Spermatozoa and seminal plasma enzyme activity in Persian sturgeon, Acipenser persicus, in relation to short-term storage
An important component of semen examination is the comparison between the characteristics of fresh and stored semen (Lahnsteiner, Berger, Weismann & Patzner 1996). The relationships among the parameters of fresh and cryopreserved semen may be determined to identify markers for prediction of the effectiveness of the cryopreservation procedure for fish semen (Lahnsteiner et al. 1996). As the quality of cryopreserved semen is significantly lower than that of fresh semen, such markers are important for successful cryobanking of fish semen (Piros, Glogowski, Kolman, Rzemieniecki, Domagala, Horvath, Urbanyi & Ciereszko 2002).
The handling and storage of gametes is central to many assisted reproductive procedures and may be broadly considered as either short-term storage, at temperatures above 0°C, or long-term storage at temperatures below 0°C. Short-term storage of fish gametes may be necessary when the availability of male and female gametes for in vitro fertilization is asynchronous (Lahnsteiner, Weismann & Patzner 1997) or if the sources of gametes are spatially separated. Preservation may also be necessary during transport to a facility with the capacity to cryopreserve gametes for long-term storage, in certain instances when spermatozoa and oocytes have been removed from moribund or recently-dead animals in the field. Several factors can, however, affect the quality and viability of the stored sperm. In this regard, the assessment of the biochemical characteristics of sperm, particularly in terms of enzyme characteristics, can provide new insights into spermatozoa motility and fertilization ability, thus creating opportunities for improving artificial reproduction and germplasm resource conservation procedures (Li, Hulak & Linhart 2008).
The enzymology of fish gametes is not well known, when compared with that associated with mammalian semen. Detailed results of research on the enzymes of fish spermatozoa are available with respect to aspartate aminotransferase (AST), lactate dehydrogenase (LDH), acid phosphatase (AcP), β-N-acetylglucosaminidase (β-N AGase) and arylsulfatase (AS) (Schmehl, Graham & Erdhal 1987; McNiven, Gallant & Richardson 1992; Ciereszko & Dabrowski 1994; Piros et al. 2002;). AS and β-N AGase are among the key enzymes, localized within the mammalian acrosome, that play a pivotal role during penetration of the oocyte (Nikolajczyk & O'Rand 1992; Brandon, Srivastava, Heusner & Fayer-Hosken 1997). The activity of AS and β-N AGase during fertilization in sturgeon can provide useful information regarding species-specific fertilization and prevention of polyspermy (when a single egg is fertilized by more than one sperm) as postulated by Cherr and Clark (1985). AcP, one of the enzymes found in sperm, has been described in mammalian spermatozoa and seminal plasma (Yousef, Diamandis, Jung & Diamandis 2001) and has also been observed in spermatozoa and in the seminal plasma of teleostean and chondrostean species. The role of this enzyme in sturgeon reproduction is unknown, but in mammals AcP plays an important role in phosphorylation and dephosphorylation reactions during the fertilization process (for example, during capacitation, hyperactivation, acrosome reaction and sperm–egg fusion) (Urner & Sakkas 2003). Lactate dehydrogenase is an enzyme that catalyzes the conversion of pyruvate (the final product of glycolysis) to lactate, resulting in the production of ATP during glycolysis (Miki 2007). Previous studies have indicated that AcP activity, as well as the activity of AS and β-N AGase, may be an important indicator of the stability of the sperm membrane during long- and short-term storage of sturgeon semen (Piros et al. 2002).
As a consequence of their life-history characteristics, including slow growth and late age-at-maturity, sturgeons are particularly sensitive to exploitation and habitat destruction. Consequently, a number of sturgeon species, including the Persian sturgeon Acipenser persicus, are listed as endangered or critically endangered on the IUCN Red List of Threatened Species (Rochard, Castelnaud & LePage 1990; Bemis & Kynard 1997; Havelka Kaspar, Hulak & Flajshans 2011). Knowledge of the reproduction of wild populations of sturgeon is therefore essential, to ensure the survival of this ancient group of animals (Haxton 2006; Aramli, Kalbassi & Nazari 2013). In the present study we studied storage of undiluted sperm and seminal plasma to investigate possible sources of sperm quality degradation during in vitro storage. We evaluated the effect of short-term storage of spermatozoa from Persian sturgeon, in terms of the enzyme activities of AS, β-N AGase, AcP and LDH.
Seven wild-caught Persian sturgeon (total length:143.38 ± 5.47 cm; mean body weight: 15.81 ± 0.57 kg) were captured (using gillnets, length 18 m, width 5.4 m, mesh size 15 cm) from the south western Caspian Sea. These were transported to the Rajaee Sturgeon Hatchery Center (Sari, Mazandaran, Iran; Lat 36°37′ N, Long 53°05′ E) during March–April 2011. The fish were treated with luteinizing hormone-releasing hormone agonists (LHRH-A2), at a dosage of 5 μg kg−1 body weight by injection into the muscle between the dorsal and lateral scutes. The temperature of the water during the experiment was between 12.5°C and 14.0°C. Spermiation occurred within 18 h after hormonal injection and sperm was collected using a syringe with attached rigid tubing that was inserted into the urogenital opening. Special care was taken to avoid contamination with urine, mucus, faeces or water. Sperm samples were taken from each of the treated males. Semen volume of Persian sturgeons ranged from 20 mL to 50 mL. Sperm samples from each fish (n = 7 per species) were dispensed into 250-mL cell culture containers and kept under aerobic conditions at 4°C. Aliquots (3 mL) were removed from each sample at 24 h (1 day), 48 h (2 days), 72 h (3 days), 144 h (6 days) and 216 h (9 days) after collection, for assessment of enzyme activity in the semen. Seminal plasma was immediately separated from spermatozoa by centrifugation, using a two-step method (500 g for 2 min and, subsequently, at 2500 g for 10 min, using a Labnet Spectrafuge 16 M: Sigma-Aldrich Co., Woodbridge, NJ, USA) and transferred to 1.5-mL microtubes for further analysis.
Acid phosphatase activity was determined using 5 mM p-nitrophenylphosphate in 20 mM citrate buffer (Glogowski, Babiak, Goryczko & Dobosz 1996). After 30 min incubation at 37°C, the reaction was terminated by addition of 0.1 M NaOH and the absorbance at 410 nm was measured (Glogowski et al. 1996). The activity of LDH was determined using an ultraviolet (UV) method with pyruvate and NADH. The reaction mixture consisted of 1.6 mM sodium pyruvate and 0.2 mM NADH in 80 mM Tris–HCl buffer containing 200 mM NaCl. Absorbance at 339 nm was measured. The sample was then incubated for 5 min at 30°C (Vassault 1983). The activity of AS was determined using 0.2 mL 20 mM p-nitrocatechol sulphate as a substrate in 0.5 M sodium acetate buffer of pH 4.9. After 30 min of incubation at 37°C the reaction was terminated, by addition of 2.5 mL 1 M NaOH, and the absorbance at 515 nm was measured (Yang & Srivastava 1974). The activity of β-N AG was measured using p-nitrophenyl β-N-glucosoaminide (0.5 mM) as a substrate in 0.1 M citrate buffer, pH 5.0. After 60 min of incubation at 37°C, the reaction was terminated, by the addition of 0.1 M NaOH, and the absorbance at 410 nm was measured (Jauhiainen & Vanha-Perttula 1986). Normality was assessed by the Kolmogorov test and the Bartlett test was used to determine homogeneity of variance. All data were expressed as the means ± SE. Differences in these values among durations of storage were analyzed by one-way anova, followed by the Duncan test. All analyses were performed at a significance level of 0.05, using the spss statistical software package (Version 11.5, Chicago, IL, USA).
Understanding the effects of short-term storage on semen quality is an important aspect of cryopreservation research, which is essential for the establishment of sperm cryobanks that play a crucial role in the genetic management and conservation of aquatic resources. Although some research has been carried out on the biochemical composition (for example, on the types of enzymes) of the semen in sturgeons, there is a paucity of information on the enzyme activity of semen during short- or long-term storage. This lack of data makes it difficult to compare results from various reports, but some similarities can be noted. The purpose of the this study is to facilitate further understanding of the effects of short-term storage on enzyme activity in A. persicus sperm.
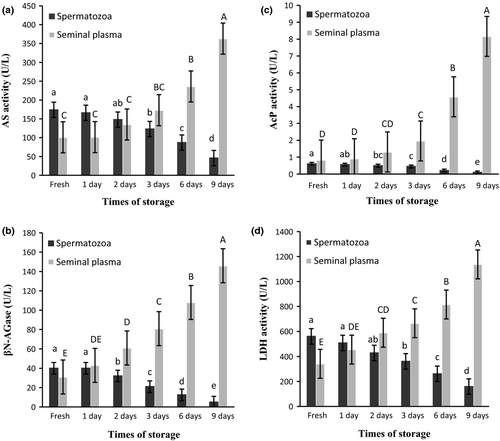
The activities of AcP, LDH, AS and β-N AGase in the spermatozoa and seminal plasma of A. persicus, immediately after semen collection, are shown in Table 1. Based on a statistical analysis, the activity levels of AcP and β-N AGase were not significantly different (P < 0.05) between spermatozoa and seminal plasma samples (Table 1), but those of LDH and AS were significantly different (P > 0.05). With the exception of AcP, all enzymes that we analyzed indicated higher activity in fresh semen than in the seminal plasma. These results are in agreement with those obtained by Piros et al. (2002) for sperm of the Siberian sturgeon, Acipenser baeri and the sterlet, Acipenser ruthenus. We identified AS and β-N AGase in the sperm of Persian sturgeon, which had been anticipated, considering that the active acrosome is present in sturgeon spermatozoa. According to our results, the activity of AS decreased significantly in spermatozoa of Persian sturgeon and increased significantly in seminal plasma after 3 days of sperm storage. During 2 days of storage no significant difference in activity of AS between fresh sperm and semen samples was observed (Fig. 1a). On the other hand, the activity of β-N AGase in seminal plasma increased significantly after 2 days of storage, but the activity of this enzyme in spermatozoa decreased during storage. The lowest enzyme activity was observed after 9 days of storage. No significant difference, in terms of β-N AGase activity, was observed between fresh sperm and semen samples during 1 day of storage (Fig. 1b). This observation concurs with results from a number of other workers (Piros et al. 2002; Sarosiek, Ciereszko, Kolman & Glogowski 2004) who also noted higher concentrations of these enzymes in the seminal plasma of sturgeon, when compared with concentrations in the supernatant of the frozen–thawed sperm sample, thus providing evidence for their presence in both seminal plasma and spermatozoa. The activity of AS and β-D-glucuronidase in seminal plasma and spermatozoa of A. baeri and A. ruthenus has been related to sperm damage. Sarosiek et al. (2004) showed that arylsulfatases exist in sturgeon spermatozoa. Using molecular techniques, they also demonstrated that the similarity of seminal plasma AS to the spermatozoa enzyme is an indication that seminal plasma AS may originate from damaged spermatozoa. The β-N AGase has been purified from the spermatozoa and seminal plasma of Siberian sturgeon (Sarosiek, Kowalski & Glogowski 2008). In mammalian sperm, both of these function as key acrosomal enzymes that play a role in binding of the sperm to the zona pellucida (Brandon et al. 1997; Carmona, Weerachatyanukul, Soboloff, Fluharty, White, Promdee, Ekker, Berger, Buhr & Tanphaichitr 2002). In this study, we observed increasing activity of AcP in the seminal plasma during storage, which increased significantly after 1 day of storage. The highest and lowest activity of this enzyme was observed after 9 days of storage in the seminal plasma and spermatozoa respectively. As was also the case for β-N AGase, no significant differences between fresh sperm and semen samples during 1 day of storage were observed (Fig. 1c). Another enzyme detected in this study was LDH, which is similar to AS. The activity of LDH decreased significantly in the spermatozoa of Persian sturgeon and increased significantly in seminal plasma after 3 days of sperm storage. No significant differences in activity of LDH between fresh sperm and semen samples during 2 days of storage were observed (Fig. 1d). As is also the case in teleost fish, the leakage of LDH and AcP has been found to be a useful marker for estimating damage to spermatozoa in sturgeons. These enzymes are released from spermatozoa after freeze–thawing. For this reason these enzymes are good potential candidates as markers of cryo-injury, as has been described for teleost fish (Glogowski et al. 1996; Lahnsteiner et al. 1996; Babiak, Glogowski, Luczynski & Luczynski 1997). Acid phosphatases, which bind to cell membranes, are thought to be important in the functioning of mammalian semen (Salzberger, Lewin & Shalgi 1992; Visconti & Kopf 1998; Tardiff, Dube, Chevalier & Bailey 2001): they may play a role in regulating protein transphosphorylation and could therefore influence the capacitation and acrosomal reaction processes. Sarosiek, Wysocka, Wysocki, Kowalski and Glogowski (2006) suggested the existence of at least two forms of AcP, with molecular weights of 39 and 19 kDa, in the spermatozoa of Russian sturgeon. In another study, Piros et al. (2002) showed a significant increase in LDH in the supernatant of frozen–thawed spermatozoa of Siberian sturgeon and sterlet. They suggested that LDH probably originates mostly from the spermatozoa and that the disruption of energy supply during cryopreservation subsequently affects sperm motility. These enzymes are probably released from spermatozoa, especially when samples are stored for a prolonged period of time prior to centrifugation. Finally, comparison of enzyme activity in fresh and stored semen is useful for examining the effect of cryopreservation on Persian sturgeon semen. In this study however, the enzyme activity decreased in sperm and increased in seminal plasma during refrigerated storage, even without cryopreservation. With respect to AS and LDH activity, these changes were noted to be significant after 2 days of storage, whereas changes in the activity levels of AcP and β-N AGase were significant after 1 day of storage. All of these enzymes therefore have the potential for use as markers of semen quality. Moreover, the presence of enzymes such as acid phosphatase, β-N AGase and AS in sturgeon sperm may indicate an important role of these enzymes in fertilization, despite the presence of micropyles in sturgeon eggs. We therefore recommend that future studies of short-term effects in sturgeon semen (including the Persian sturgeon) should focus on sperm morphology and on the mechanism of the above-mentioned key enzymes during the fertilization process.
| Enzyme | Fresh sperm | Seminal plasma |
|---|---|---|
| Lactate dehydrogenase (U L−1)a | 516 ± 31.6 | 341 ± 21.07 |
| Acid phosphatase (U L−1) | 0.61 ± 0.01 | 0.83 ± 0.01 |
| Arylsulfatase (U L−1)a | 174 ± 5.17 | 101 ± 3.78 |
| β-N-acetylglucosaminidase (U L−1) | 40.5 ± 2.5 | 31.3 ± 1.33 |
- a Significant at 0.05% level.
Acknowledgments
This study was financed by Tarbiat Modares University, Tehran, Iran. The authors thank all the technical staff of Rajaee Sturgeon Hatchery Center, (Sari, Iran) for their help and support during the conduction of this experiment.




