, high trophic level (i.e. top predators) and long life span. The decline of lepisosteid populations has led to the development of captive culture techniques to reduce fisheries pressure and restore natural populations. It is especially important to develop more efficient larval rearing protocols, because the highest mortality occurs during the larval phase (Mendoza et al. 2002, 2008a).
A critical factor determining success in fish aquaculture is the mass production of high quality embryos, which results in increased success at first-feeding, and normal development and growth of larvae. Thyroid hormone (TH) administration to broodfish or eggs has been associated with elevated hormone content in eggs, and are reported to accelerate early development and improve larval survival (Kang & Chang 2005). Prior to the maturation of the larval thyroid gland, fish embryos and, subsequently, the larval yolk sack contain significant amounts of TH of maternal origin (Kobuke, Specker & Bern 1987; Tagawa & Hirano 1987). This source of TH is likely important for the physiological regulation of growth and development in larvae prior to the development of functional endogenous thyroid follicles (Einarsdóttir, Silva, Power, Smáradóttir & Bjaörnsson 2006).
Although the role of TH in fish development had long been obscure, a wide body of knowledge about TH in fish development, mostly in teleosts, has been accumulated in the last two decades (Yamano 2005). Most studies of TH have been conducted with juvenile salmonids, relatively slow-growing, cold water species (Sullivan, Iwamoto & Dickhoff 1987 and Sullivan, Bernard, Hara & Dickhoff 1989). In contrast, little information is available on the effects of TH on warm water, fast-growing species. Such fish may be expected to exhibit characteristics of thyroid function different from salmonids due to differences in temperature optima, nutrient requirements, or salinity tolerance (Moon et al. 1994). In addition, the paucity of data on thyroid physiology in ancient fish (Youson 2007) makes further description of the role of TH in lepisosteid larval development especially important, and may provide alternatives to improve larval robustness and survival. Furthermore, hormonal manipulation may reduce costs and increase productivity of lepisosteid culture.
Thyroid hormones, thyroxine (T4) and triiodothyronine (T3) are important molecules in early development and metamorphosis of all vertebrates. In fish, TH of maternal origin are transferred to eggs and are essential for embryonic and larval development (Lam 1994; Brooks, Tyler & Sumpter 1997; Power, Llewellyn, Faustino, Nowell, Björnsson, Einarsdottir, Canario & Sweeney 2001). When administered exogenously to several fish species, these hormones promote a significant increase in embryo pigmentation, hatching and growth rates, higher incidence of successful swimbladder inflation, muscle development and larval metabolic capacity, accelerated metamorphosis and organ differentiation, enhanced immune system development and improved survival rate (Lam 1980; Inui & Miwa 1985; Sullivan et al. 1987; Brown, Doroshov, Cochran & Bern 1989; Tagawa, Tanaka, Matsumoto & Hirano 1990; Ayson & Lam 1993; Huang, Specker & Bengtson 1996; Tachihara, Khalil, Ishimatsu & Tagawa 1997; Kang & Chang 2004; Lam, Sin, Gong & Lam 2005; Walpita, Geyten, Rurangwa & Darras 2007; Mendoza et al. 2008a; Landines, Sanabria, Senhorini & Urbinati 2010).
This study describes the impact of TH on larval gar development by two methods for transferring exogenous TH into fish embryos: (1) the exposure of alligator gar embryos to a specific concentration of T3 by balneation. Alligator gar were selected because of the species' threatened status in Mexico (Mendoza et al. 2008a); (2) and the injection of spotted gar broodstock during induced spawning to enhance transfer of TH to embryos. Spotted gar were selected as a subrogate species for alligator gar because of the greater availability of adults, smaller size and smaller space requirements in captivity.
Materials and methods
Spawning induction and application of thyroid hormones
Alligator gar (Atractosteus spatula)
Alligator gar broodfish used in this study were maintained at the Ecophysiology Laboratory of the Biological Sciences Faculty at the Universidad Autónoma de Nuevo León, Mexico. In total, three females [total length (TL) 1.45 ± 0.19 m, body weight (BW) 9.6 ± 1.02 kg] and nine males (TL 1.10 ± 0.12 m, BW 4.7 ± 0.62 kg) were used. The gender of broodstock was assessed by ELISA-vitellogenin method (Mendoza, Santillán, Revol, Aguilera & Cruz 2012). To induce spawning, all three females were injected intramuscularly with a synthetic analogue of gonadotropin releasing agent and a dopamine inhibitor (Ovaprim®; Syndel Laboratories, Vancouver, BC, Canada) at a dose of 0.5 mg kg−1 BW (Mendoza et al. 2002). Fertilized eggs from a single spawn were collected the next day after the spawn took place and were divided into six groups of 200 fertilized eggs each. Three of these groups were exposed in a water bath with T3 (3,3′,5-triiodo-l-thyronine; Sigma 564605, St. Louis, MO, USA) at 1 ppm for 2 h. T3 was dissolved in 0.5 mL of dimethyl sulfoxide (DMSO; Sigma 275865) before it was added to the water. Three groups of 200 fertilized eggs were exposed to the same concentration of DMSO without the T3 and were used as control treatment. Due to the scarcity of alligator gar broodfish only one TH administration method was used. This also limited the utilization of different dosages.
Spotted gar (Lepisosteus oculatus)
Spotted gar broodfish were collected from Bayou Chevreuil, St. James Parish, LA, USA. Broodstock spawning was conducted at the Nicholls State University farm, Thibodaux, Louisiana. In total, 59 females (TL 0.60 ± 0.1 m, BW 0.93 ± 0.46 kg) and 103 males (TL 0.52 ± 0.02 m, BW 0.56 ± 0.1 kg) were used. To induce spawning, fish were injected intramuscularly with Ovaprim® at a dose of 0.5 mg kg−1 BW (Mendoza et al. 2002). For this trial, immediately following Ovaprim® injection, TH were injected intramuscularly into broodstock using DMSO as an injection vehicle. Three treatments and a control group were used: T3 (20 mg kg−1 BW), T4 (20 mg kg−1 BW; l-Thyroxine, Sigma T1775; Tachihara et al. 1997; Kang & Chang 2004), thyroid-stimulating hormone (TSH; 4 IU kg−1 BW; Thyrotropic hormone from bovine pituitary; sigma T8931) and DMSO (0.5 mg kg−1 BW). For each spawn, 12 individuals were placed in round 1600 L plastic tanks with a ratio of 3:1 male to female based on snout morphology (Love 2002). Three independent spawns were used per treatment. Each independent spawn counted as a treatment replicate.
Larval rearing and sampling
For the first trial, fertilized eggs and larvae of alligator gar were incubated and reared in 25 L aquaria (29 ± 0.22°C; 5.5 mg L−1 O2; 50% daily water exchange). Hatching rate was calculated by counting the number of unhatched eggs. For the second trial, fertilized eggs and larvae of spotted gar from each spawn were incubated and reared in 90 L rearing tanks (23.6 ± 0.36°C; 5.5 mg L−1 O2; recirculation using biofiltration). Fertilized egg samples (30 eggs per spawn) from both species were collected to measure diameter (mm) and to quantify T3 and T4 concentration. Eggs were not dechorionated and sampled eggs were always carefully rinsed after diameter measures. Larvae of both species were fed ad libitum a commercial diet four times daily [Silver Cup®; Nelson's Silver Cup Fish Feed, Salt Lake City, UT, USA (Skretting, Tooele, UT, USA) starter diet; 52% protein, 16% lipids]. Feed was first offered when exogenous feeding began [3 days after hatching (DAH) for alligator gar and 5 DAH for spotted gar]. Samples of alligator gar larvae were collected every two days (0, 2, 4, 6, 8 and 10 DAH), while spotted gar larvae were collected (30 larvae per day) every three days (0, 3, 6, 9, 12 and 15 DAH). Fertilized eggs and larvae were photographed with a digital camera for later morphometric analysis using The University of Texas Health Science Center at San Antonio Image Tool 3.0 software®. Morphometric parameters that were measured included egg diameter (mm), yolk volume [(4/3) × π × (yolk diameter/2)3], TL (mm) and snout–body length proportion [(snout length/total length) × 100]. Samples were frozen at −80°C for quantification of TH, digestive enzymes activity and nucleic acids content.
As the initial number of fertilized eggs from spotted gar from each spawn was not known, hatching rate was calculated using 7.6 L-round plastic containers (21.7 ± 0.03°C) with 100 fertilized eggs (three containers per spawn). In addition, 100 larvae (0 DAH) were placed in similar containers to determine the number of days until 50% and 100% mortality occurred. These larvae were not fed and dead larvae were removed daily. From the number of surviving larvae and survival duration, the survival activity index (SAI) as described by Emata and Borlongan (2003) was calculated.
Thyroid hormone extraction and quantification
The TH extraction procedure was a modification of the previously described methods by Kobuke et al. (1987) Greenblatt et al. (1989) and Klaren, Wunderink, Yúfera, Mancera and Flick (2008). Samples (5 embryos or 3 larvae) were homogenized in 1 mL ethanol with 6-N-propyl-2-thiouracil 1 mM (Sigma P3755) to block T4-T3 enzymatic conversion. The resulting homogenates were centrifuged (1700 g, 10 min, 4°C). This procedure was repeated twice and the supernatant was collected and dried at 37°C. Resolubilization of TH was made in 50 μL ethanol and 450 μL barbital buffer 0.11 M pH 8.6. Extracted samples were processed at the Endocrinology Laboratory of ‘Dr. Jose Eleuterio Gonzalez’ Hospital of the Universidad Autónoma de Nuevo León where T3 and T4 concentration (ng per individual) were quantified by an electrochemiluminescence immunoassay.
Digestive enzyme activity
Dissected digestive tracts from 10 larvae were pooled and homogenized in 1 ml of distilled water at 4°C. The resulting homogenates were centrifuged (16 000 g, 30 min, 4°C) and supernatants were stored in 200 μL aliquots at −70°C to be used later as enzymatic extracts. Digestive enzyme activities evaluated were: total alkaline proteases activity using azocasein (2%) as substrate (Sarath, de la Motte & Wagner 1989), total acid proteases activity using haemoglobin (2%) as substrate (Sarath et al. 1989), trypsin-like activity using N-α-benzoyl-dl-arginine 4-nitroanilide as substrate (Erlanger, Kokowsky & Cohen 1961) and leucine-aminopeptidase activity using l-leucine-p-nitroanilide as substrate (Appel 1974). All assays were performed in triplicate and were conducted at 37°C, except for trypsin assays that were conducted at 30°C. Enzymatic activities were expressed as activity units (U), where 1 U was defined as the increase of 0.01 units of absorbance per minute per larva. The extinction coefficient used was 0.01 for total proteases and trypsin and 0.1 for leucine-aminopeptidase.
RNA:DNA ratio
Nucleic acid quantification procedure used was based on the fluorometric method described by Kaplan, Leamon and Crivello (2001). Lyophilized caudal peduncles from 5 larvae were pooled, weighed, homogenized and digested for 2 h at 55°C in a solution containing 0.1 M NaCl, 0.2% sodium dodecylsulfate and 10 mg mL−1 proteinase K. Following digestion, samples were centrifuged (13 000 g, 10 min). PicoGreen (Invitrogen P7589, Carlsbad, CA, USA) was used to determine double-stranded DNA (dsDNA) concentration in the samples by comparison with a dsDNA standard curve (0–500 ng, Lambda DNA Standard, Invitrogen PicoGreen dsDNA Assay Kit P7589). RNase-free DNase I (Fermentas EN0521) and RiboGreen (Invitrogen R11490) were used to determine total RNA concentration in the samples by comparison with a RNA standard curve (0–500 ng, Escherichia coli 16s ribosomal RNA, Invitrogen RiboGreen RNA Assay Kit R11490). Samples were analysed using a BioTek (Winooski, VT, USA) Synergy fluorescence plate reader (500 nm excitation; 535 nm emission). RNA:DNA ratio, DNA:Dry weight ratio and RNA:Dry weight ratio were calculated.
Statistical analysis
Data were analysed by nested anova to detect differences among treatments (P < 0.05) considering the inherent variation of culture tanks and different spawns (Ruohonen 1998). Significant nested anova were followed by a post hoc Ryan-Einot-Gabriel-Welsch multiple-range test (REGWQ) using the SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Prior to nested anova analysis, data expressed as percentages were arcsine transformed.
Results
T3 and T4 concentration in embryos and larvae
Levels of T3 (113.8 ± 22.8 ng per individual) and T4 (3.83 ± 0.2 ng per individual) were higher in alligator gar embryos that were exposed by balneation to T3, compared with embryos of the control treatment (T3 = 17.1 ± 8.5; T4 = 1.57 ± 0.15 ng per individual), however, no significant differences between treatments were observed in larvae. Levels of T4 were decreased at hatching (0 DAH), however, at 2 DAH T4 levels had increased and remained relatively constant throughout larval development, while levels of T3 had decreased at hatching (0 DAH) and remained low throughout larval development.
In spotted gar, T4 levels were highest in embryos from the T4 (5.97 ± 1.7 ng per individual) and T3 (5.06 ± 0.34 ng per individual) treatments but were not different in larvae among different treatments. Levels of T3 were significantly higher in embryos from the T3 treatment (4.18 ± 1.5 ng per individual) and in 0 DAH larvae from the T4 treatment (4.97 ± 1.5 ng per individual). However, no significant differences were observed among treatments after 3 DAH.
Hatching rate, morphometric analysis and survival rate
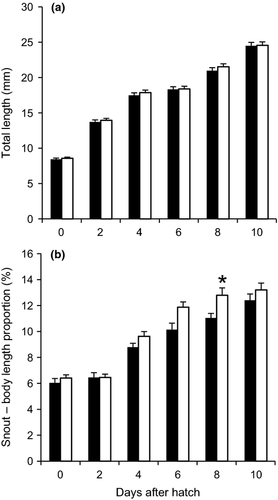
Immersion in T3 had no significant effect on hatch rate of alligator gar embryos. Diameter of T3-exposed fertilized eggs was significantly greater than for control eggs, however, yolk volume of larvae from the T3 treatment at hatching was significantly lower compared with larvae hatched from the control treatment. In addition, a higher, but not significant, larval survival rate was observed in larvae from the T3 treatment compared with the control treatment (Table 1). Finally, TL of larvae throughout development was not affected by T3 exposure of the embryos (Fig. 1a). However, a significant increase in snout–body length proportion was found in the T3 treatment larvae at 8 DAH (Fig. 1b).
| T3 | Control | |
|---|---|---|
| Hatching rate | 94.33 ± 0.57 | 94.18 ± 1.34 |
| Fertilized egg diameter | 4.43 ± 0.09A | 4.09 ± 0.07B |
| Yolk volume at hatching | 30.53 ± 0.53B | 33.27 ± 1.03A |
| Survival rate | 78.19 ± 3.84 | 72.86 ± 2.11 |
- Different letters indicate a significant difference among treatments (P < 0.01)
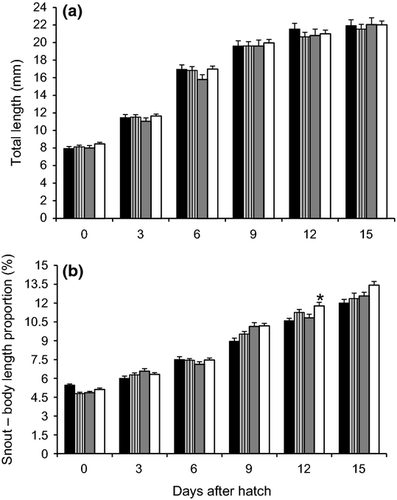
In spotted gar, a positive effect of TH on reproductive success was observed. The percentage of successful spawns was higher in all TH treatments [T4 100% (four spawns), T3 80% (four of five spawns) and TSH 75% (three of four spawns)] compared with the control treatment [42.86% (three of seven spawns)]. Moreover, a significant improvement in hatching rate was observed in the TH treatments when compared with the control group (Table 2). Fertilized egg diameter was significantly higher in the control and T3 treatments, whereas yolk volume at hatching was higher in the T3 treatment. In addition, a significantly lower SAI, as well as number of days to 50% and 100% mortality was observed in the T3 treatment compared with the control group (Table 2). Similar to alligator gar, no significant increase in TL of larvae from TH-treated broodfish was observed (Fig. 2a). However, a significant increase in the snout–body length proportion was observed in the T3 treatment larvae at 12 DAH (Fig. 2b).
| T3 | T4 | TSH | Control | |
|---|---|---|---|---|
| Hatching rate | 84.22 ± 6.01A | 79.88 ± 7.03A | 79.33 ± 5.16A | 64.83 ± 2.91B |
| Fertilized egg diameter | 4.09 ± 0.04A | 3.95 ± 0.03B | 3.94 ± 0.02B | 4.10 ± 0.03A |
| Yolk volume at hatching | 10.01 ± 0.17A | 9.22 ± 0.27B | 9.78 ± 0.37AB | 9.32 ± 0.12B |
| Days to 50% mortality | 17.0 ± 0.29B | 19.6 ± 0.33A | 18.8 ± 0.95AB | 19.8 ± 0.45A |
| Days to 100% mortality | 18.6 ± 0.29B | 21.6 ± 0.50A | 20.7 ± 1.21AB | 21.7 ± 0.73A |
| Survival activity index | 296.1 ± 7.6B | 383.8 ± 13.8AB | 379.6 ± 31.1AB | 419.9 ± 20.2A |
- Different letters indicate significant differences among treatments (P < 0.05)
Digestive enzymes activity
In alligator gar, trypsin-like activity was significantly higher at 4 DAH in the T3 treatment (23.0 ± 3.6 U per larva) than in the control group (13.94 ± 4.3 U per larva). However, later in development no significant differences between treatments were observed. A similar pattern was observed for total alkaline proteases, where activities of these enzymes at 4 DAH were significantly higher in T3 treatment larvae (33.7 ± 5.3 U per larva) compared with the controls (18.03 ± 5.2 U per larva). Finally, the leucine-aminopeptidase (LAP) activity increased at 4 DAH, and T3-treated larvae at 6 DAH showed a significantly higher activity (6.49 ± 0.3 U per larva) compared with the control group (3.61 ± 0.96 U per larva).
In spotted gar, trypsin-like activity gradually increased during development. Trypsin-like activity of TH treatments larvae was significantly higher for T3 and T4 treatments at 6 DAH (T3 = 3.57 ± 1.09; T4 = 2.85 ± 0.46 U per larva) and 9 DAH (T3 = 10.28 ± 1.04; T4 = 10.07 ± 1.09 U per larva) than that of the control group (6 DAH = 0.7 ± 0.02; 9 DAH = 6.98 ± 0.6 U per larva). However, similar to alligator gar, later in development no significant differences among treatments were observed. Total alkaline proteases activity was detected at hatching in all treatments and significantly increased at 9 DAH in the TH treatment (T3 = 6.48 ± 0.9; T4 = 6.74 ± 1.79; TSH = 6.68 ± 2.22 U per larva) as compared with the control group (2.28 ± 0.87 U per larva). Total acid protease activity was initially detected at 6 DAH, and in contrast to other enzymatic activities, no significant effect of TH was observed during larval development. Finally, LAP activity increased gradually during development, and larvae from the T3 and T4 treatments had a significantly higher activity at 3 DAH (T3 = 2.0 ± 0.34; T4 = 2.14 ± 0.38 U per larva) and 9 DAH (T3 = 3.93 ± 0.18; T4 = 3.59 ± 0.08 U per larva) compared with the control group (3 DAH = 0.82± 0.15; 9 DAH = 1.71 ± 0.54 U per larva) and TSH treatment (3 DAH = 1.3 ± 0.12; 9 DAH =2.52 ± 0.06 U per larva).
Nucleic acids
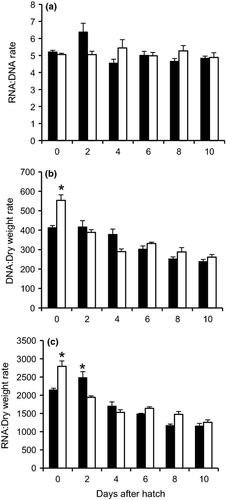
No significant effect of T3 was found in the RNA:DNA ratio in alligator gar, and this ratio remained fairly constant throughout larval development (Fig. 3a). DNA:Dry weight and RNA:Dry weight ratios were significantly higher at hatching in the T3 treatment and tended to decrease as development progressed, however, no significant differences among treatments were observed thereafter (Fig. 3b and c).
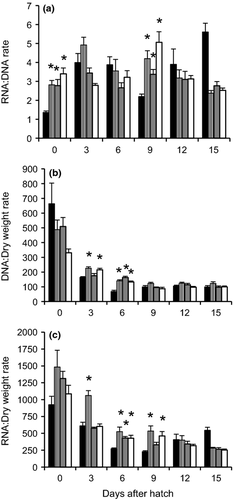
The RNA:DNA ratio in spotted gar larvae at 0 and 9 DAH from TH treatment was significantly higher than that of the control group (Fig. 4a). Similar to the alligator gar, this ratio remained fairly constant throughout larval development. DNA:Dry weight ratio increased significantly by 3 and 6 DAH in larvae from TH treatments (Fig. 4b). While RNA:Dry weight ratio was significantly increased by 3 DAH in larvae from TSH treatment, and at 6 and 9 DAH from TH treatments (Fig. 4c). Overall, DNA:Dry weight and RNA:Dry weight ratios in both species decreased throughout larval development, although in spotted gar this reduction was more conspicuous.
Discussion
Thyroid hormone transference to eggs
Both methods of TH administration resulted in a significant increase in the concentration of TH in embryos of both species. T3 increased by approximately 5 times in both species compared with their respective controls. However, it should be noted that the quantity of T3 available to the embryos of alligator gar was higher (17-fold) compared with spotted gar, considering that the eggs of both species were approximately the same size. This may be explained by differences in larval development among lepisosteid species (Revol, Garza Rodríguez, Hernández Montenegro, Aguilera, Barrera Saldaña & Mendoza 2005; Mendoza et al. 2008a). In this regard, it has been stated that the amount of TH and ratio of T4 to T3 in eggs varies among species, as well as between stocks of the same species (Yamano 2005). Importance of T3 is consistent with the presence of T3 at greater concentrations than T4 before vitellogenin uptake, suggesting an earlier uptake of T3 into oocytes (Tagawa, Ogasawara, Sakamoto, Miura, Yamauchi & Hirano 1994). The more or less constant levels of T4 and decreasing levels of T3 have been observed in other species (Brooks et al. 1997). Although, the dose of hormone and the method of administration may be critical factors influencing results (Moon et al. 1994; Power et al. 2001). Despite the greater increase in TH concentration in embryos exposed to balneation, several researchers have reported that applying TH directly to fish embryos or larvae may result in malformations in larvae and decreased survival rates, probably as a result of an overdose (Nacario 1983; Mendoza et al. 2002; Walpita et al. 2007). Thus, one should be cautious when applying this method, particularly when exposing larvae, as the animal would be exposed to a virtually unrestricted and constant source of hormone, that may overwhelm the ability of the animal to metabolize and excrete the hormone (Raine, Cameron, Vijayan, Lamarre & Leatherland 2004).
In this study, the significant elevation of T3 concentrations in the eggs from the hormone-injected spotted gar females, as compared with controls, points to the transfer of T3 from the maternal circulation into oocytes, as has been observed in other fish species (Ayson & Lam 1993). Moreover, in studies where TH have been extracted from the developing gonads, or when T3 has been administered to broodfish, TH accumulates in oocytes during ovarian maturation (Power et al. 2001; Kang & Chang 2004).
When injecting TSH into spotted gar females, no significant increase in TH concentration was observed in the embryos, contrary to other reports that showed that the administration of TSH allowed increased TH production in broodfish, which was later transferred to the embryos (Yamano 2005). TH may have been regulated by endogenous mechanisms in broodfish (Lam 1994), and are likely transferred at a rate well within the ability of the embryo to maintain TH homeostasis (Raine et al. 2004).
The positive reproductive effects of TH in fish have been well documented. For example, the direct stimulation of T4 secretion by GnRH can occur widely in teleost fish (Chiba, Amano, Yamada, Fujimoto, Ojima, Okuzawa, Yamanome, Yamamori & Iwata 2004). Moreover, in female rainbow trout (Oncorhynchus mykiss), T3 supply with or without gonadotropins (Gn), led to a significant increase in the gonadosomatic index (Cyr & Eales 1996). It has also been reported that T3 can act synergistically with Gn to improve ovary development, amplifying Gn action possibly by increasing the number of receptors for these glycoproteins, and enhancing Gn stimulation of E2 production (Cyr & Eales 1988). Furthermore, T3 has potentiated oocyte maturation [germinal vesicle breakdown (GVBD)] in response to Gn (Sullivan et al. 1989). In addition, T3 increased the activity of adenylate cyclase and the consequent amplification of Cyclic adenosine monophosphate levels in follicular cells, which simultaneously promotes the synthesis of testosterone and estradiol, hormones involved in vitellogenesis and the final maturation of the oocytes and ovulation (Eales & Brown 1993; Bjornsson, Halldorsson, Haux, Norberg & Brown 1997). Based on the increased percentage of successful spawns observed in spotted gar injected with TH, a significant role of TH in lepisosteids spawning was observed. This is in agreement with the close correlation between gonadal maturation and thyroidal status reported for other ancient fish, such as sturgeons (Cyr & Eales 1996). Consequently, TH may improve spawning protocols for lepisosteids. To avoid excessive manipulation of females, a single injection of TH is recommended as a viable alternative for future experiments.
Concentration of TH through larval development
Exposing alligator gar fertilized eggs to T3 resulted in a significantly elevated level of both T3 and T4 in the embryos. T3 was likely taken up by diffusion due to lipophilicity, a process confirmed by Tagawa and Brown (2001). Because T4 is a precursor of T3, the artificially high T3 levels may have prevented the conversion of naturally occurring T4 to T3 resulting in elevated T4 levels. This could be the result of the early inhibition of the 5′-monodeiodinase system, which prevents the conversion of T4 to T3, as described in zebrafish embryos by Walpita et al. (2007) and may explain the elevated T4 levels in the alligator gar embryos exposed to T3. This contention is supported by Eales and Finnson (1991) who observed that exogenous T3 suppresses endogenous T3 production, and other authors (Eales & Brown 1993; Finnson & Eales 1999) have demonstrated that the exogenous administration of T3 depressed hepatic outer-ring deiodination of T4. Within this context, the presence of 5′-monodeiodinase (and potentially also 5-monodeiodinase) would permit the metabolism of TH, thus facilitating some maintenance of internal somatic embryonic TH levels (Raine et al. 2004). The former authors also suggested that the embryo's ability to enzymatically degrade the exogenous T3 is limited, which would contribute to explain the high levels of T3 after hatching.
For the spotted gar, the down regulation mechanism of broodfish may not have allowed the accumulation of important levels of T3 in the embryos, resulting in elevated T4 levels in the broodfish that were thereafter incorporated into the eggs. The conversion of T4 into T3 in fish has been previously reported (Ayson & Lam 1993). In spotted gar, it was only after hatching that T3 levels increased significantly, suggesting that the activation process of 5′monodeiodinases takes place at hatching. Altogether, these results confirm not only the maternal transference of TH but also the great ability of incorporation of TH into lepisosteid eggs.
Activation of 5′monodeiodinases at hatching would explain the rapid utilization of TH, as confirmed by the decrease in the concentration levels of T4 and T3 at hatching, a process that has been observed in several species (Tagawa et al. 1990; Crane, Pickford, Hutchinson & Bown 2004). Moreover, this suggests a significant role in fish embryogenesis, supported by the expression and activity of TH receptors (TR) during blastula and gastrula stages of embryogenesis (Power et al. 2001), and the greater affinity of TR for T3 than for T4 (Nowell, Powerb, Canario, Llewellyna & Sweeneya 2001).
Thyroid hormone concentration throughout larval development of alligator gar and spotted gar did not differ significantly between the treatment and control groups early in development. These results differ from those reported for other fish species, where TH application has resulted in a significantly higher concentration of thyroid hormones in fish larvae up to 6 to 15 DAH (Brown et al. 1989; Ayson & Lam 1993; Tachihara et al. 1997; Kang & Chang 2004). Unlike the several fish species studied to date, lepisosteids have fast growth rates, reaching up to 5.06 mm day−1 (Aguilera, Mendoza, Rodríguez & Márquez 2002). As a consequence, alligator gar and spotted gar embryos may have used the surplus TH quicker than slower growing species. The sharp increase in endogenous T4 in alligator gar at 2 DAH may be indicative of the early development of the thyroid gland, as suggested by Mendoza et al. (2002). Based on the timing of endogenous TH production in different fish species, the onset of endogenous TH production usually occurs at or around the end of yolk sac absorption (Brooks et al. 1997; Yamano 2005). In this study, yolk sac contents of alligator gar and spotted gar were consumed 3 and 5 DAH respectively, approximately when TH production began. The early development of thyroid follicles and endogenous TH secretion, coupled with the exogenous application of hormones may have interfered with the endogenous secretion causing variability in the levels of TH during the first weeks of development. This variability was particularly conspicuous in levels of T3 observed during larval development among replicates of both gar species. Similar variations have been reported in other fish species and have been also attributed to the activity of 5′-monodeiodinase enzymes, which are affected by several factors such as temperature, salinity, stress, starvation, pH, but mostly by levels of postprandial glucose and cortisol (Farbridge & Leatherland 1992; Eales & Brown 1993; Eales, MacLatchy & Sweeting 1993).
Effects of TH in larval development
The smaller spotted gar egg diameter in the TSH and T4 treatments compared with the T3 and control treatments may be related to the inherent variability of different spawns. For the alligator gar, the effect that T3 had on fertilized egg diameter was possibly due to the direct exposure of embryos to T3 balneation, specially since eggs were obtained from a single spawn. As T3 is involved in protein metabolism (Tanaka, Tanangonan, Tagawa, de Jesus, Nishida, Isaka, Kimura & Hirano 1995), it is possible that the concentration of amino acids and small peptides in fertilized eggs may have increased, thus increasing the solute concentration inside the eggs and resulting in a water influx and associated increased in fertilized egg diameter.
Walpita et al. (2007) demonstrated that applying TH to zebrafish mature females resulted in increased hatch rates and concluded that fish embryos possess the required mechanism to use TH in early stages of development. In this study, hatch rate was very high and similar in both alligator gar treated with T3 and the control, while a substantial increase in hatch rate was observed in spotted gar compared with the control. In this regard, effectiveness of TH in increasing hatching rates seems to be exclusive of freshwater fish, as there is almost no effect in most marine fish (Yamano 2005).
The response of yolk sac volume at hatching to treatments was not similar for both lepisosteid species. Alligator gar larvae treated with T3 had lower yolk sac volume at hatching than did control larvae. The faster yolk utilization could result from the exposure to TH, as has been reported in other species (Reddy & Lam 1992; Tanaka et al. 1995) and may account for the higher mortalities in the treated larvae than in the control as reported with other species (Huang et al. 1996; Tachihara et al. 1997). No differences in yolk sac volume were observed among treatments for spotted gar larvae. This may be attributed to differences in the total amount of TH transferred to embryos of this species. Nevertheless, spotted gar larvae from the T3 treatment under starvation conditions died earlier and had a lower SAI, presumably due to a faster utilization of the yolk sac contents. Accelerated yolk resorption after exposure to THs has been reported in other fish species (Nacario 1983). In the same sense, the lower mortality of larvae from the T4 treatment, similar to the control, could be the result of the regulation of deiodinases.
Thyroid hormones did not affect TL of larvae in either gar species. In contrast, several authors reported a significant increase in growth expressed as TL of fish larvae exposed to TH (Inui & Miwa 1985; Ayson & Lam 1993). However, Kuz′mina, Levin, Vei and Rusanova (2010) and Landines et al. (2010) observed that growth rates in Rutilus rutilus and piracanjuba (Brycon orbignyanus) declined after T3 exposure, despite the marked effects of T3 effects on organ differentiation. The absence of anabolic effect on other fast-growing warm water species has already been reported (Moon et al. 1994). Moreover, Mendoza et al. (2002) reported smaller size and the occurrence of deformities in alligator gar larvae after a long exposure to T3 (0.1 ppm for 15 days). Alterations in the skeletal ontogeny of sea bream larvae after exposure to T3 have also been reported (Power et al. 2001). Altogether, these results may reflect species specific effects of TH on growth and may also be related to dose.
Mendoza et al. (2002) reported that TL of larval lepisosteids is not as good of an indicator of growth and development as are other morphometric variables such as body depth at pectoral fin, caudal depth and snout–body length proportion. Irrespective of not finding a significant effect of TH on TL, both alligator gar and spotted gar had faster snout development, confirming the effect of TH in lepisosteid metamorphosis and development. Results of this research and the previous one show that the exposure of eggs or larvae to TH leads to similar results in terms of a faster snout development. Moreover, these results are in agreement with those reported by the same authors who showed that snout development in alligator gar larvae decreased significantly when larvae were exposed to an antithyroid agent (Thiourea), which may be a viable option to reduce cannibalism, one of the main problems in the culture of lepisosteids. Other reports stated that TH stimulate growth differentially during development, so that the proportions of different parts of the body are altered (Ali 1961; Reddy & Lam 1992). Furthermore, a positive effect of TH application in alligator gar was the low variability of larval size towards the end of the study period, which can contribute to improved survival by reducing cannibalism.
The roles that TH have in development and tissue differentiation in fish larvae, including the digestive system have been well documented (Sullivan et al. 1987; Huang et al. 1996). For example, TH increase protein synthesis in specific tissues, including the synthesis of digestive enzymes (Kuz′mina et al. 2010). Faster digestive tract development and higher digestive enzymes activities during larval development may result in a higher capacity to digest more complex nutrients, promoting growth and increasing nutrient storage, increasing survival rates of fish larvae. The pattern of enzymatic activity in alligator gar and spotted gar was similar to that previously reported in lepisosteids (Comabella, Mendoza, Aguilera, Carrillo, Hurtado & García-Galano 2006; Mendoza, Aguilera, Carreón, Montemayor & González 2008b; Aguilera, Mendoza, Iracheta & Márquez 2011). In addition, a significant increase in the alkaline enzymatic activity of TH-treated larvae was observed, primarily at the onset of the enzyme synthesis and secretion (4 and 6 DAH in alligator gar and 6 and 9 DAH in spotted gar). This suggests that TH accelerated development of the digestive structures, which increased digestive enzyme production at the beginning of exogenous feeding. This advanced digestive function induced by TH imparts improvements in food utilization during the critical time of first-feeding.
Finally, the RNA:DNA ratio, a valuable biochemical indicator of the physiological condition of fish larvae during development (Westerman & Holt 1994; Kaplan et al. 2001; Hook, Gorokhova & Hansson 2008) was measured in this study. The amount of DNA is independent of a larvae's physiological condition, is virtually constant in cells of somatic tissues, and can be an index of total cell number. RNA concentration in cells is proportional to the rate of protein synthesis and increases with metabolism (Buckley 1984). Although, no significant effect of T3 on the RNA:DNA ratio was detected in alligator gar, RNA:Dry weight and DNA:Dry weight ratios increased significantly in T3-treated larvae at hatching. In contrast, the RNA:DNA ratio increased significantly at hatching in spotted gar larvae from the TH treatment compared with the control group. The increase in these ratios reflects the effects of TH in larvae protein synthesis and cellular proliferation. The effect on protein synthesis could be observed in a higher enzymatic activity at 6 and 9 DAH in spotted gar which are in agreement with a higher RNA:DNA and RNA:Dry Weigth ratios.
Considering the results of the experiment with the spotted gar, T4 would be the best option among TH in practical terms, taking into account the results achieved in spawning rate, hatching rate and larval survival.
Both TH balneation and maternal injection methods resulted in a significant increase in T3 and T4 concentration in embryos of alligator gar and spotted gar. Furthermore, TH contributed to a faster larval development of alligator gar and spotted gar, since a significant increase in the snout development was observed, as well as an augmentation in the activity of digestive enzymes at the beginning of exogenous feeding, and in the RNA:DNA, RNA:Dry weight and DNA:Dry weight rates at hatching.
Acknowledgments
The authors wish to acknowledge Consejo Nacional de Ciencia y Tecnología (CONACYT Ciencia Básica 103562 Project), Bayousphere Research Laboratory and Nicholls State University. The authors have no conflict of interest to declare.




