RNA/DNA ratio is an early responding, accurate performance parameter in growth experiments of noble crayfish Astacus astacus (L.)
Abstract
Recent intensification efforts of astacid culture considerably depend on the appropriate assessment of the animal's physiological condition both for research and application. We conducted a 4 weeks feeding experiment to assess temporal resolution and accuracy of different response parameters (RNA/DNA ratio, RNA per wet weight, carapace length, wet weight, specific growth rate). Juvenile noble crayfish were exposed to five feeding regimes that differed in feeding frequency and food availability. Continuous growth was detected in all feeding regimes with individual increase up to 90% (wet weight) and 17% (carapace length), respectively. Morphometric parameters allowed separation of three weight-groups or two length-groups. During the experimental period RNA/DNA ratios showed both decrease (−17%) and increase (+35%), with superior accuracy than morphometric parameters, separating four groups. Based on RNA/DNA ratios, different feeding regimes were detected earlier, with two groups separated already after 3 weeks. RNA/DNA ratio was clearly superior to RNA per unit wet weight, as the latter failed to detect any differences between groups. In conclusion, RNA/DNA ratio is a valuable tool in nutritional studies with freshwater crayfish if overall growth is the key variable of interest.
Introduction
Exploitation of freshwater crayfish stocks has a long tradition in Europe (Ackefors & Lindqvist 1994; Swahn 2004) and currently, table size crayfish represent a luxury food item of high commercial value. Often, market demands for noble crayfish Astacus astacus (Linnaeus) cannot be satisfied because natural populations are declining due to the dispersion of the crayfish plague Aphanomyces astaci, habitat loss and competition with non-indigenous species (Westman & Savolainen 2001; Jussila & Mannonen 2004). In the last decades, production of noble crayfish has been practised in semi-intensive pond systems. Recently, rising demand for both (re-)stocking material and especially table size crayfish has led to novel attempts to intensify culture in Europe. These primarily focus on introduced signal crayfish Pacifastacus leniusculus (Dana) and native noble crayfish A. astacus (e.g. González, Celada, González, García, Carral & Sáez-Royuela 2010; Kouba, Kanta, Buric, Policar & Kozak 2010). The latter species is more economically auspicious, as animals can be brought to market for both stocking and consumption purposes. Production of table size crayfish requires intensification of culture, which depends considerably on the appropriate assessment of the physiological condition of an individual (e.g. well fed or starving).
Researches promoting culture intensification in crayfish apply somatic growth rate described as an increase in wet weight or body length. However, high individual variation within an age group along with intermittent growth (related to the moulting cycle) result in a restriction of temporal resolution and loss of accuracy.
As an alternative approach, various biochemical indicators to assess physiological condition have been applied. Weber, Higgins, Carlson and Janz (2003) suggest that a strict combination of various biochemical indices representing a certain aspect of the animals' physiological condition allows accurate assessment of condition and growth potential of wild fish. These indices include whole body total lipids and triglycerides, muscle RNA/DNA ratio and muscle protein. Similar conclusions are drawn by Gilliers, Le Pape, Désaunay, Morin, Guérault and Amara (2006) for assessing habitat quality of nursery grounds for juvenile fish based on a combination of different bio-indicators that were measured on individual fish. However, when looking at overall growth capability, quantification of nucleic acids has great potential, as those have a major function in growth and developmental processes. Protein biosynthesis, which may lead to changes in somatic growth, is regulated over two pathways: either increase in the amount of RNA or increased ribosomal activity (Clemmesen 1996; Malzahn, Clemmesen & Rosenthal 2003). Even though increased ribosomal activity may uncouple the positive relationship between RNA concentration and protein biosynthesis, measurements of RNA or DNA per unit wet weight or per individual (Wagner, Campbell, Boudreau & Durbin 2001; Desai & Anil 2002) or RNA/Protein ratio (Moss 1994a,b; Parslow-Williams, Atkinson & Taylor 2001) are successfully used. For fish larvae (Clemmesen 1993, 1994) and crustaceans (Moss 1994a,b; Parslow-Williams et al. 2001; Wagner et al. 2001; Desai & Anil 2002), RNA/DNA ratio has been shown to be a good indicator of their nutritional status. Also Wolf (2004) and Stumpf, Calvo, Castillo Díaz, Valenti and López Greco (2011) used RNA/DNA ratio to assess physiological condition of juvenile crayfish. In adult noble crayfish, this parameter has been applied in a field survey to estimate individual growth rates (Olsson, Nyström, Stenroth, Nilsson, Svensson & Granéli 2008). Though RNA/DNA ratio reflects capacity of protein synthesis normalized to DNA content, to some extent it still is weight and stage dependent, due to metabolic (protein production rate and amount of active ribosomes) and physiological changes (e.g. white muscle protein to body mass ratio) (Ciotti, Targett, Nash, Batty, Burrows & Geffen 2010). Nevertheless, within a developmental stage RNA/DNA ratio is known to reduce noise caused by individual variation in body size and it has been shown to be a reliable parameter (Moss 1994a,b; Wagner et al. 2001; Desai & Anil 2002; Vrede, Persson & Aronsen 2002). In a recent review, Koop, Winkelmann, Becker, Hellmann and Ortmann (2011) suggest RNA/DNA ratio as an instantaneous and less time consuming method for measurements of invertebrates' physiological condition in the field.
We investigated the feasibility of RNA/DNA ratio as an alternative performance parameter in juvenile A. astacus. Since food supply is an effective factor for controlling somatic growth and has direct effect on the organism's physiological condition, we designed an experiment with different feeding regimes. Using this setup we compared the relationship between somatic growth (carapace length, wet weight, specific growth rate) and nucleic acid based indicators (RNA/DNA ratio, RNA per unit wet weight). We expected RNA/DNA ratio to reflect chosen feeding regimes more accurately and with higher temporal resolution than the standard morphometric parameters carapace length and wet weight. Further, using juveniles with a high moulting frequency and higher growth rates (e.g. Kouba et al. 2010) allows shorter experimental periods. The fluorimetric measurement of RNA content and RNA/DNA ratio established by Clemmesen (1993) and modified by Malzahn et al. (2003) has frequently been applied and therefore was our method of choice.
Materials and methods
Experimental set-up and study animals
We used 4 weeks old juvenile crayfish obtained from crayfish eggs incubated under controlled conditions (for details see Järvenpää 1995; Jeske 2007). Until starting the experiment, the animals were kept in communal tanks under experimental conditions in a temperature controlled room at 12 h : 12 h light dark cycle at Kiel University, Department of Limnology.
The experimental system consisted of 10 recirculating 112 L tanks filled with conditioned tap water (19.0 ± 1.1°C, pH 8.4 ± 0.1, 9.2 ± 0.1 mg L−1 O2, 6.0 ± 0.2 mmol L−1 alkalinity). Each tank was equipped with five 2.5 L net cages (mesh size 0.6 mm) housing the animals. Structure and shelter were provided by woody brown coal (Xylit, Vattenfall Europe Mining AG, Spremberg, Germany) added to the net cages. Five feeding regimes with 10 replicates were set up in randomized block design with each 112 L tank representing one block.
Prior to stocking, wet weight (WW) of all individuals was determined (R160P balance, Sartorius AG, Göttingen, Germany) and carapace length (CL) was measured using an eyepiece micrometer with a Zeiss stereo microscope (Stemi SV11; Carl Zeiss Microscopy GmbH, Göttingen, Germany). 400 juvenile A. astacus (WW 45.65 ± 2.56 mg, CL 6.95 ± 0.15 mm) were stocked in groups of eight randomly selected individuals into the net cages at an initial density of 400 Ind m−2.
The animals were fed frozen Daphnia sp. bred at the outdoor facilities of the Department of Limnology. Daphnia sp. were caught every third day and feeding portions were kept frozen at −20°C. The five feeding regimes differed in feeding frequency (feeding 1 fed once a week, feeding 2 fed twice a week up to feeding 5 which was fed five times a week) and therefore in the amount of food available. One feed unit was equal to 4% of the wet weight of stocked crayfish (Chybowski 2007). Once a week, the amount of food was adjusted for growth, dead and removed individuals. After 4 weeks the experiment was terminated.
Assessment of performance parameters
We used five different parameters to assess physiological condition of crayfish in response to feeding regime. One individual from each of the 10 replicate net cages was removed weekly, thereby equally reducing density in all net cages to 200 Ind m−2 at the end of the experiment. WW and CL were determined immediately and the animal was frozen (−80°C) for further analysis. Analysis of RNA and DNA contents were conducted at the facilities of GEOMAR Helmholtz Centre for Ocean Research Kiel, applying a modified fluorescence technique (Clemmesen 1993; Malzahn et al. 2003).
From the frozen or defrosted individual a small amount of tissue (approximately 1 mg) was removed from the ventral side of the abdominal muscle, weighed and returned to -80°C until further use. For extraction of nucleic acids, 400 μL Tris EDTA-SDS 0.01% buffer and some glass-beads were added to the tissue sample and homogenized in a shaking-mill (type: MM2, Retsch GmbH & Co. KG, Haan, Germany) for 20 min at room temperature. Samples were centrifuged at 3830 g and 0°C for 8 min (type: 3–18 K, Sigma, Osterode am Harz, Germany). 300 μL of the supernatant (homogenate containing nucleic acids) were transferred to a new tube and the precipitate was discarded. All fluorimetric measurements were made using a fluorimeter (Fluoroskan Ascent; type 374, Thermo Scientific, Dreieich, Germany) and a 96-well micro titre plate with ethidium bromide as specific nucleic acid fluorescent dye (Clemmesen 1993). We used 16S and 23S ribosomal RNA from E. coli as RNA standard (average slope ratio: 30.92 ± 1.36 (n = 10), 0.25 mg mL−1, Boehringer, 206938) and λ-phage DNA as DNA standard (average slope ratio: 45.63 ± 1.79 (n = 10), 4.0 mg mL−1, Roche Molecular Biochemicals, Mannheim, Germany, 745782). Prior to each measurement, autofluorescence of the homogenate was determined to correct the DNA and RNA fluorescence. To measure DNA content of homogenates, RNA was broken down using RNase A (from bovine pancreas, Serva, Heidelberg, Germany). RNA content was calculated as the difference between the corrected fluorescence of the homogenate and corrected DNA-fluorescence (Clemmesen 1993).
As additional WW based parameter, we calculated specific growth rate (SGR) as suggested by Evans and Jussila (1997):
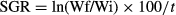
Data analysis
Data were analyzed using two-way Analysis of Variances (anova) with feeding regime and week as the two independent variables and WW, CL, RNA/WW or RNA/DNA ratio as the dependent variable respectively. Normality was analyzed using Shapiro-Wilk statistics; Levene–Median Test was used to test for homogeneity of variances and log transformation was applied for WW and RNA/WW to meet the assumptions of the statistical test (i.e. normality, homogeneity of variances). Data for WW violated the assumption of homogeneity of variances despite transformation. Heteroscedasticity of data is likely to cause type 1 error (i.e. rejecting the null hypothesis even if it is true) but anova is robust to violation of the assumption of homogeneity of variances if sample sizes are equal (Quinn & Keough 2001), which was the case for WW in our study. Therefore, we applied multiple comparisons of two-way anova for log transformed WW data and additionally calculated Kruskal–Wallis one-way anova on Ranks to validate the results. Treatments were compared by Holm-Sidak post-hoc test and differences were considered significant at P < 0.05. To analyze the cause of the variation found in WW, simple linear regressions were used. All analyses were performed using SigmaPlot™ 12.3 (Erkrath, Germany).
Results
Morphometric growth parameters
During the experimental period, a continuous increase in WW and CL was detected. Individuals which were fed more often also had a significantly faster increase for both parameters (Fig. 1). WW increased between 30% (feeding 1) and 90% (feeding 5), CL increased between 5% (feeding 1) and 17% (feeding 5). Results of the two-way anova indicated a significant main effect of both feeding regime and week on CL and WW (all P < 0.001), but no interaction between the two factors was detected (all P > 0.423). However, significant differences between feeding regimes were apparent after 4 weeks. Based on WW, three feeding regimes could be identified (Fig. 1). Feedings 4 and 5 showed a significant increase compared to feedings 1 and 2 (P < 0.05). Multiple comparisons based on the untransformed, ranked data confirmed these results. When comparing CL (Fig. 1), statistically significant differences between feeding 5 and feedings 1–3 (P < 0.05) were found.
Nucleic acid based physiological indicators
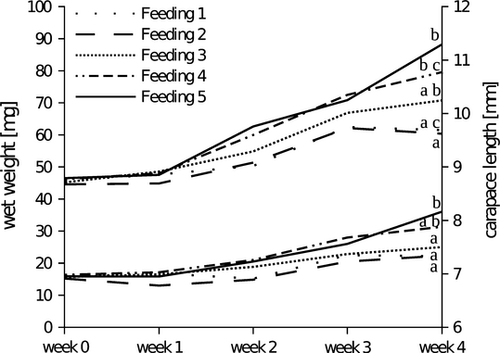
Individually measured RNA/DNA ratios ranged from 0.33 (feeding 2, week 1) to 1.52 (feeding 5, week 4) with RNA amounts between 0.27 μg mg−1 WW (feeding 2, week 2) and 1.61 μg mg−1 WW (feeding 5, week 4) and DNA amounts between 0.52 μg mg−1 WW (feeding 3, week 3) to 2.11 μg mg−1 WW (feeding 1, week 4). Over the experimental period, changes in RNA/DNA ratio ranged from −17% to +35% (Fig. 2) with a decrease in feedings 1 and 3 and an increase in feedings 4 and 5. No differences were observed for feeding 2 between the beginning and end of the experiment (Fig. 2). Results of the two-way anova indicated a significant main effect of feeding regime (P < 0.01) and week (P < 0.001) on RNA/WW, but no interaction between the two factors was detected (P = 0.858). For RNA/DNA ratio, a significant main effect of feeding regime and week (both P < 0.001) as well as an interaction between the two factors was found (P < 0.05).
Accuracy and temporal resolution of response parameters
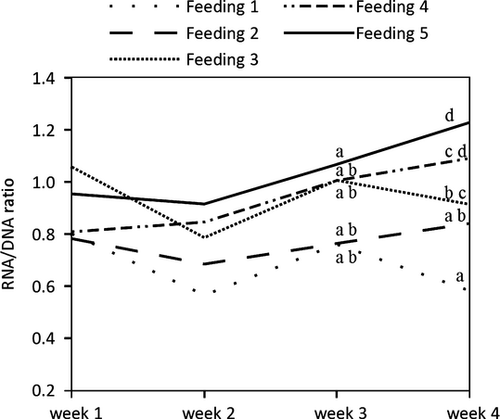
At the end of the experiment, there were clear differences between the feeding regimes for all response parameters. The means were ascending throughout the feeding regimes with feeding 1 having the lowest and feeding 5 having the highest values (Table 1). WW was more accurately reflecting feeding regime than CL, separating three groups, while CL only separated two (Table 1). Calculated SGR values show similar accuracy as CL, detecting only two groups (Table 1). Using RNA/DNA ratio, four different feeding regimes could be detected. Comparing the relationship between SGR and RNA/DNA ratio or RNA per unit WW (Fig. 3), simple linear regressions show that RNA per unit WW only explains 57% whereas RNA/DNA ratio explains 89% of the variation found in specific growth rate (Fig. 3). Furthermore, RNA per unit WW fails in detecting differences in feeding regime (Table 1). RNA/DNA ratio shows differences earlier than the other response parameters (Figs 1 and 2). WW and CL only indicate differences between treatments in week 4, whereas two feeding groups can be separated based on RNA/DNA ratio already after 3 weeks.
| Feeding regime | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Carapace length [mm] | 7.3a (±0.5) | 7.3a (±0.3) | 7.5a (±0.4) | 7.9ab (±0.6) | 8.2b (±0.6) |
| Wet weight [mg] | 61.5ac (±9.5) | 60.4a (±6.7) | 70.7ab (±9.8) | 79.5bc (±18.1) | 88.1b (±24.3) |
| SGR [day−¹] | 1.0a (±0.6) | 1.1a (±0.4) | 1.6ab (±0.5) | 1.9ab (±0.8) | 2.2b (±0.9) |
| RNA/DNA ratio | 0.59a (±0.08) | 0.84ab (±0.25) | 0.91bc (±0.14) | 1.09cd (±0.21) | 1.23d (±0.08) |
| RNA [μg mg−1 wet weight] | 0.84a (±0.33) | 0.74a (±0.15) | 0.75a (±0.20) | 0.96a (±0.26) | 0.99a (±0.34) |
Discussion
Preference of juvenile crayfish for invertebrate food is well documented (Momot 1995) and several studies have demonstrated enhanced growth rates when the animals were fed a diet including zooplankton (Brown, Wetzel, Spacie & Konopka 1992; Verhoef, Jones & Austin 1998; Parkyn & Collier 2002; Sáez-Royuela, Carral, Celada, Pérez & González 2007; González, Celada, González, García, Carral & Sáez-Royuela 2008). Growth rates of juvenile A. astacus obtained on a diet of Daphnia sp. in the present study are in the range reported by others for juvenile noble crayfish under laboratory conditions (e.g. Kouba et al. 2010), demonstrating the suitability of our experimental design for the animals.
Our results show high accuracy and temporal resolution of RNA/DNA ratio in comparison to carapace length (CL), wet weight (WW) and associated SGR. Differences found in RNA/DNA ratio between feeding regimes were not temporarily stable but increased with time (Fig. 2) and RNA/DNA ratio was the only parameter that showed an interaction between feeding regime and time of measurement. This indicates the suitability of this method when studying temporal effects of food supply on the growth potential of juvenile noble crayfish, as these changes in RNA/DNA ratio reflect the integrated effects of the last week's food supply (Clemmesen 1996). For CL and WW differences between feeding regimes did also increase with time, (Fig. 1) but both response variables did not sufficiently indicate these temporal changes to result in a significant interaction between feeding regime and time of measurement.
RNA/DNA ratio explains 89% of variation found in SGR (Fig. 3). Similar accuracy was found by Moss (1994b) for the white shrimp where RNA/DNA ratio accounted for 80% of the variation in growth rate. A slightly higher correlation value (R² = 0.94) is reported on RNA/DNA ratio and specific growth rate (SGR) of Daphnia (Vrede et al. 2002). The correlation between SGR and RNA/DNA ratio has been intensively discussed by Ciotti et al. (2010) who emphasize biological (DNA- content of cells) or even methodological (extraction procedure) reasons may lead to the high correlation of SGR and RNA/DNA ratio. Potential bias due to errors associated with laboratory extraction procedure are considered minor due to the fact that RNA and DNA are measured in the same aliquot and are expressed as ratio. The influence of DNA concentration on the correlation seems to be low compared to temperature or developmental stage. Ciotti et al. (2010) conclude that the correlation is a good measure for physiological condition, which could potentially be sensitive to many more factors than can be controlled for. In our experiment the often applied nucleic acid based parameter RNA per wet weight shows no separation of feeding regimes (Table 1). However, lack of correlation reported in our study is likely due to the use of wet instead of dry weight in combination with a small tissue sample. In a study by Moss (1994b), RNA per wet weight was well correlated with growth rate and accounted for 76% of variation found in growth rate. Nevertheless, as indicated above, RNA/DNA ratio performed even better. Interestingly, Parslow-Williams et al. (2001) found the highest correlation with growth rate of lobsters in RNA per dry weight. This parameter performed better then RNA/Protein and RNA/DNA ratio. One could assume that for small tissue samples, the use of RNA per dry weight is superior to RNA per wet weight as the adhering water might be a source of error in the latter.
The separation of four feeding groups after 4 weeks (compared to three or two groups separated by WW and CL respectively) indicates that RNA/DNA ratio reflects the feeding regime more accurately than WW and CL. It also shows that the variation found in WW and CL does not affect the measurement of RNA/DNA ratio to a greater extend then the actual feeding regime does. Nevertheless, this parameter probably does not completely eliminate the effect of body size or developmental stage (Clemmesen 1994; Malzahn et al. 2003). Clemmesen (1994) showed that herring larvae needed to reach a size of about 30 mm to show no length dependency in their RNA/DNA ratios. Due to the fact that DNA concentration is inversely related to cell size, metabolic or physiological developments may affect RNA/DNA ratios. According to Wolf (2004), the measurement of RNA/DNA ratios is a reliable method for measuring the physiological response of signal crayfish to the changes in their metabolism. However, he also suggests that the best time for the measurement would be the most stable intermoult period, as during moulting water absorption into the cells might change the ratio between DNA content and cell size. Wagner et al. (2001) were able to show a stage dependency of the RNA/DNA ratio in Calanus finmarchicus (Gunnerus) and suggested a calibration for each stage. We measured individuals at different time points rather than comparing different developmental stages and did not undertake calibrations concerning developmental stage or moulting period. Faster increases in WW and CL at week 2 and week 3 (Fig. 1) indicate that moulting has most likely taken place during the experimental period. Furthermore, parts of the exoskeleton were found more frequently in the net cages during these 2 weeks. Whether changes in RNA/DNA ratios (Fig. 2) are also due to the moulting events could not be determined, as moulting was not measured in our study. Even without taking stage-dependent adjustments into account, we were able to reach high accuracy and temporal resolution with this method after a short experimental period (4 weeks). Consequently, we conclude that comparison of individuals of similar developmental stage based on relative changes in RNA/DNA ratio between treated or observed groups is feasible.
Temperature is another important and widely discussed variable (Buckley et al. 2008, Ciotti et al. 2010) that influences metabolic activity by principally affecting RNA concentrations and RNA activitiy. Therefore, RNA/DNA ratios as well as the relationship between RNA/DNA ratio and SGR are modified by temperature. Growth models, which include temperature and interaction terms (temperature with RNA/DNA ratio) have been suggested for different species (Wagner et al. 2001; Buckley et al. 2008, Ciotti et al. 2010). We did not consider this temperature dependency of SGR-RNA/DNA relationship, as individuals reared at suitable temperatures (Kouba et al. 2010) are comparable among each other (Buckley et al. 2008). Therefore, we suggest the effects of temperature on our results can be neglected and food supply had the main influence on the assessed performance parameters.
Furthermore, for stock assessment and field monitoring, this method has great potential (reviewed in Koop et al. 2011), even though calibration to developmental stage may be necessary. Many authors have reported age dependent within species variation (Wagner et al. 2001; Desai & Anil 2002; Lemos, Garcia-Carreňo, Hernández & Navarette del Toro 2002), but as shown, within an age group RNA/DNA ratio correlates very well with food supply and therefore may be a valuable tool to assess physiological conditions related to food availability of either wild or farmed stocks of freshwater crayfish. For this purpose, an exact and minimal invasive measurement of the animal's physiological condition is desirable. Clear advantages of using RNA/DNA ratio are its accuracy and sensitivity allowing to process tissue samples down to 10 μg dry weight (Clemmesen 1996). Furthermore, changes in the amount of RNA can be measured rather quickly within 24 h for some crustacean species (Moss 1994b). According to Clemmesen (1996) limits for significantly distinguishable changes within the RNA/DNA ratio are, depending on species, 4–9 days. Due to this time lag, measurements do not show the effects of the last diet provision, but rather the integrated effects of the last weeks food supply. This time period is shorter than one moulting cycle in juvenile noble crayfish, thus showing physiological changes earlier than measurements of WW and CL would.
Another important benefit of applying RNA/DNA ratio to assess physiological condition arises for research related to crustacean culture. A major objective for intensive culture lies in the development of adequate feeds, highlighted by numerous studies on this topic (e.g. Ackefors, Castell, Boston, Räty & Svensson 1992; D'Agaro 2004; González et al. 2008; Garza de Yta, Davis, Rouse, Ghanawi & Saoud 2012; Saoud, Garza de Yta & Ghanawi 2012). But with few exceptions (e.g. Wolf 2004) those are relying on weight increase as a measure of somatic growth rate, thereby necessitating long (several weeks to months) experimental duration. Likely therefore, the vast majority of studies is dealing with juvenile crayfish, as adults will moult less often, requiring even longer experiments. We can show that RNA/DNA ratio correlates very well with food supply for crayfish. Since the method is useful to show ongoing anabolism without needing a moulting event, its application will facilitate shorter experiments and consequently more thorough testing of feeds which is especially important when aiming at producing table size crayfish.
Several controversial findings to whether RNA per weight, RNA/Protein or RNA/DNA ratio is the most accurate performance parameter have been published (Clemmesen 1993; Moss 1994b; Parslow-Williams et al. 2001; Wagner et al. 2001; Desai & Anil 2002). On the basis of our experimental study, we suggest that for noble crayfish, RNA/DNA ratio is an accurate performance parameter that responds rapidly to differences in food supply.
Acknowledgments
We thank Helmut Jeske, Krebszucht Oeversee for providing the crayfish, Sabine Geisler and Burkhard von Dewitz for laboratory assistance. Vattenfall Europe Mining AG Spremberg, Germany kindly provided Xylit substrate. Financial support for the study was provided by a European Fisheries Fund grant (No. SH-363E.35) to Heinz Brendelberger.




