Larval development and salinity tolerance of Japanese flounder (Paralichthys olivaceus) from hatching to juvenile settlement
Abstract
Salinity tolerance and growth of Japanese flounder Paralichthys olivaceus at different developmental stages were evaluated, including newly hatched larvae (nhl), yolk sac larvae (ysl), oil droplet larvae (odl), post oil droplet larvae (podl), premetamorphic larvae (preml) and prometamorphic larvae (proml), at 11 salinities from 5 to 55 g L−1 for 96 h. The ontogenesis during the early life of P. olivaceus was investigated under hatchery salinity 35 g L−1. The results showed that suitable salinities for nhl, ysl, odl, podl, preml and proml larvae were 10 to 25 g L−1, 10 to 30 g L−1, 20 to 30 g L−1, 30 g L−1, 10 to 30 g L−1, 15 g L−1, respectively, demonstrating an ontogenetic variation of salinity tolerance. The salinity tolerance of nhl, ysl, preml was higher than that of odl, podl and proml. The ysl and preml larvae displayed wide salinity tolerances. The present findings demonstrate that the suitable salinity for larviculture of P. olivaceus is 20–25 g L−1 before the depletion of oil droplet; after that, higher salinity (30 g L−1) should be ensured for the post-oil droplet larvae; the premetamorphic larvae can be cultured at a wide salinity range (10–30 g L−1), and the metamorphosed larvae should be reared at salinity about 15 g L−1.
Introduction
Salinity is one of the most important abiotic factors which can affect the survival and distribution of fish in coastal and estuarine waters. The effects of salinity variation are important considerations during early life stage of fish (Schreiber 2001). Salinity can strongly influence physiological processes and morphological developments of larval fish, and has a direct effect on growth and survival by expending the amount of energy needed for osmoregulation (Boeuf & Payan 2001; Varsamos, Nebel & Charmantier 2005). Salinity also affects egg hatching (Martínez-Palaciosa, Morteb, Tello-Ballinasa, Toledo-Cuevasa & Rossb 2004; Yang & Chen 2006; Zhang, Shi, Zhu, Liu & Zang 2010), yolk consumption efficiency (Swanson 1996), larval growth and survival (Murashige, Bass, Wallace, Molnar, Eastham, Sato, Tamaru & Lee 1991; Tandler, Anav & Choshniak 1995; Estudillo, Duray, Marasigan & Emata 2000; Madrones-Ladja 2002; Sampaio & Bianchini 2002; Fielder, Bardsley, Allan & Pankhurst 2005; Sampaio, Freitas, Okamoto, Louzada, Rodrigues & Robaldo 2007).
The successful establishment of a species in a given habitat depends on the ability of each developmental stage to cope with changes in salinity through osmoregulation (Varsamos et al. 2005). Because osmoregulation is an energy demanding process, in some fish species, energetic budget is thought to be lower at iso-osmotic salinities (Holliday 1969), and more energy is available for growth and/or survival (Sampaio & Bianchini 2002). Some marine teleost larvae are able to osmoregulate (Hiroi, Kaneko, Seikai & Tanaka 1998; Miyazaki, Kaneko, Hasegawa & Hirano 1998; Hiroi, Kaneko & Tanaka 1999) and hold their body-fluid ion concentrations at levels between 11 and 14 g L−1 (Alderdice 1988). The mechanisms and organs for osmoregulation probably changes with development stage in larval fish (Hwang 1987; Alderdice 1988; Banks, Holt & Wakeman 1991; Hiroi et al. 1999; Schreiber 2001). In hypo-osmoregulating fish larvae, chloride cells located on the skin are the main site for excreting ions entering the larvae by diffusion, whereas the water lost by osmosis is regained by water-drinking behavior (Tytler & Blaxter 1988a,b). Some work on the effects of salinity on growth and survival of fish larvae, such as scad Caranx mate (Cuvier 1833) (Santerre 1976), brown-spotted grouper Epinephelus tauvina (Forsskål 1775) (Akatsu, Al-Abdul-Elah & Teng 1983), gilthead sea bream Sparus aurata (Linnaeus 1758) (Tandler et al. 1995) and Brazilian flounder Paralichthys orbignyanus (Valenciennes 1839) (Sampaio et al. 2007) larvae, showed an increase in survival and/or growth at intermediate salinities (>15 g L−1 but <30 g L−1), supporting the above concept. Others found improved growth or survival at higher salinities (>34 g L−1), such as those conducted with milkfish Chanos chanos (Forsskål 1775) larvae (Swanson 1996), southern flounder Paralichthys lethostigma (Jordan and Gilbert 1884) (Henne & Watanabe 2003; Moustakas, Watanabe & Copeland 2004) and common snook Centropomus undecimalis (Bloch 1792) (Rhody, Nassif & Main 2010). The lack of consistent results among species is further illustrated by cobia Rachycentron canadum (Linnaeus 1766) larvae (Faulk & Holt 2006), where no significant differences in growth were observed among different salinities. Thus, results vary among species and across developmental stages.
In recent years, many researchers have paid much attention to the salinity tolerance during ontogeny in coastal marine fish. Larval southern flounder P. lethostigma was able to tolerate a reduced salinity of 20 g L−1, but salinity of 10 g L−1 caused a decrease in survival (Daniels, Berlinsky, Hodson & Sullivan 1996). In summer flounder Paralichthys dentatus (Linnaeus 1766), a decline in tolerance to acute changes in salinity occurred after larvae metamorphosized (Schreiber & Specker 1999). In greenback flounder Rhombosolea tapirina (Günther 1862), egg fertilization, incubation and yolk sac resorption were dependent on salinity, with salinity of 28 g L−1 resulting in the best overall performance (Hart & Purser 1995). There is evidence that low-salinity tolerance of larval summer flounder P. dentatus increases as development progresses from hatching through metamorphosis (Specker, Schreiber, McArdle, Poholek, Henderson & Bengtson 1999). However, effects of salinity on growth and survival of Japanese flounder Paralichthys olivaceus (Temminck and Schlegel 1846) larvae at early stage from hatching to metamorphic stages received little attention.
Japanese flounder P. olivaceus is an euryhaline teleost that inhabits coastal waters and estuaries, and is capable of adapting to substantial changes in environmental salinity. In natural environments, P. olivaceus has a short distance migratory habit, returning back to shallow waters for breeding, and their juveniles naturally occur in estuaries where salinity is usually low. Many studies showed that some flounder fish experience some crucial early developmental events, such as transition from endogenous to exogenous nutrition and metamorphosis, are very sensitive to the environmental salinity variation (Conides & Glamuzina 2001; Schreiber 2001). At present, P. olivaceus is a popular food flatfish with high current market value, accordingly the marine-based aquaculture industry in this fish has been widely developed. However, mass mortality during larviculture has been frequently reported and poses a bottleneck for aquaculture of this species. To date, the larviculture of this species has not been well-studied, especially the effects of salinity on growth and survival in different early stages are still unclear. Knowing which salinity is more appropriate may enhance larval survival and growth by channeling energy saved by less osmotic work, to larval development and growth. The objective of this study was to evaluate the effect of salinity on the survival and growth and understand salinity tolerance of P. olivaceus in different early developmental stages, finding out the suitable salinity range for larviculture in this species.
Materials and methods
Egg fertilization, incubation and larvae rearing
All brood stocks of flounder P. olivaceus were selected from Beidaihe Central Experiment Station of Chinese Academy of Fishery Sciences (CAFS), Hebei, China. Female brood fish with soft and distended abdomen from which matured eggs were stripped off by gentle hand pressure were selected. Ovulated eggs and milt were procured from the mature female and male by hand stripping on fish belly respectively. Then, the egg mass and milt were mixed in 500 mL beaker for 3 min thoroughly. Fertilization was conducted by adding clean seawater to the mixture of eggs and milt and gently mixing in a plastic container. This process was continued for 2 min, and the fertilized eggs were rinsed twice by clean seawater. All fertilized eggs were incubated in 500-L tank filled with clean seawater. The incubation tank was held at temperature 17.0–19.0 C and dissolved oxygen 7.0–8.0 mg/L under natural photoperiod. Natural seawater (35 g L−1) used for fertilization and incubation was filtered by a sand filter.
Thirty thousand larvae were hatched out after 56 h. During the incubation, hydrostatic cultivation was adopted until the larvae were hatched out for 5 days. After that, the larvae were averagely transferred to three 500-L tanks which were circulated by flowing seawater. After 2 days post-hatching (dph), larvae were fed with live rotifers, and soon after Artemia nauplii. The larvae of 20 dph began to ingest commercial dry diets. In the whole rearing process, the uneaten diets and feces were cleaned by siphon and the water was half changed every single day. Larvae were kept in the above culture conditions before being used for the following experiments.
Experiment 1: larval development and growth
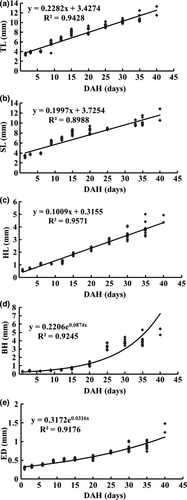
Random samples of 15 larvae were taken from each tank everyday for 42 days from the hatching day. Morphological development was observed and photographed with camera. Growth performance of flounder larvae reared in natural salinity of 35 g L−1 was investigated, including volumes of yolk sac and oil droplet, total length, standard length, head length, body height and eye diameter. The larvae from 1 to 15dph were measured under a dissecting microscope (JAPAN ASONE, IS/Mill-E, China) equipped with TSView software. The larvae from 16–42dph were photographed using a camera (Panasonic ZS10, Osaka, Japan). Growth of larvae as a function of age (days after hatching) was calculated using linear equations for total length, standard length, head length, where y = ax + b; body height and eye diameter were calculated using a function of x, where y = aebx (Martínez-Lagos & Gracia-López 2009).
Experiment 2: salinity tolerance of earlier larvae
Table 1 shows the selected developmental stages of P. olivaceus in the whole study according to Martinez and Bolker (2003). Earlier larvae at four important developmental stages were chosen to test the salinity tolerance, including newly hatched larvae (nhl), yolk sac larvae (ysl), oil droplet larvae (odl), and post oil droplet larvae (podl). At the first four specific developmental stages, the larvae were sampled randomly for the following experimental treatments.
| Stage | Abbr. | dph | Criteria |
|---|---|---|---|
| A-Newly hatched larva | nhl | 1 | Newly hatched larva with yolk-sac and oil droplet |
| B-Yolk-sac larva | ysl | 3 | Yolk-sac larva with mouth opened |
| C-Oil droplet larva | odl | 4 | Larva with oil droplet |
| D-Post oil droplet larva | podl | 6 | Larva without oil droplet |
| E-Premetamorphic larva | prel | 25 | Postflexion larva before eye migration |
| F-Prometamorphic larva | prol | 33 | Metamorphosis completed and transformed to juvenile |
Eleven salinities with three duplicates were selected for salinity tolerance test, including 5, 10, 15, 20, 25, 30, 35, 40, 45, 50 and 55 g L−1. Original seawater (35 g L−1) was either diluted with freshwater to reach the salinities below 35 g L−1 or concentrated with crystal sea salt to obtain the desired higher salinities. Salinity was determined using a handheld refractometer. At each specific developmental stage, 990 larvae were sampled from the rearing tanks, and allocated into thirty three plastic containers containing 950 mL water with 11 salinities (three containers for each salinity). The larvae were kept in static water without feeding, the water temperature was 18.0°C and the dissolved oxygen was maintained at 7.0–8.0 mg/L. Survival was monitored at 0.5, 1.5, 3, 6, 12, 24, 48, 72 and 96 h. Dead fish were removed out immediately when they were found. Meanwhile, larval growth in different salinity treatments was investigated after 96 h as described in Experiment 1.
Experiment 3: salinity tolerance of premetamorphic and prometamorphic larvae
Two tests were designed to assess the effects of salinity on survival and growth of premetamorphic (25 dph) and prometamorphic (33 dph) larvae. The two developmental stages were assigned based on the position of the translocating right eye described previously (Schreiber & Specker 1998). In brief, premetamorphic larvae (prel) are bilaterally symmetrical, and eyes of prometamorphic larvae (prol) are completely on the left side. After the complete metamorphosis, the eye is in its final location and the dorsal canal is closed, indicating the fish is a juvenile.
Likewise, 990 larvae were sampled from the rearing tank at 25 dph (prel) and 33dph (prol) respectively. Fish larvae were randomly allocated into thirty three plastic containers containing seawater with 11 salinities as described above. The experimental measurements were the same as experiment 2.
The index for salinity tolerance used for the studies was the median lethal salinity-96 h (MLS-96). It is defined as the salinity at which survival falls to 50%, 96 h (4 days) following direct transfer from the original salinity to test salinities. It is regarded as the final survival (%) in experimental salinity and calculated as the sum of the number of individual survived, divided by the initial number of fish. Percentage of survival was plotted against the test salinity and the 96-h median lethal salinity (MLS-96) was determined as the salinity at which survival falls to 50% (Watanabe, Kuo & Huang 1985).
Statistical analysis
Data on 96 h salinity tolerance at six stages were statistically analysed using two-way analysis of variance (anova). Percentage data were arcsine transformed. When interactions between the two main factors, i.e. salinity and stage, were present, one-way anovas to test for differences among levels of one factor were tested at each level of the other factor, and Tukey's HSD post hoc multiple range tests were performed to determine which treatments were different. Data on other evaluated parameters were statistically analysed using one-way anova, differences were considered significant at P < 0.05, and Tukey's HSD post hoc multiple range tests were carried out to determine which treatments were different. Prior to the analysis, normality of the data was evaluated by using the Shapiro-Wilk's W-test and homogeneity of variances was checked by Levene's test using the statistical software spss 17.0. The results are expressed as the means ± SD of the data.
Results
Larval development and growth
According to the standard of postembroyonic developmental stages in flatfish, the development of Japanese flounder P. olivaceus from hatching to complete metamorphosis was described in Fig. 1.

At Stage 1 (day 1, TL = 2.66 ± 0.26 mm, Fig. 1a): the newly hatched larva had a big yolk sac with a little pigmentation, and motor activity was weak with short spasmadic movements; in this stage, no fins were detected and the larvae used the primordial fin-fold that border the notochord for locomotion. The yolk sac began slightly from the forward edge of the head and extended almost to the middle part of the body. Most of larvae had a single oil droplet, which was located in the posterior region of the yolk sac. The mouth was not formed and the digestive tract showed little development.
At Stage 2 (day 3, TL = 3.58 ± 0.14 mm, Fig. 1b): the mouth was opening, and the pigmentation and lens of eyes began. The digestive tract was observed under the notochord. Melanophores appeared in both sides of the notochord region in the body. The body was narrow in the rear region and the fin-fold showed more development.
At Stage 3 (day 4, TL = 3.85 ± 0.11 mm, Fig. 1c): The larvae opened their mouth, moving from a sub-terminal to a terminal position, and the head had completely lifted off the yolk sac. Peristaltic movement occurred in the digestive tract and food was seen in the middle part of the gut. In this period, the larvae reacted to external stimuli.
At Stage 4 (day 5, TL = 3.92 ± 0.07 mm, Fig. 1d): the retina is clear and the cornea and nostrils are well pigmented. In this period, the yolk sac disappeared, only the drop of oil was observed. The digestive system was partitioned into intestine and stomach in some sort.
At Stage 5 (day 6, TL = 4.07 ± 0.12 mm, Fig. 1e): the oil droplet disappeared, indicating the endogenous nutrition was complete, the shape of digestive system changed dramatically.
At Stage 6 (day 25, TL = 9.39 ± 0.39 mm, Fig. 1f): the dorsal fin and the pelvic fins were apparent and the body color became fuscous. The body with much pigmentation was bilaterally symmetrical. The liver was visible in the anterior portion of the digestive tract and the stomach and intestine had twisted into a convolved formation. The most dramatic morphological change during metamorphosis was migration of the right eye to the left side of the head.
At Stage 7 (day 33, TL = 10.04 ± 0.56 mm, Fig. 1g): the right eye was in its final position adjacent to the left eye, and the body was left-right asymmetry. The metamorphosis was just complete, and was defined by the start of eye migration, visible as a slight asymmetry, with the right eye somewhat higher than the left.
At Stage 8 (day 42, TL = 12.09 ± 1.05 mm, Fig. 1h): the juveniles were not distinguishable from adults except in their size and maturity. There was also an evidence of the eye groove which was formed as a result of the resorption of the supraorbital bar.
The growth curves of P. olivaceus in terms of TL, SL, HL, BH and ED in early life stages reared at salinity of 35 g L−1 were shown in Fig. 2.
Salinity tolerance of different developmental stages of larvae
During the test of salinity tolerance of larvae in different developmental stages, survival rates decreased with time. Salinity and developmental stage significantly affected survival rates of larvae, and there was an interactive effect between the two factors (Table 2; Fig. 3). For the salinity effect (Table 3), after 96 h, all larvae held in 55 g L−1 died in every developmental stage. In 5 g L−1, ysl and prel larvae did not die completely within 96 h, no larvae survived in other stages within 96 h. For nhl larvae, the survival rates at 10–25 g L−1 were significantly higher than other salinities. For the ysl larvae, the survival rates at 10–35 g L−1 were significantly higher than other treatments. The survival rates of odl larvae reared at salinity 20–30 g L−1 were significantly higher than other salinity treatments. However, for the podl larvae, the only favourable salinity was 30 g L−1. Larvae of preml under salinity 10–35 g L−1 showed higher survival rates than those in other treatments, but for proml larvae, the better salinity for survival was only 10–15 g L−1. For the developmental stage effect (Table 3), larvae in stages of nhl, ysl and preml showed a comparable eurysalinity, the odl larvae showed a moderate one, and larvae of podl and proml were found to have the narrowest suitable salinity, showing low-grade survivals in different salinities. The MLS-96 h values for different developmental stages were listed in Table 3.
| Source | d.f. | MS | F | P-value |
|---|---|---|---|---|
| Stage | 5 | 2.379 | 386.015 | <0.001 |
| Salinity | 10 | 2.365 | 383.699 | <0.001 |
| Stage*Salinity | 50 | 0.200 | 32.372 | <0.001 |
| Error | 132 | 0.006 | ||
| Total | 198 |
| Salinity (g L−1) | Survival rates in different early development stages for 96 h (%) | |||||
|---|---|---|---|---|---|---|
| nhl | ysl | Odl | podl | Preml | proml | |
| 5 | 0aA | 4.4 ± 1.9aB | 0aA | 0aA | 6.7 ± 3.3aB | 0aA |
| 10 | 88.9 ± 5.1dC | 94.4 ± 1.9cdC | 4.4 ± 1.9aA | 0aA | 85.6 ± 6.9cdC | 48.9 ± 5.1cdB |
| 15 | 81.1 ± 1.9cdC | 95.6 ± 1.9cdD | 57.8 ± 5.1bB | 0aA | 94.4 ± 1.9dD | 51.1 ± 6.9 dB |
| 20 | 84.4 ± 5.1cdC | 92.2 ± 3.9cdC | 92.2 ± 7.7dC | 1.1 ± 1.9aA | 93.3 ± 3.3dC | 34.4 ± 1.9bB |
| 25 | 78.9 ± 6.9cdB | 98.9 ± 1.9dC | 90.0 ± 6.7 dB | 13.3 ± 6.7bA | 90.0 ± 3.3 dB | 38.9 ± 3.9bcA |
| 30 | 75.6 ± 6.9cBC | 95.6 ± 5.1cdD | 81.1 ± 3.8cdC | 55.6 ± 1.9cAB | 91.1 ± 5.1dCD | 30.0 ± 6.7bA |
| 35 | 55.6 ± 5.1bB | 86.7 ± 8.8cC | 71.1 ± 8.4bcBC | 15.6 ± 1.9bA | 77.8 ± 7.7cC | 8.9 ± 3.9aA |
| 40 | 18.9 ± 6.9aB | 51.1 ± 6.9bC | 1.1 ± 1.9aA | 12.2 ± 5.1bAB | 58.9 ± 1.9bC | 4.4 ± 3.9aA |
| 45 | 12.2 ± 5.1aA | 25.6 ± 8.4abB | 0aA | 1.1 ± 1.9aA | 41.1 ± 3.9bC | 8.9 ± 1.9aA |
| 50 | 13.3 ± 3.3aB | 4.4 ± 5.1aA | 0aA | 0aA | 2.2 ± 1.9aA | 0aA |
| 55 | 0a | 0a | 0a | 0a | 0a | 0a |
| MSL | ||||||
| Ls | 7.8 | 7.5 | 14.3 | 29.3 | 7.7 | 14.8 |
| Hs | 34.5 | 41 | 37 | — | 42.6 | — |
- Mean values within a column followed by different superscript lowercase letters indicate significant difference (P < 0.05) between salinity treatments, and mean values within a line followed by different capital letters indicate significant difference (P < 0.05) between developmental stages. ‘—’ indicates larvae in this treatment have no oil droplet.
- Hs, High salinity; Ls, Low salinity.
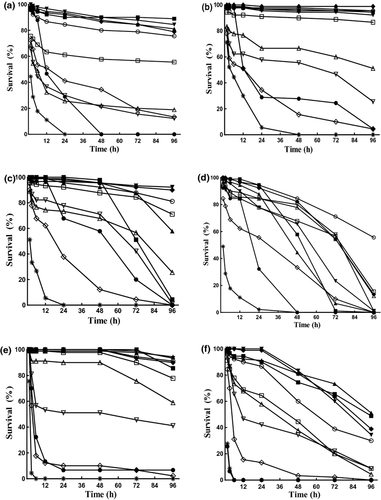
 5 g L−1
5 g L−1  10 g L−1
10 g L−1  15 g L−1
15 g L−1  20 g L−1
20 g L−1  25 g L−1
25 g L−1  30 g L−1
30 g L−1  35 g L−1
35 g L−1  40 g L−1
40 g L−1  45 g L−1
45 g L−1  50 g L−1
50 g L−1  55 g L−1.
55 g L−1.Effects of salinity on the growth of larvae at different stages for 96 h
Salinity had significant effects on all growth parameters of the nhl larvae, with higher values in low salinities. For ysl and odl larvae, salinity significantly affected the TL, SL, HL and BH, those values were higher in low salinities for ysl larvae, but were higher in median salinities for odl larvae, and no significant effect was found in ED. The TL and SL of preml larvae were also influenced by salinity, with higher values in low salinities, and no significant effects were found in BH, HL and ED. However, for podl and proml larvae, salinity showed no significant effect on any growth parameters.
Effects of salinity on yolk sac and oil droplet
The yolk sac volume of nhl larvae were significantly affected by salinity after 24 h, and the yolk sac decreased with the increase in salinity (Table 4). However, the diameter of oil droplet was only affected by salinity in ysl larvae after 96 h, with higher values in low salinities (Table 4).
| Salinity (g L−1) | Oil droplet diameter of nhl | Oil droplet diameter of ysl | Oil droplet diameter of odl | Volumes of yolk sac of nsl | ||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 96 h | 24 h | 96 h | 24 h | 96 h | 24 h | 96 h | |
| 5 | 0.1024 ± 0.0064 | / | 0.0548 ± 0.0067 | 0.0389 ± 0.0067b | 0.0454 ± 0.0055 | / | 9.76 ± 1.04d | / |
| 10 | 0.1018 ± 0.0061 | 0.0452 ± 0.0063 | 0.0527 ± 0.0051 | 0.0318 ± 0.0047ab | 0.0463 ± 0.0050 | — | 6.34 ± 0.81c | 3.60 ± 0.98a |
| 15 | 0.1018 ± 0.0034 | 0.0453 ± 0.0055 | 0.0532 ± 0.0086 | 0.0310 ± 0.0045ab | 0.0457 ± 0.0067 | — | 6.40 ± 0.92c | 3.99 ± 0.61a |
| 20 | 0.1018 ± 0.0057 | 0.0416 ± 0.0055 | 0.0531 ± 0.0052 | 0.0306 ± 0.0063a | 0.0440 ± 0.0037 | — | 6.20 ± 1.19c | 3.50 ± 0.89a |
| 25 | 0.1012 ± 0.0087 | 0.0408 ± 0.0060 | 0.0522 ± 0.0072 | 0.0283 ± 0.0041a | 0.0435 ± 0.0055 | — | 5.64 ± 0.89bc | — |
| 30 | 0.0996 ± 0.0062 | 0.0412 ± 0.0033 | 0.0506 ± 0.0109 | — | 0.0427 ± 0.0067 | — | 4.75 ± 0.87ab | — |
| 35 | 0.1011 ± 0.0066 | 0.0401 ± 0.0046 | 0.0512 ± 0.0093 | — | 0.0406 ± 0.0058 | — | 5.26 ± 0.74bc | — |
| 40 | 0.0986 ± 0.0067 | 0.0372 ± 0.0076 | 0.0514 ± 0.0072 | — | 0.0415 ± 0.0084 | / | 4.65 ± 0.95ab | — |
| 45 | 0.0975 ± 0.0074 | 0.0380 ± 0.0036 | 0.0496 ± 0.0074 | — | 0.0431 ± 0.0084 | / | 4.52 ± 0.89ab | — |
| 50 | 0.0905 ± 0.0083 | 0.0370 ± 0.0056 | 0.0476 ± 0.0088 | — | 0.0437 ± 0.0046 | / | 3.79 ± 0.90a | — |
| 55 | / | / | 0.0472 ± 0.0096 | / | 0.0437 ± 0.0060 | / | / | / |
- Different letters in the same column indicate a significant difference among treatments (P < 0.05). ‘/’ indicates all larvae died, and ‘—’ indicates larvae in this treatment have no oil droplet.
Discussion
The measurement of short-term tolerance to environment salinity in fish through ‘salinity tolerance test’ is an effective tool for determining the osmoregulatory capacity at different early developmental stages (Fashina-Bombata & Busari 2003). The present study pointed out that the effect of salinity on the early life of Japanese flounder P. olivaceus was dependent on development stages, which was similar to the African catfish Heterobranchus longifilis (Valenciennes 1840) (Fashina-Bombata & Busari 2003) and Brazilian flounder P. orbignyanus (Sampaio et al. 2007). The tolerance of flounder to different salinity demonstrated experimentally was in line with its distribution in the natural environment (Smith, Denson, Heyward, Jenkins & Carter 1999a; Smith, McVey, Jenkins, Denson, Heyward, Sullivan & Berlinsky 1999b). After hatching, flounder larvae undergo metamorphosis in such habitat, and sometimes encounter low-salinity water. Thus, flounder larvae are likely to tolerate low salinity in early life stage. Post-larvae and juveniles of species within the genus Paralichthys showed an euryhaline tendency (Yamashita, Tanaka & Miller 2001). The observations made in this study further demonstrate that P. olivaceus falls into the euryhaline group.
In this study, earlier flounder larvae i.e. nhl, ysl and odl were able to adapt to a wide and mutative range of environmental salinities. Based on our results, low (5 g L−1) and high (55 g L−1) salinities were harmful to the newly hatched larvae of P. olivaceus. The high survivals for nhl occurred at salinities of 10 to 25 g L−1, which was slightly lower than southern flounder P. lethostigma with high survival in salinities between 15 g L−1 and 35 g L−1 (Smith et al. 1999b). Banks et al. (1991) reported that 1-day-old larvae of spotted sea trout, Cynoscion nebulosus (Cuvier 1830), could tolerate salinity ranges of 4–40 g L−1; 3-day-old larvae 8–32 g L−1 and 9-day-old larvae 8–48 g L−1. From the present results, high survivals of Japanese flounder were observed within the salinity of 10 to 25 g L−1 in the early period, but were quite low in high salinities of more than 35 g L−1, which was similar to the European sea bass Dicentrarchus labrax (Linnaeus 1758) (higher at 28 g L−1versus 37 g L−1) (Barnabe & Guissi 1993), and the sea bream (higher at 25 g L−1versus 32 or 40 g L−1) (Tandler et al. 1995). However, there are some contrary states on the salinity tolerance of newly hatched larvae. For example, early-stage southern flounder P. lethostigma larvae were not entirely euryhaline, showing reduced survival and markedly lower growth rates at 25 g L−1 compared to larvae reared in 34 g L−1 seawater (Henne & Watanabe 2003; Moustakas et al. 2004), and newly hatched larvae of southern flounder died soon afterwards at salinity of 10 g L−1 (Smith et al. 1999a,b). In summer flounder, yolk sac larvae showed better growth at 36 g L−1 than at 31 g L−1 and 26 g L−1 (Watanabe, Feeley, Ellis & Ellis 1998). Better growth in full-strength seawater than at lower salinities was also observed in other larval marine finfish species such as scad C. mate (Santerre 1976), greenback flounder R. tapirina (Hart, Hutchinson & Purser 1996) and Atlantic halibut Hippoglossus hippoglossus (Linnaeus 1758) (Lein, Tveite, Gjerde & Holmefjord 1997). These authors suggested that osmoregulation at very early stages occurs mostly through the skin. Mass mortality of larvae in low or high salinity water could be related to the lack of differentiated gills. Metabolic demand of larvae increases in hyp/hyper-osmotic environments as they attempt to maintain homeostasis of body fluids (Alderdice 1988), and larvae in hyp/hyper-osmotic environments need to divert more energy into osmoregulation (Fielder et al. 2005).
Higher survival at the period of the yolk sac stage was found when flounder larvae were raised at salinities of 10–35 g L−1, showing a wide range of salinity adaptation. However, the suitable salinity range for survival in odl larvae was reduced to 20–30 g L−1. In contrast, increases in salinity tolerance from yolk sac larvae to oil droplet larvae have been documented for other species, such as trout C. nebulosus (Banks et al. 1991) and mangrove red snapper Lutjanus argentimaculatus (Forsskål 1775) (Estudillo et al. 2000). During this period, the mouth and gut of larval flatfish are neither fully formed nor functional for digestion until the yolk sac is completely resorbed (Blaxter, Danielssen, Moksness & Oistead 1983; Bisbal & Bengtson 1995). It has been suggested that the initial tolerance to low salinity is due to the extreme low permeability of ions and water through the skin of larvae without a mouth opening. With the development of skin, gill and intestines, the forms of energy supply and osmoregulation are different to earlier stage, and larvae may comport different salinity tolerance when the homeostasis loses balance. Nevertheless, some flatfish larvae at yolk-sac stage were able to drink water and adjust drinking rate to different salinities (Tytler & Blaxter 1988a,b). Survival of Japanese flounder during podl period was quite low, which may coincide with their first feeding, a critical event for marine fish in general and also verified for Brazilian flounder P. orbignyanus (Sampaio et al. 2007). It is possible that during this period larvae died due to depletion of endogenous nutrition, without exogenous feeding timely and not as a consequence of the salinity stress itself. Development associated with first feeding could cause lower tolerance to abrupt salinity change, which was also reported in sea trout C. nebulosus (Banks et al. 1991).
The premetamorphic larvae displayed a wide salinity tolerance with survival rates above 50% within the salinity of 10 g L−1 to 40 g L−1. Similarly, larvae of mangrove red snapper L. argentimaculatus on 28 dph also showed an increase in salinity tolerance (Estudillo et al. 2000). Post-metamorphic larvae of southern flounder (50-day-old) showed no significant difference in survival at salinities ranging from 5 to 30 g L−1 (Smith et al. 1999a,b). In summer flounder, pre and post-metamorphic larvae were not adversely affected by salinities as low as 14 g L−1 and 8 g L−1 (Specker et al. 1999). This could be attributed to the development of the gills, which have been reported by various authors to be the site for ionic regulation (McCormick 1995). According to the description by Hiroi et al. (1998), cutaneous chloride cells are common in the skin of premetamorphic larvae, during the metamorphic period, these cells disappear in metamorphic climax larvae and the branchial chloride cells begin to increase. In later metamorphosis, when the gills are completely formed with fully functional juvenile-type mitochondria-rich cells, the osmoregulatory performance and salinity tolerance of juveniles increase (Hiroi, Sakakura, Tagawa, Seikai & Tanaka 1997; Sampaio et al. 2007; Bodinier, Sucré, Lecurieux-Belfond, Blondeau-Bidet & Charmantier 2010). However, our results are not consistent with this theory. At premetamorphosis, the tolerance of Japanese flounder to salinity increases apparently, while prometamorphic larvae express low salinity tolerance, and the prometamorphic larvae showed a lower survival and tolerance in the present study. In our experiment, prometamorphic larvae were sampled just after metamorphosis, the energetic cost of osmoregulation may be potentially very high at this period, and more exogenous nutrition should be supplied. Due to the high-energy consumption and poor nutritional status (without feeding), the energy used for other physiological activities is insufficient. Thus, Japanese flounder exhibited low survivals at various salinities after metamorphosis.
Substantial information is available on the short-term effects of salinity changes on growth of fish larvae (Banks et al. 1991; Cioni, de Merich, Cataldi & Cataudella 1991; Avella, Berhaut & Bornancin 1993; Moser & Miller 1994). The results of the present study showed that growth of flounder was better at salinities less than 25 g L−1 before the depletion of oil droplet and metamorphosis. Likewise, the intermediate salinity resulted in better growth and survival performance in many fish species during early life stage (Lee & Menu 1986; Murashige et al. 1991; Tandler et al. 1995; Gaumet, Boeuf, Severe, le Roux & Mayer-Gostan 1995; Fielder & Bardsley 1999; Saillant, Fostier, Haffray, Menu & Chatain 2003; Varsamos et al. 2005; Okamura, Yamada, Mikawa, Horie, Utoh, Kaneko, Tanaka & Tsukamoto 2009; Zhang et al. 2010). The results of many previous studies support the hypothesis that the energetic cost of osmoregulation and standard metabolic rate are lower in an isoosmotic environment, where ionic and osmotic gradients between body fluid and environmental water are minimal (Boeuf & Payan 2001). Fish that experience salinity fluctuation have higher energy costs of osmotic and ionic regulation, and therefore less energy is allocated to growth (Kinne 1960; Sampaio & Bianchini 2002). According to Takei, Kawakoshi, Tsukada, Yuge, Ogoshi, Inoue, Hyodo, Bannai and Miyano (2006), the isosmotic salinity for fish larvae is around 10 g L−1, and optimum growth is often observed under conditions of intermediary salinity (Boeuf & Payan 2001). From a physiological point of view, isosmotic rearing conditions decrease the energy expenditure required to maintain ionic homeostasis, and this phenomenon may also explain the better survival observed at moderate salinities. Metabolic demand of larvae increases in hyp/hyper-osmotic environments as they attempt to maintain homeostasis of body fluids (Alderdice 1988), and larvae in hyp/hyper-osmotic environments need to divert more energy into metabolism rather than growth (Fielder et al. 2005). The effect of salinity on survival and growth is species specific, and euryhaline fish has been known to change throughout ontogenetic development (Rhody et al. 2010). As yet, there appears to be no general salinity range for rearing fish larvae, therefore more comprehensive studies are needed to examine the salinity tolerance for various fish larvae throughout their developmental stage. This information is important for the development of optimal culture techniques aimed at increasing larval growth and survival.
The present study demonstrated the difference in salinity tolerance during different early stages of development in Japanese flounder. Environmental salinity affected the survival of flounder larvae particularly at the end of the oil droplet and prometamorphic stage, and the preml larvae showed the strongest adaptation to salinity. In general, the high survival occurred at 10 g L−1 to 25 g L−1, while the optimal salinity was 15 g L−1 to 25 g L−1. Understanding the environmental salinity adaptability of P. olivaceus during the early life will help farmers control the rearing conditions initiatively and create the optimal rearing environment. Thus, the current salinity condition (35 g L−1) in larviculture of Japanese flounder should be improved, and it is beneficial to reduce salinity moderately.
Acknowledgments
This work was supported in part by Science & Technology Committee of Shanghai (11PJ1404500), National Natural Science Foundation of China (31072228), Shanghai Education Commission grant (10ZZ102), the Doctoral Program of Higher Education of China (20113104110002) and also Shanghai Universities First-class Disciplines Project of Fisheries.




