Efficacy of 17 α-methyl testosterone and letrozole on sex reversal of protogynous grouper, Epinephelus tauvina (Forskal, 1775) and spawning performance of sex-reversed males
Abstract
This study aimed to test the efficacy of 17 α-methyl testosterone (17 α-MT) alone and in combination with letrozole, an aromatase inhibitor, for the induction of sex reversal in protogynous greasy grouper, Epinephelus tauvina. Further, the long-lasting effects of these treatments and spawning performance of sex-reversed males were also investigated. Greasy grouper with oocytes in the perinucleolus stage were implanted with 5 mg 17 α-MT kg−1 body weight (T1), 5 mg 17 α-MT and 0.2 mg letrozole kg−1 body weight (T2) and 5 mg 17 α-MT with 0.4 mg letrozole kg−1 body weight (T3) and no androgens/enzyme inhibitor implanted (C). The 17 α-MT alone and in combination of letrozole-induced sex reversal in greasy grouper, whereas untreated control fish (C) showed normal ovarian development. However, T2 and T3 group showed 100% sex reversal and completion of spermatogenesis up to functional male phase in 2 and 3 months, respectively, whereas T1 group resulted in only 66.67% functional male with motile spermatozoa after 4 months. Sex-reversed males successfully fertilized the eggs during induced spawning. There were significant differences on fertilization and hatching rates between T2 group (79.00 ± 4.36%; 77.67 ± 2.87%, respectively) and T1 group (57.67 ± 3.17%; 63.87 ± 2.91%, respectively). The result suggested that 17 α-MT (5.0 mg kg−1 BW) in combination with letrozole (0.2 mg kg−1 BW) has the potential to produce 100% sex-reversed male in short period in greasy grouper, which might greatly help in seed production of greasy grouper.
Introduction
Groupers of the genus Epinephelus are an ideal candidate species for intensive aquaculture in tropical and subtropical waters. They are fast-growing, disease-resistant, efficient feed convertor and can withstand crowded environment. Grouper farming appears to have great potential for coastal cage culture as well as pond culture. However, the expansion of aquaculture with this species is hindered by an unreliable and limited supply of seeds as the grouper aquaculture relies mostly on wild-caught juvenile fish, with more than 60% of cultured groupers originating as wild-caught fry (Sadovy, Donaldson, Graham, McGilvray, Muldoon, Phillips, Rimmer, Smith & Yeeting 2003).
Mass production of grouper seed from hatcheries under controlled conditions would certainly be the key to the success of this industry. Collection of mature broodstock of both sexes is required simultaneously for reliable seed production of grouper. But obtaining males from the wild for spawning is problematic because groupers are protogynous hermaphrodites, i.e., fishes which are born female and at some point in their life span change sex to male (Smith 1965; Tan & Tan 1974; Chen, Hsieh & Chang 1980; Shapiro 1987; Brusle-Sicard, Debas, Fourcault & Fuchs 1992; Shapiro, Sadovy & McGehee 1993; Sadovy & Colin 1995). Otherwise rearing juveniles or adults until they naturally transform into males is time-consuming, economically prohibitive or unfeasible for greasy grouper, due to their extremely long time taking process of sex reversal, as reported by Tan and Tan (1974). According to them, the transition from female to male begins at the age of 7 years in Epinephelus tauvina, and the proportion of sex-inverted males increases thereafter up to 100% at the age of 10 years. Therefore, the male broodstock are generally obtained through induced sex reversal at a relatively early age.
Several studies have been conducted on the use of androgens to induce sex reversal in groupers in captive condition (Chen, Chow, Chao & Lim 1977; Roberts & Schlieder 1983; Kuo, Ting & Yeh 1988; Chao & Lim 1991; Tan-Fermin 1992; Tan-Fermin, Garcia & Castillo 1994; Hassin, de Monbrison, Hanin, Elizur, Zohar & Popper 1997; Quinitio, Caberoy & Reyes 1997; Tanaka, Tsuchihashi & Kuromiya 2000; Yeh, Dai, Chu, Kuo, Ting & Chang 2003; Yeh, Kuo, Ting & Chang 2003a; Yeh, Kuo, Ting & Chang 2003b). In E. tauvina, functional males, at the age of three were obtained when a dose of 145 mg 17 α-methyl testosterone (MT) per kg body weight was given orally (Chen et al. 1977) and artificial fertilization was achieved by using these sex reversed males. However, high dose or long-term treatment with MT might result in higher proportion of females in fish (Piferrer & Donaldson 1991; Piferrer, Zanuy, Carrillo, Solar, Devlin & Donaldson 1994). This might be due to aromatization of exogenous androgen to oestrogen and thus the production of female fish.
Letrozole (CGS 20267), with commonly used brand name Femara, is a nonsteroidal triazole derivative and one of the most potent aromatase inhibitors (AIs) (Smith, Andrews & Sinclair 1999). It has potential for use both to prevent the conversion of androgenic steroids to oestrogens and to prevent or diminish the side effects of androgenic steroid (Haynes, Dowsett, Miller, Dixon & Bhatnagar 2003). Letrozole is capable of inhibiting aromatase 98–99% and reducing serum concentrations of oestrone and E2 beyond the limit of detection in patients (Smith et al. 1999). It induced sex reversal in the protogynous red-spotted grouper, Epinephelus akaara (Li, Liu & Lin 2005); Oreochromis mossambicus (Das, Rather, Basavaraja, Sharma & Udit 2012) and inhibited oocyte growth in the Japanese medaka, Oryzias latipes (Sun, Zha, Spear & Wang 2007).
Several studies have demonstrated the use of androgens to induce sex reversal in groupers in captive condition, and few reports are available about the use of letrozole for sex reversal in fish (Li et al. 2005; Sun et al. 2007; Das et al. 2012). However, synergistic effect of androgens and letrozole implantation has not been studied in grouper especially in E. tauvina. Therefore, this study was carried out to assess the effect of 17 α-MT alone and in combination with different doses of letrozole in the induction of sex reversal and spawning performance of sex-reversed males of greasy grouper, E. tauvina. Further, the long-lasting effects of these treatments were also analysed.
Materials and methods
Experimental fish
The greasy grouper, E. tauvina, adults weighing 2.0–5.0 kg body mass were procured from the commercial catches from fishermen and transported to the mariculture hatchery of Visakhaptnam Regional Centre of Central Marine Fisheries Research Institute, Visakhapatnam, Andhra Pradesh (India). The fish were given prophylactic treatment and kept in 10 t cement tank filled with filtered seawater and further stocked into 6 m dia. floating sea cage off Visakhapatnam coast. During the early morning, the fish were anaesthetized with 200 ppm of 2-phenoxyethanol for 2 min or until the opercular movement was significantly reduced. Then, the fish were cannulated to collect gonadal tissue using flexible catheter of 1 mm inner dia (Feeding tube CH 6, local medical shop, Visakhapatnam, Andhra Pradesh, India) passing it through the genital pore and examined microscopically to determine the gonadal status. Fish (n = 24) of 3.01 ± 0.15 kg with the ovary at the stage of perinucleolus oocyte were chosen for the experiment.
Experimental design
Each fish was individually tagged with a tag transponder (PIT TAG FS 2001) for identification and stocked in a floating sea cage of 3.5 m depth and 6.0 m dia. Then, the fish (n = 24) were randomly divided into four groups, each containing six individuals. Test dose for androgen was selected on the basis of earlier work published in different species of fish (Tan-Fermin et al. 1994; Hur, Lim, Hwang, Kim, Ryu, Hur, Song, Jeong, Baek, Takemura & Lee 2012), whereas test dose for AI was selected on our own unpublished data. The treatment group T1 was implanted with 17 α-MT pellets alone at the rate of 5.0 mg kg−1 BW, where as treatment groups T2 and T3 were implanted with a combination of 17 α-MT (5.0 mg kg−1) and letrozole at the rate of 0.2 and 0.4 mg kg−1 BW respectively. No hormonal implantation was carried out for treatment group C, and it was kept as control. The 17 α-MT and letrozole were procured from Sigma-Aldrich (St. Louis, MO, USA). The pellet for implantation was prepared by mixing 17 α-MT, cholesterol and gum acacia in the ratio of 1:2:1. The required quantity of pellet was implanted into the tissue right below the dorsal fin of each fish in January 2012. The implantation of pellet was carried out at regular interval of 2 months to each fish.
After 4 months of experimental period, fish of each group were divided into two subgroups (a and b). First subgroup of each treatment groups (T1a, T2a and T3a) were continued with pellet implantation at regular interval of 2 months, whereas the second subgroups (T1b, T2b and T3b) did not receive any pellet implantation. These subgroups (T1b, T2b and T3b) were maintained without pellet implantation to assess the long-lasting effect of pellet in producing functional male. Water quality parameters during experimental period were pH: 7.89 ± 0.04, salinity: 32.00 ± 0.71 g L−1, temperature: 28.42± 0.31°C, while dissolved oxygen, nitrite and total ammonia were 4.16 ± 0.08, 0.005 ± 0.001 and 0.029 ± 0.005 mg L−1 respectively.
Feeding
Fish were fed twice daily at 0700 and 1600 h with sardine and squid at the rate of 5% of body weight. Moreover, vitamin E (400 mg) capsule (Merck, Goa, India) and Vitamin-mineral mixture were supplemented twice a week along with feed.
Examination of functional male
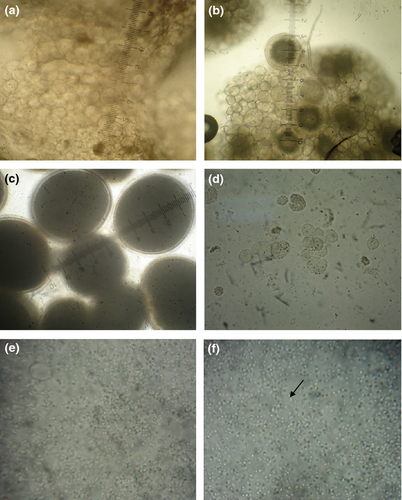
The gonadal stages of the treated as well as control fish were assessed monthly by cannulation using catheter. The biopsy was examined under a trinocular microscope (Lawrence and Mayo, Mumbai, India) and photographed using a digital camera (Sony Corporation, Tokyo, Japan). The gonadal stage of female was classified into F1, F2 and F3 and male into M1 and M2, whereas I denoted intersex stage (Peatpisut & Bart 2010).
F1: The presence of transparent oocytes (<100 μm diameter).
F2: The presence of some opaque oocytes (100–400 μm diameter).
F3: The presence of oocytes with transparent circle surrounding the yolk (>400 μm oocyte diameter).
I: The presence of some oocytes as well as few sperms in biopsy tissue.
M1: The presence of sperm cells but not motile upon activation.
M2: The presence of motile spermatozoa.
Spawning performances of sex-reversed males
The spawning performance trials were conducted in hapa (2 m dia and 3 m depth) of mesh size 500 μm in floating sea cage. The mature female broodstock-containing vitellogenic oocytes with the mean diameter larger than 450 μm were selected as female broodstock (3.0–8.0 kg). The sex-reversed males from three different treatments (T1, T2 and T3) were used for induced spawning. The sex ratio for each induced spawning trial was 1:1. One male and one female were used in each spawning trial, and three trials were performed for each treatment group. The females were injected with two doses of human chorionic gonadotropin (hCG) at the rate of 500 IU kg−1 body weight (BW) at 24-h interval where as sex-changed males were given only one injection of hCG at the rate of 500 IU kg−1 BW on the second day. The female spawned after 12–14 h of second injection. The floating eggs were collected and kept for hatching in glass aquarium. The numbers of floating eggs collected were counted; fertilization rate and hatching rate in each spawning trial were estimated in triplicate. The hatching rates were estimated in respect of fertilized eggs stocked in the tank.
Statistical analysis
All the data were analysed using one-way anova using SPSS V. 16 Software (SPSS, Chicago, IL, USA) followed by Duncan's multiple range tests was used to compare difference at 5% (P ≤ 0.05) level. All the data are expressed as the mean ± SEM.
Results
The processes of the gonadal development and sex reversal were classified into six stages (F1, F2, F3, I, M1 and M2) as shown in Figure 1. The gonadal stages observed in the greasy grouper after initial implantation of 17 α-MT and letrozole during 4 months are summarized in Table 1. All control fish (C) remained as females with different stage of their gonadal development during the experimental period. Intersex stages were found in all implanted fish on 30th day of implantation. 17 α-MT implanted fish showed 16.67% of intersex on 30th day, where as 33.33% intersex with 66.67% sex-reversed male in M1 stage was recorded in treatment T2 and 66.67% intersex with 33.33% sex-reversed male in M1 stage was recorded in T3. Treatment T2 recorded 100% sex reversed male in M2 stage from 60th day onwards. All fish were found to be sex reversed males in T3 on 60th day. However, 50% was in M1 stage and rest 50% in M2 stage in group T3, whereas only 33.33% fish were found to be in sex-reversed male with M1 stage only in T1 group on 60th day. Treatment group T1 showed 33.33% fish in each stage of intersex stage, M1 stage and M2 stage on 90th day. T1 and T2 groups showed 100% sex converted functional male (M2 stage) on 60th and 90th day, respectively, whereas M2 stage sex converted male were only 66.77% after the 4 months of 17 α-MT implantation in T1 group.
| Stages of development – day after implantation | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | Days 30 | Days 60 | Days 90 | Days 120 | ||||||||||||||||||||||||
| F1 | F2 | F3 | I | M1 | M2 | Male (%) | F1 | F2 | F3 | I | M1 | M2 | Male (%) | F1 | F2 | F3 | I | M1 | M2 | Male (%) | F1 | F2 | F3 | I | M1 | M2 | Male (%) | ||
| T1 | 6 | 5 | – | – | 1 | – | – | 0 | – | – | – | 4 | 2 | – | 0 | – | – | – | 2 | 2 | 2 | 33.33 | – | – | – | 1 | 1 | 4 | 66.67 |
| T2 | 6 | – | – | – | 2 | 4 | – | 0 | – | – | – | – | – | 6 | 100 | – | – | – | – | – | 6 | 100 | – | – | – | – | – | 6 | 100 |
| T3 | 6 | – | – | – | 4 | 2 | – | 0 | – | – | – | – | 3 | 3 | 50 | – | – | – | – | – | 6 | 100 | – | – | – | – | – | 6 | 100 |
| C | 6 | 5 | 1 | – | – | – | – | 0 | 4 | 1 | 1 | – | – | – | 0 | 2 | 3 | 1 | – | – | – | 0 | 1 | 2 | 3 | – | – | – | 0 |
- F1, Female stage 1; F2, Female stage 2; F3, Female stage 3; I, Intersex-transitional stage; M1, Male stage 1; M2, Male stage 2; Male (%), M2.
Once the sex-reversed males reached to the functional male phase M2, the spawning performance was conducted (Table 2). The floating eggs were collected in the range of 0.32–0.43 million eggs per spawning. There was no significant difference in number of egg spawned in between spawning trials. In spawning trial, the fertilization rate was significantly different between T2 or T3 male than T1 male. The hatching rate was significantly higher (77.67 ± 2.87%) in spawning trial where male was used from treatment T2 compared with treatment T1 (63.87 ± 2.91%).
| Source of the male | Number of floating eggs in millionNS | Fertilization rate | Hatching rate |
|---|---|---|---|
| T1 | 0.32 ± 0.027 | 57.67b ± 3.17 | 63.87b ± 2.91 |
| T2 | 0.40 ± 0.040 | 79.00a ± 4.36 | 77.67a ± 2.87 |
| T3 | 0.43 ± 0.037 | 73.33a ± 4.67 | 68.07ab ± 3.41 |
- NS, not significant.
- Means bearing different superscripts are significantly (P ≤ 0.05) different.
The gonadal stages of fish of treatment groups T1b, T2b and T3b during post-implantation period (after 4 months) are summarized in Table 3. Sex-reversed males in these treatment groups were in M2 stage on 30th and 60th days. But on 90th day, the reversion of M2 stage males back to M1 stage males was found to be 100% in T1b, whereas it was only 33.33% in T2b and T3b. On 120th day, 100% functional female has been recorded in T1b group; however, only 66.67% and 33.33% were found to be functional females in T2b and T3b group respectively.
| Treatment group | 30 days | 60 days | 90 days | 120 days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | I | F | |
| T1b | 100 | – | 100 | – | – | 100 | – | – | – | 100 |
| T2b | 100 | – | 100 | – | 66.67 | 33.33 | – | – | 33.33 | 66.67 |
| T3b | 100 | – | 100 | – | 66.67 | 33.33 | – | – | 66.67 | 33.33 |
- M1, Male stage 1; M2, Male stage 2; I, Intersex-transitional stage; F, Female.
Discussion
The aim of this study was to assess the efficacy of 17 α-MT alone and in combination with non-steroidal AI letrozole in sex reversal of greasy grouper, E. tauvina for breeding in controlled conditions. The present studies demonstrated that 17 α-MT alone and in combination with letrozole were able to induce temporary sex reversal in greasy grouper, while the control fish remained as females that exhibited normal ovarian development during the course of study.
In the present study, sex reversal into males (66.67%) was recorded within 120 days in MT implanted fish (T1). Similarly, several authors have reported sex – inversion in fish by use of MT. By oral administration, a dose of 145 mg MT kg−1 BW for 1 year (Chen et al. 1977) and 120 mg MT kg−1 BW for 7 months (Chao & Chow 1990) in E. tauvina, 70 mg MT kg−1 BW in Epinephelus fario and 104 mg MT kg−1 BW in Epinephelus salmonoides (Kuo et al. 1988) successfully induced sex reversal in grouper. By injection, a total 30 mg MT kg−1 BW (0.8–1.5 kg BW, six biweekly injections) induced functional males in grouper Epinephelus suillus (Tan-Fermin et al. 1994). Chao and Lim (1991) reported a 2 mg MT-silastic capsule is effective in transforming mature female grouper (3–4 kg) to functional male after 4 months of implantation. However, these MT-implanted males could not fertilize the eggs (Chao & Lim 1991), whereas in the present study, 17 α-MT-implanted males successfully fertilized eggs. This might be due to the application of proper dose of 17 α-MT (5 mg kg−1 BW) for sex reversal and possibly resulted in production of better quality of milt. However, T1 group resulted in only 66.67% of functional male after 4 months. This might be due to aromatization of 17 α-MT to estrogen.
Aromatase inhibitors are steroidal and non-steroidal compounds that inhibit aromatase activity, which catalyses the biosynthesis of estradiol-17β (E2) from its precursor testosterone, resulting in reduced estrogen production (Steele, Mellor, Sawywer, Wasvary & Browne 1987). In the present study, a combination of letrozole and 17 α-MT (T2 and T3) resulted in the production of 100% males with M2 stage. This might be due to inhibition of conversion of 17 α-MT into oestrogen by letrozole (Piferrer et al. 1994). This may also be due to letrozole preventing androgen production of E2 by the ovaries. The combination of 0.2 mg of letrozole and 5 mg of 17 α-MT kg−1 BW (T2) yielded 100% male of M2 stage from 60th day onwards, whereas 5 mg 17 α-MT kg−1 BW alone (T1) resulted only in 66.67% M2 stage after 120 days, suggesting that the letrozole has important role in preventing the aromatization of 17 α-MT which lead to early transformation (60 days) of females to sex-reversed males. This may be the shortest term treatment of 60 days ever recorded to get successful sex reversal of greasy grouper.
Treatment with 17 α-MT probably increases the androgen level in the blood. But since 17 α-MT can be converted to oestrogen by P450 aromatase, it may also increase the oestrogen level (Kwon, McAndrew & Penman 2002). In addition to the exogenic steroid supplied to the system by implantation, treatment with an AI increases the androgen level by blocking the transformation of androgen to oestrogen thereby increasing the androgen/oestrogen ratio. Therefore, the efficacy of aromatse inhibitor treatment with exogenic steroids to induce male differentiation is higher than the efficacy of treatment with 17 α-MT alone.
During this study, although the fish of all three-second subgroups (T1b, T2b and T3b) were in sex-reversed male stage M1 (100% in T1b, 33.33% in T2b and T3b) after 90 days of last implantation, the motility competent sperm cells were not obtained in any of these fish. This might be due to the receding effect of MT and or letrozole because the pellet was not implanted to these subgroups for 90 days. Thus, the present study demonstrated that the induced sex reversal in greasy grouper is reversible in the absence of hormonal induction. The result obtained attributed that the hormonal treatment is necessary to maintain the male phenotype at smaller size of fish as the same phenomenon reflected in natural stock (Tan & Tan 1974). However, fish of first subgroups T1a, T2a and T3a continued to be in M2 stage while receiving the pellet on regular interval of 60 days. Similar to the present study, Chao and Chow (1990) also reported that about 50% sex-inversed males from mature female reverted back to functional females after the MT-treatment was discontinued for about 3 months. Even Yeh et al. (2003) and Yeh et al. (2003a,b) reported that the release of androgens from implant could last long for 60 days in E. coioides. However, in the present study, functional sex-reversed males (M2 stage) have reverted back to male M1 stage only in MT-implanted fish (T1) after 3 months of discontinuation, not to functional females as reported by Chao and Chow (1990). This might be due to difference in method of hormonal administration followed. In the present study, hormone was implanted into the muscle which might have a long-lasting releasing effect than its oral administration to the fish through feed. Methyl testosterone implanted fish reverted back to female more quickly than the fish implanted with a combined dose of MT and letrozole. This showed that use of letrozole in addition to MT is capable of maintaining sex-reversed functional male for prolonged period.
The induced spawnings with the help of hCG were successfully obtained in the present experiment with the sex-reversed functional males. Similar findings by using androgen to induce sex reversal followed by hCG for inducing breeding with artificial fertilization (by stripping) were reported in E. tauvina and E. fuscoguttatus (Chao & Lim 1991) and E. coioides (Yeh et al. (2003); Yeh et al. (2003a,b); Peatpisut & Bart 2010). However, in the present study, eggs were fertilized naturally during induced spawning. This might be due to the good spawning response of sex-reversed and induced males during breeding trials. The fertilization and hatching rates were also successfully obtained from sex-reversed males. In the present study, the sex-reversed males treated with a combination of letrozole and 17 α-MT (T2 and T3) resulted in significantly higher fertilization and hatching rates than the sex-reversed male treated with only 17 α-MT (T1). This might be due to the development of a large number of motility competent sperms in males of T2 and T3 than those of T1. It was very difficult to use natural male as control in spawning experiments because we did not get any natural male from wild during the fish collection and it takes at least 8 years to obtain a natural sex-changed male of this species in captivity (Tan & Tan 1974).
The present study indicated that combination of 17 α-MT and letrozole are more effective in sex-reversal of greasy grouper with the production of 100% functional male in short period. The development of this protocol for sex reversal might have a significant influence on greasy grouper seed production.
Acknowledgments
The authors are thankful to Dr. G. Syda Rao, Director, CMFRI for providing necessary facilities. Thanks to Dr. G. Gopakumar, HOD, Mariculture Division, CMFRI and Dr G. Maheswarudu, SIC, RC of CMFRI, Visakhapatnam for their constant encouragement and support extended during the period of study. We are thankful to ICAR, New Delhi for financial support.




