Depletion of florfenicol amine in tilapia (Oreochromis sp.) maintained in a recirculating aquaculture system following Aquaflor®-medicated feed therapy
Abstract
Aquaflor® [50% w w−1 florfenicol (FFC)], is approved for use in freshwater-reared warmwater finfish which include tilapia Oreochromis spp. in the United States to control mortality from Streptococcus iniae. The depletion of florfenicol amine (FFA), the marker residue of FFC, was evaluated after feeding FFC-medicated feed to deliver a nominal 20 mg FFC kg−1 BW d−1 dose (1.33× the label use of 15 mg FFC kg−1 BW d−1) to Nile tilapia O. niloticus and hybrid tilapia O. niloticus × O. aureus held in a recirculating aquaculture system (RAS) at production-scale holding densities. Florfenicol amine concentrations were determined in fillets taken from 10 fish before dosing and from 20 fish at nine time points after dosing (from 1 to 240 h post-dosing). Water samples were assayed for FFC before, during and after the dosing period. Parameters monitored included daily feed consumption and biofilter function (levels of ammonia, nitrite and nitrate). Mean fillet FFA concentration decreased from 13.77 μg g−1 at 1-h post dosing to 0.39 μg g−1 at 240-h post dosing. Water FFC concentration decreased from a maximum of 1400 ng mL−1 at 1 day post-dosing to 847 ng mL−1 at 240 h post-dosing. There were no adverse effects noted on fish, feed consumption or biofilter function associated with FFC-medicated feed administration to tilapia.
Introduction
The antibacterial agent florfenicol (FFC; [R-(R*,S*)-2,2-dichloro-N-[1-fluoromethyl-2-hydroxy-2-(4-methylsulphonylphenyl)] ethyl acetamide]; Fig. 1a), is a fluorinated analogue of thiamphenicol (Fukui, Fujihara & Kano 1987) specifically developed for use in veterinary medicine, including fish. It is available for use in fish under a variety of trade names including Aquaflor®/Aquafen®/Florocol® and is registered in more than 50 countries globally for use to control susceptible bacterial pathogens of fish. Where available (registrations vary by jurisdiction), it is distributed as a feed premix containing FFC (50% w w−1) for administration in medicated feed under voluntary feeding at a dose rate of 10 or 15 mg FFC kg−1 bodyweight (BW) d−1 for 10 consecutive days. As an example, FFC is presently approved in the United States (US FDA 2012a) for administration in medicated feed to control mortality in freshwater-reared finfish from the following indications: enteric septicaemia in catfish (10–15 mg kg−1 BW d−1); coldwater disease and furunculosis in trout (10 mg kg−1 BW d−1); columnaris disease (warmwater fish, 10–15 mg kg−1 BW d−1; all other fish, 10 mg kg−1 BW d−1); streptococcal septicaemia in warmwater fish (15 mg kg−1 BW d−1). Florfenicol was approved in the United States as a veterinary feed-directive (VFD) drug. The VFD regulations (21 CFR 510, 514, and 558, US FDA 2008a,b; 2013) presently limit the pathogens and animal species for which a VFD drug may be prescribed to only those which the US Food and Drug Administration (FDA) has explicitly included in the drug registration – extra-label use by a veterinarian, whether at alternate dose rates, to control other susceptible pathogens or for use in species other than those listed on the label for the specified disease indication is not authorized.
Streptococcosis, caused by Streptococcus iniae, is a leading cause of mortality in tilapia, especially when reared in recirculating or flow-through systems. Estimates of world-wide annual economic loss due to streptococcosis in tilapia suggest that losses had increased ~2.5 fold by the late 2000s (Klesius, Shoemaker & Evans 2008) from approximately US $100 million in the 1990s (Shoemaker & Klesius 1997). Although vaccines have been developed for use in prevention of streptococcosis in tilapia, disease occurrence has been reported in vaccinated tilapia (Creeper & Buller 2006) with S. iniae recovered from vaccinated tilapia. Despite advances in vaccine development, a critical need remains for effective antibiotics for the control of mortality in tilapia and other warmwater fish.
Florfenicol has been shown to be effective in controlling streptococcosis in tilapia Oreochromis sp. and in hybrid striped bass Morone saxatilis × M. chrysops (Bowker, Ostland, Carty & Bowman 2010; 10 mg FFC kg−1 BW d−1 for 10 days; Darwish 2007, 2010; 5, 10, 15 or 30 mg FFC kg−1 BW d−1 for 10 days; Gaunt, Endris, McGinnis, Baumgartner, Camus, Steadman, Sweeney & Sun 2010; 5, 10 or 15 mg FFC kg−1BW d−1 for 10 days; US FDA 2012b; 15 mg FFC kg−1 BW d−1 for 10 days). Although FFC was found to be effective at multiple dose levels in both tilapia and hybrid striped bass (Darwish 2007, 2010; Gaunt et al. 2010) compared with nonmedicated controls, treatment at 15 mg kg−1 BW appeared to substantially increase survival of treated fish over that of fish treated at 10 mg kg−1. Florfenicol has been found to be generally well-tolerated in a variety of fish species at dose levels exceeding 15 mg kg−1 BW (Atlantic salmon Salmo salar, Inglis, Richards, Varma, Sutherland & Bokken 1991; channel catfish Ictalurus punctatus, Gaikowski, Wolf, Endris & Gingerich 2003; hybrid striped bass Morone saxatilis × M. chrysops, Straus, Bowker, Bowman, Carty, Mitchell & Farmer 2012; yellow perch Perca flavescens, J. Bowker, US Fish and Wildlife Service, personal communication; tilapia, Gaikowski, Wolf, Schleis, Tuomari & Endris 2013)
The pharmacokinetics of FFC in plasma and FFC residues in tissues have been studied in a number of fresh and saltwater fish species following administration by bolus or voluntary feeding (Table 1). In a metabolism study in Atlantic salmon, florfenicol amine (FFA; [R*,S*0]-α-(1-amino-2-fluoroethyl)-4-(methylsulphonyl)-benzenemethanol; Fig. 1b) was identified as the major metabolite of 14C-FFC in muscle, exceeding the FFC concentration by 2 days after dosing (Horsberg, Martinsen & Varma 1994).
| Reference | Species | Dose (mg FFC kg−1 BW) | Duration (days) | Routea | Resultb |
|---|---|---|---|---|---|
| Pharmacokinetics | |||||
| Martinsen, Horsberg, Varma & Sams 1993 | Atlantic salmon Salmo salar | 10 | 1 | i.v. | t½, 12.2 h |
| Horsberg et al. 1994 | Atlantic salmon | 10 | 1 | p.o. | Cmax, 6.1 μg g−1 |
| Yanong, Curtis, Simmons, Battaram, Gopalakrishnan, Ketabi, Nagaraja & Derendorf 2005 | Koi carp Cyprinus carpio | 25 | 1 | i.m. | t½, 13.9 h |
| 50 | 1 | p.o. | t½, 7.6 h | ||
|
Threespot gourami Trichogaster trichopterus |
25 | 1 | i.m. | t½, 2.5 h | |
| 50 | 1 | p.o. | t½, 6.6 h | ||
| Horsberg, Hoff & Nordmo 1996 | Atlantic salmon | 10 | 1 | i.v. | t½, 14.7 h |
| Pinault et al. 1997 | Rainbow trout Oncorhynchus mykiss | 10 | 1 | p.o. | Cmax, 3.23 μg mL−1 |
| Samuelsen, Bergh & Ervik 2003 | Cod Gadus morhua | 10 | 1 | i.v | t½, 1.6 |
| Feng, Jia & Li 2008 | Tilapia | 10 | 1 | p.o. | t½, 24.41 h |
| Lim, Kim, Hwang, Song, Park & Yun 2010 | Olive flounder Paralichthys olivaceus | 10 | 1 | i.v. | t½, 40.43 h |
| 20 | 1 | i.m. | t½, 43.00 h | ||
| Zhao, Zhang, Bai, Zhu, Shan, Zeng & Sun 2011 | Crucian carp Carassius auratus cuvieri | 40 | 1 | p.o. | t½, 2.17 h |
| 1 | i.m. | t½, 38.2 h | |||
| Gaunt, Langston, Wrzesinski, Gao, Adams, Crouch, Sweeney & Endris 2011 | Channel catfish Ictalurus punctatus | 10 | 1 | i.v. | t½, 8.25 h |
| 10 | 1 | p.o. | t½, 9.11 h | ||
| Residue depletion | |||||
| Wrzesinski et al. 2006 | Channel catfish | 10 | 10 | p.o. | Time to tolerance, 4 days |
| Bowser et al. 2009 | Tilapia | 15 | 10 | p.o. | Time to tolerance 8.6–12.7 days |
| Kosoff et al. 2009 | Hybrid striped bass Morone chrysops x M. saxatilis | 10 | 10 | p.o. | Time to tolerance, 0.7–2.6 days |
| Tilapia | 10 | 10 | p.o. | Time to tolerance, 4.1–6.1 days | |
| Walleye Sander vitreus | 10 | 10 | p.o. | Time to tolerance, 9.7–12.6 days | |
| Gaikowski et al. 2010 | Tilapia | 15 | 10 | p.o. | Time to tolerance, 6.14 days |
- a i.m., intramuscular injection; i.v., intravenous injection; p.o., administered as medicated feed.
- b t½, half-life; Cmax, concentration maximum.
Muscle (skin-on fillet), by regulation, is considered the edible tissue of most scaled fish. Florfenicol amine was selected as the marker residue of FFC based on a common moiety assay originally established in cattle tissues and subsequently employed for all food animals including fish. In the common moiety assay, FFC and metabolites other than FFA, including bound residues, are converted to FFA through acid hydrolysis (Wrzesinski, Crouch, Gaunt, Holifield, Betrand & Endris, 2006). The maximum residue limit for FFA for all fish is 1 μg g−1 in the USand in the European Union (EMEA 2000; US FDA 2005).
Studies describing FFA depletion in fillet following dosing at either 10 or 15 mg FFC kg−1 BW in tilapia reared in flow-through systems have been published (e.g. Bowser, Kosoff, Chen, Wooster, Getchell, Craig, Lim, Wetzlich, Craigmill & Tell 2009; Kosoff, Chen, Wooster, Getchell, Bowser, Clifford, Craig, Lim, Wetzlich, Craigmill & Tell 2009; Gaikowski, Mushtaq, Cassidy, Meinertz, Schleiss & Endris 2010). Data describing the depletion of FFA from tilapia following administration of FFC-medicated feed in a recirculating aquaculture system (RAS), in which FFC or FFA could accumulate in the water and alter FFA tissue depletion, were not available prior to this study.
The study objectives were to determine in a GLP-compliant study (1) the decline of FFA in the edible tissue (scaled, skin-on fillet) of market-weight tilapia (Oreochromis sp.) and the (2) accretion and decline of FFC in water of a RAS in which the system fish were fed FFC–medicated feed. To insure consumption of at least 15 mg kg−1 BW d−1, the tilapia in this study were offered FFC-medicated feed at a concentration and rate sufficient to achieve a dose of 20 mg kg−1 BW d−1 for 10 days if 100% of the offered feed was consumed. The target dose in this study was thus 1.33× the maximum label dose of 15 mg kg−1 BW d−1.
Methods
Test article and analytical standards
Aquaflor® was supplied by Merck Animal Health, Summit, NJ and was used in the preparation of the medicated feed. Florfenicol [high performance liquid chromatography (HPLC) purity 99.7%] and FFA (HPLC purity 97.3%) were supplied by Merck Animal Health (Summit, NJ, USA) and were used as the standards in the analysis of FFC content in feed and water matrices and of FFA level during tissue analysis.
Test fish and rearing conditions
Phenotypic male Nile O. niloticus × O. niloticus and hybrid O. niloticus × O. aureus tilapia were obtained from a US commercial tilapia farm. As fry, these fish had been fed feed containing 17α-methyltestosterone by the fry supplier to produce a nearly complete phenotypic male tilapia population. All fish were used regardless of gender or strain. Tilapia (282 fish split evenly between the test tanks) were ~240 days old and weighed on average 447.3 g when placed into the RAS and were allowed to grow to the desired size (~500 g) as the biofilter adjusted to the increased biomass.
Approximately 6 months prior to fish transfer to UMESC, a commercial RAS (Aquatic Eco-Systems Fish Farm™ II, Apopka, FL; Fig. 2) consisting of twin ~1900 L (500–gal) polyethylene tanks (Tanks A and B) which shared mechanical (clarifier and suspended solids filter) and biological filters was established. The RAS was filled with UMESC well water (total system volume of ~3350 L); replacement UMESC well water (~12°C) used during daily maintenance was allowed to equilibrate to ambient temperature (~21°C) to minimize RAS temperature fluctuation. Sodium bicarbonate (Aquatic Ecosystems, about 172 to about 462 g d−1) was added to replacement water to maintain RAS alkalinity and pH. Supplemental oxygen was supplied to each RAS tank when tilapia were present to maintain dissolved oxygen concentrations above 5 mg L−1. The RAS biofilter was prepared to receive study tilapia by inoculation with commercially available bacterial cultures (Bacta-Pur®, Aquatic Ecosystems, Apopka, FL, USA; Turbo Start #700, Fritz Industries, Dallas, TX, USA) and by rearing non-study tilapia in the system for ~4 months prior to placement of test fish into the RAS (non-study tilapia were removed from the test tanks 1 day before test fish were placed in the tanks). Ammonium chloride was occasionally added to the RAS during biofilter preparation to maintain ammonia levels of ~1 mg NH3-N L−1.
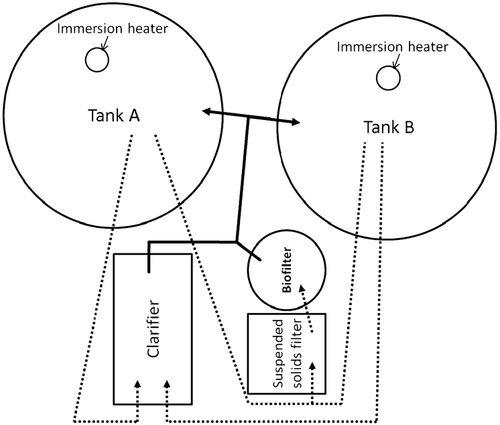
Test fish were placed into the RAS after water chemistry (ammonia, nitrite) values stabilized. The recommended system maximum fish load was ~136 kg or ~0.04 kg L−1. The nominal fish load of tilapia at transfer to the RAS was ~0.038 kg L−1 and ~0.033 kg L−1 at the initiation of dosing. Tilapia were acclimated to test conditions by maintaining them in the RAS tanks for 38 days before they were offered FFC-medicated feed. Fish were held under a 24-h light photoperiod using overhead lighting, photoperiod and light intensity (~32 lux at the RAS tank water surface) approximated that of the source farm.
Water temperature, dissolved oxygen and pH were recorded once daily through the acclimation, dosing and post-dosing period. Total ammonia, nitrite and nitrate concentration were determined once daily in samples collected from each tank prior to tank cleaning. Hardness and alkalinity were determined weekly in a water sample collected from each RAS tank. A water sample was collected from the RAS 4 days before dosing and analysed for metals and volatile and semi-volatile organics (Davy Laboratories, La Crosse, WI, United States); contaminants were below levels of concern. The RAS tanks were cleaned daily by draining and clearing solids from the clarifier and suspended solids filter. Tank cleaning was completed before the first feeding and the volume of water removed and the volume of water used to replenish the RAS was recorded.
Nonmedicated and medicated feed and feeding
Nonmedicated tilapia grower diet (extruded pellet 3.2–6.4 mm; 35% protein; 6% fat) manufactured at Land O’ Lakes, Owatana, MN, United States was used briefly during the initial acclimation of tilapia to the test conditions and during transition to the experimental feeds. Medicated and nonmedicated feeds used during the acclimation, dosing and post-dosing periods were prepared at the Delta Western Research Center (DWRC; Indianola, MS, USA) where feed pellets (4.7 mm) were prepared by extrusion. The DWRC nonmedicated control feed was formulated first and was prepared to be nutritionally similar to the Land O'Lakes nonmedicated feed. The medicated feed batch was nutritionally equivalent to the DWRC nonmedicated feed except that FFC was added prior to extrusion to achieve a FFC concentration of 2.667 g FFC kg−1. This FFC concentration would deliver a dose of 20 mg kg−1 BW when offered at a rate of 0.75% BW d−1. Nonmedicated and medicated feeds were stored separately at ~−20°C.
Florfenicol concentration in the medicated ration, and its absence in the control ration, was confirmed before and after the dosing period. Florfenicol concentrations were determined according to methods adapted from Hayes (2005) at Eurofins AvTech Laboratories, Kalamazoo, MI, USA. The mean florfenicol level of the medicated feed before dosing was 98.9% of nominal and was unchanged after dosing. Florfenicol concentrations in feed are summarized in Table 2. Proximate and contaminant (e.g. other antibiotics, inorganic (arsenic, cadmium, lead, mercury) and organic (aflatoxin, chlorinated hydrocarbons, N-methyl carbamates, organophosphates, oxidized lipids) contaminants) analyses were completed by Eurofins Scientific Inc, Des Moines, IA. Florfenicol feed assay analyses conformed to GMP regulations (21CFR225). The control ration nutrient content was similar to that of commercial tilapia diets. Organic and inorganic contaminants were not at levels of concern to the overall outcome of the study.
| Nominal dose (mg FFC kg−1 BW d−1) | Nominal feed concentration (g FFC kg−1feed) | Measured concentration (g FFC kg−1 feed) | |
|---|---|---|---|
| Before dosing | After dosing | ||
| 0 (control) | 0 | <LODa | <LOD |
| 20 | 2.667 | 2.638 | 2.560 |
- a <LOD – method limit of detection = 0.0002 g kg−1
The Land O'Lakes feed was offered upon receipt of the test fish. Thirty-five days before dosing, the Land O'Lakes feed was incrementally replaced by the DWRC nonmedicated feed over a 5-d period. Test fish were offered the DWRC nonmedicated feed for the remainder of the acclimation and post-dosing periods. The target feed rate during the acclimation period ranged from 0.25% to 1% BW d−1. The target feed rate during the post-dosing period was 0.75% BW d−1.
The medicated feed was the sole feed offered during the 10-d dosing period. The target feed rate during the dosing period was 0.75% BW d−1 which delivered a target dose rate of 20 mg kg−1 BW d−1 for the 10-d dosing period. This feed rate was selected to ensure consumption of all medicated feed offered during the dosing period. The biomass present at the initiation of dosing was estimated by determining the daily weight change between receipt at UMESC and control fish collection before the dosing period began. The biomass present during dosing was adjusted based on the daily growth rate and the amount of feed offered during the last 5 days of the dosing period was increased to account for growth during the first 5 days of the dosing period.
The ration amount offered was calculated separately for each tank based on estimated fish weight and the number of fish in the tank. Feed offered was weighed and the actual amount offered recorded. Feed was pre-weighed and stored in sealed containers at ambient temperature for up to 5 days during the acclimation period and up to 4 days during the dosing period. The daily ration was divided into three equivalent portions and fed three times daily by hand over a ~8–h period with a minimum of 4 h between feed offerings. The first daily feed offering was at least 1 h after tank cleaning, water chemistry measurement and water sampling. The feed amount was adjusted for mortalities as needed.
Observations
Fish were observed a minimum of twice daily during the work week and once daily on the weekend and holidays for mortality, abnormal behaviour, abnormal coloration, external lesions and other clinical signs. Feeding activity was visually assessed at each feeding period during the acclimation, dosing and post-dosing periods. Feeding activity was assessed as either active (fish immediately fed on pellets) or inactive (fish not responsive to feeding, did not take pellets from surface or were not observed to feed within five minutes of placing feed into tank). Medicated feed consumption was to be monitored by collecting the remaining uneaten feed after each feed offering to estimate the amount of feed consumed during the dosing period. This was not completed as the tilapia consumed all the medicated feed offered during each feeding.
Water sample collection and FFC analysis
Water samples for florfenicol analysis were collected (1) concurrent with the control fillet collection, (2) prior to tank cleaning during the dosing period (~1-h prior to the first daily feeding), (3) just prior to the second and third daily feeding intervals, (4) 4-h after the third daily feed interval and (5) concurrent with tissue collection during the post-dosing period except that a water sample was not collected at the 1-h post-dosing fillet collection. At each collection interval, one water sample (~50 mL) each was taken from the clarifier and from the suspended solids filter (Fig. 2). Each sample was placed into a separate, labelled, screw-capped polypropylene container and hand mixed (inverted ~10 times). A portion of the sample was placed into a disposable syringe fitted with a syringe filter (Millipore Cat. No. SLHVM25NS, Durapore® [PVDF, 0.45 μm] membrane, Millipore, Billerica, MA, USA) then five ~2-mL aliquots were filtered into HPLC vials. Bulk water samples and filtered aliquots were stored at −80°C until analysed.
Florfenicol concentrations were determined by ultra high-pressure liquid chromatography with mass spectrometric detection (UPLC-MS/MS) on an AB SCIEX 4000 mass spectrometer using an atmospheric pressure ionization interface. The procedure was previously validated to FDA bioanalytical guideline standards (US Food & Drug Administration (US FDA) 2001a; US FDA 2001b) to determine FFC concentration in RAS water ranging from 10 to 5000 ng mL−1 and up to 20 000 ng mL−1 after dilution (S. Charles, Merck Animal Health, personal communication).
Each filtered water sample was analysed by a single determination; samples were fortified with florfenicol-d4 as an internal standard and analysed directly. Ionic transitions of 356 to 185 m/z and 360 to 189 m/z were monitored for FFC and the internal standard respectively. Duplicate and bracketing 10−point calibration curves (10 to 5000 ng mL−1) for FFC were prepared in high-pressure liquid chromatography (HPLC) grade water and analysed with each batch of samples. Each back-calculated standard, except the lowest, was required to have accuracy of no greater than ±15% deviation from nominal (±20% for the lowest standard). The validated limit of quantitation (LOQ) was 10 ng mL−1 for FFC and corresponded to the lowest standard on the standard curve.
Method performance was assessed during sample analysis using RAS water collected prior to dosing. For each set of water samples, the Quality Control (QC) samples included RAS water collected before FFC dosing that was fortified at 0, 10, 30, 150, 2500 or 4500 ng FFC mL−1. The QCs criteria for the sample sets were mean accuracy (% difference) within ±15% (±20% at the LOQ) of nominal concentrations for calibration curves standards with at least ⅔ of the QC samples at each level within ±15% (±20% at the LOQ) of nominal and a QC precision [per cent coefficient of variation (%CV)] of ≤15% (≤20% at the LOQ).
Fillet collection and FFA analysis
Ten fish (5 per tank) were indiscriminately selected 4 days before the initiation of the dosing period to obtain control tissue. Following withdrawal of the medicated ration at the end of the third daily feeding period on the tenth day of dosing, groups of 10 fish from each tank were indiscriminately selected from the respective tank population at nine time points (Table 3). Tilapia were euthanized by electrocution, weighed and measured [total length (TL); fish remaining in the test tanks after the last fillet collection were euthanized, weighed and measured but not filleted]. Fish were processed within 2 h of euthanasia to obtain bilateral scaled, skin-on fillets. Fillets were rinsed in tap water, weighed and placed in separate labelled Ziploc® bags on ice then stored at −80°C within 4 h of collection.
| Time post-dosing (h) | Fish per tank | Fillet FFA level (μg g−1)a | |||
|---|---|---|---|---|---|
| Uncorrected | Correctedb | ||||
| Tank A | Tank B | Tank A | Tank B | ||
| Controlc | 5 | <LOQd | <LOQ | – | – |
| 1 | 10 |
14.42 (6.61) 3.51–27.78 |
13.13 (3.54) 7.54–18.51 |
15.72 (7.21) 3.83–30.30 |
14.32 (3.86) 8.22–20.19 |
|
13.77 (5.21) |
15.02 (5.68) |
||||
| 12 | 10 |
12.12 (3.62) 9.21–21.15 |
14.79 (4.86) 6.94–21.63 |
13.22 (3.95) 10.04–23.06 |
16.13 (5.30) 7.57–23.59 |
|
13.45 (4.39) |
14.67 (4.79) |
||||
| 24 | 10 |
7.35 (3.01) 3.49–12.41 |
7.98 (3.64) 2.80–12.65 |
8.02 (3.28) 3.81–13.54 |
8.70 (3.97) 3.06–13.79 |
|
7.67 (3.27) |
8.36 (3.56) |
||||
| 36 | 10 |
7.39 (2.47) 4.49–12.46 |
4.09 (1.40) 2.46–6.41 |
8.06 (2.69) 4.89–13.59 |
4.46 (1.52) 2.68–6.99 |
|
5.74 (2.58) |
6.26 (2.82) |
||||
| 48 | 10 |
4.60 (1.51) 2.50–7.46 |
5.33 (1.08) 3.59–7.11 |
5.02 (1.65) 2.73–8.14 |
5.82 (1.18) 3.92–7.76 |
|
4.97 (1.33) |
5.42 (1.45) |
||||
| 72 | 10 |
2.59 (1.02) 0.86–4.26 |
3.08 (2.15) 0.64–7.16 |
2.83 1.11) 0.94–4.64 |
3.36 (2.34) 0.70–7.80 |
|
2.84 (1.66) |
3.09 (1.81) |
||||
| 96 | 10 |
1.93 (0.68) 0.83-3.05 |
2.38 (0.85) 1.31-3.50 |
2.11 (0.74) 0.91-3.33 |
2.60 (0.93) 1.43-3.82 |
|
2.16 (0.78) |
2.35 (0.86) |
||||
| 120 | 10 |
1.40 (0.66) 0.42–2.32 |
1.37 (0.41) 0.93–2.11 |
1.52 (0.72) 0.46–2.52 |
1.49 (0.45) 1.01–2.30 |
|
1.38 (0.53) |
1.51 (0.58) |
||||
| 240 | 10 |
0.46 (0.20) 0.31–0.98 |
0.33 (0.09) 0.18–0.50 |
0.50 (0.22) 0.34–1.07 |
0.36 (0.10) 0.20–0.55 |
|
0.39 (0.16) |
0.43 (0.18) |
||||
- a Values are the tank mean (standard deviation), range and system mean (standard deviation).
- b Corrected FFA concentration (for assay recovery) = uncorrected value ÷ 0.917.
- c Control samples taken 4 days before dosing.
- d LOQ – Limit of Quantitation = 0.05 μg g−1.
Florfenicol amine (FFA) concentration was determined in the tissue samples using a validated determinative procedure (Gaikowski et al. 2010) in which FFC residues are converted to the common moiety FFA by acid-catalysed hydrolysis. Briefly, a 2 ± 0.2 g sample of ground fillet tissue was hydrolysed by adding 8 mL of 6 N HCl and heating, with agitation, at 95–100°C for approximately 2 h. The tissue-hydrolysate was extracted with ethyl acetate (ca. 20 mL) and the extracted hydrolysate centrifuged for 5 min at a relative centrifugal field of about 1200 g. The ethyl acetate was discarded and the pH of the aqueous hydrolysate adjusted to ≥12.5 by adding about 8 mL of 30% (w w−1) NaOH solution. The pH-adjusted solution was poured onto a Varian Chem Elut CE1020 sorbent column (Varian, Palo Alto, CA, USA) and allowed to adsorb for 45–60 min. The column was then eluted with three 20-mL portions of methylene chloride. The methylene chloride eluates were combined and evaporated to dryness at 45–50°C with a nitrogen stream. The dried residue was dissolved in 2 mL of 10-mM potassium phosphate buffer, pH 4.0, containing 1% (v v−1) acetonitrile and the resulting solution was filtered through a 0.45-μm filter to yield the final extract which was analysed by HPLC using UV detection at 220 nm. Collected fillets were processed through extraction within the established storage stability period for tilapia fillet (6 months at −20°C; Gaikowski et al. 2010).
A calibration curve range of 0.05 to 2.4 μg FFA mL−1 (six concentrations prepared in HPLC mobile phase; Gaikowski et al. 2010), equivalent to 0.05 to 2.4 μg FFA g−1 in tissue, was confirmed by triplicate injections of each of the standard levels before beginning sample analysis. The coefficient of determination described by Gaikowski et al. (2010) was used with the following additional criteria: each back-calculated standard must have a precision of no greater than 10% CV and accuracy of no greater than ± 10% deviation from nominal (those limits were 15% for the lowest standard). Calibration curve standards met all criteria; the tissue equivalent of the lowest calibration curve standard was considered the limit of quantitation (LOQ; 0.05 μg g−1) for reporting residue levels. Extracts of tissue samples with residue levels >2 μg g−1 were diluted to a concentration within the standard curve and reanalysed. Because maximum tissue concentrations (~30 μg g−1) exceeded the previous maximum method validated tissue concentration (20 μg g−1), five control samples were spiked at ~30 μg g−1 prior to assay to successfully validate the method at this level.
Method performance was assessed using tissue from untreated fish. The following QC specimens were analysed for each set of tissue samples, including control fish: one unfortified control tissue sample, two control tissue samples fortified at a low level (~0.5 μg g−1) and two control tissue samples fortified at a high level (~2 μg g−1). Analyses from a sample set was accepted if the recovery of 3 of 4 fortified samples was 80–110% and the control sample was <LOQ.
There was a total of 10 sample sets consisting of one set with fillets from 10 control fish and one set for each of the nine fillet collection events during the post-dosing period. Each control fish tissue sample was analysed one time to establish a baseline background before assaying incurred tissues. Each incurred tissue specimen was analysed by a single determination. For samples with peak responses above the highest calibration standard, aliquots of the stored (~4°C) final extracts from the initial analysis were diluted in mobile phase and reanalysed within the established extract stability of 28 days after initial preparation (Gaikowski et al. 2010). Samples in which the FFA concentration was <LOQ were reported as <LOQ. Assay recovery in the method validation study (Gaikowski et al. 2010) was 91.7% averaged over 0.5, 1 and 2 μg g−1 fortifications with 24 replicates for each level. The FFA levels in this study were reported as uncorrected and corrected for FFA assay recovery; corrected values were determined by dividing the uncorrected value by 0.917.
Statistical analysis
The residue depletion profile of FFA (uncorrected for assay recovery) in skin-on fillet of tilapia following withdrawal from the medicated diet was modelled by log-linear regression to calculate the withdrawal period. Data were fit to the linear equation LnY = mX + b, where LnY is the natural log of the FFA concentration on day X, m is the slope of the line and b is the Y intercept. Model assumptions were evaluated before analysis (Levene 1960; Shapiro & Wilk 1965; Neter & Wasserman 1974) and least square regression was used to estimate model parameters. The withdrawal period was defined as the time when the FFA residue concentration was at or below 1 μg g−1 at the 99th percentile with 95% confidence (US FDA 2006).
Data (uncorrected FFA concentrations) were also fit to a monoexponential regression model which followed a one-compartment model of elimination kinetics (Gibaldi & Perrier 1982). The data were fit to the model Yt = Aexp(−αt) where Yt is the FFA concentration in the fillet tissue on day t. The model parameters were estimated using nonlinear least-squares regression (SAS ver 9.1.3; SAS Institute, Cary, NC, USA). The elimination half-life t1/2 was calculated by = 0.693/α where α equaled the model-derived rate constant (Gibaldi & Perrier 1982).
Other statistical analyses results included calculation of means, standard deviations and coefficients of variation of tissue and water assay results.
Good laboratory practices
All phases of this study were conducted in compliance with US FDA (1987: GLP; 21 CFR 58) except that the facilities that prepared the feed and analysed the feed for FFC concentration, nutrients and possible contaminants were non-GLP-compliant facilities at the time the study was conducted.
Results
Water chemistry
During acclimation of test tilapia, 6–11% of the total RAS volume was removed and replaced daily. During the dosing and post-dosing periods, 6% to 8% and 5% to 7%, respectively, of the total RAS volume was removed and replaced daily.
Test tilapia were placed into the RAS at an initial temperature of ~24.5°C then gradually acclimated (~1°C d−1) to the test temperature (27 ± 2°C). Mean temperatures during the acclimation, dosing and post-dosing periods were 26.5, 27.5 and 26.5°C respectively. The RAS temperature was within the test temperature range except for a ~18-h period during post-dosing days 5 and 6 when the RAS temperature decreased to 22.7°C. The RAS immersion heaters were replaced and the water temperature returned to within the test limits within hours of heater replacement. The short time period during which the fish were slightly below the test temperature would not be expected to cause a significant effect on the depletion of FFA from tilapia.
Mean dissolved oxygen level during the acclimation, dosing and post-dosing periods was 7.1, 7.3 and 9.0 mg L−1 respectively. Mean pH during the acclimation, dosing and post-dosing periods was 7.48, 7.32 and 7.80 respectively. Mean alkalinity and hardness during the acclimation, dosing and post-dosing periods were 203, 127 and 218 mg L−1 as CaCO3, and 135, 179 and 174 mg L−1 as CaCO3 respectively.
Unionized ammonia, calculated from measured total ammonia concentrations, was <0.02 mg L−1 NH3-N during the acclimation, dosing and post-dosing periods. Mean nitrate concentration was 67, 81 and 75 mg NO3-N L−1 during the acclimation, dosing and post-dosing periods. Nitrite concentration occasionally exceeded the safe upper limit of 2.0 mg NO2-N L−1 but only during the acclimation period; nitrite levels were ≤0.9 mg L−1 during the 8 days prior to dosing. Nitrite levels steadily increased during the dosing period until peaking on dosing day 8 at concentrations of 1.82 and 1.69 mg L−1 in Tanks A and B respectively (Fig. 3). Nitrite concentration in the RAS water rapidly decreased after a biofilm blockage was flushed from the biofilter supply lines on dosing day 8. Nitrite concentrations remained low during the remainder of the dosing period and during the post-dosing period until spiking again on post-dosing day 7. Nitrite concentration again dropped rapidly after the biofilter supply lines were flushed on post-dosing day 7. The increased nitrite concentrations observed during the dosing and post-dosing periods were thus associated with the inadvertent buildup of biofilms in the plumbing which delivered RAS water to the biofilter, not FFC exposure.

Mortality
There were two mortalities during the FFC dosing period. The first mortality that occurred during the dosing period was removed from Tank B on the first dosing day before the first administration of FFC-medicated feed. The second mortality was removed from Tank B on the fourth dosing day but with obvious post-mortem degeneration, suggesting death preceded removal by >24 h. Although a definitive cause for the second mortality was not determined, there were no clinical changes in feeding behaviour or feed consumption during the dosing period nor was any gross pathology observed during fillet collection. Thus, we concluded that the two mortalities that occurred during the dosing period were not correlated with FFC administration.
Growth
Tilapia in this study were held in the RAS for a period of 58 days (extending from receipt at UMESC through the end of the post-dosing period). The mean weight of tilapia on receipt was 447.3 g. The mean weight of fish collected per sampling interval during the post-dosing period ranged from 485.2 to 520.5 g. The overall mean weight of fish euthanized during the post-dosing period (including fish euthanized for fillet collection during the post-dosing period and those euthanized at the termination of the study) was 504.3 g. The overall growth rate was 1.0 g d−1.
Feed consumption and delivered dose
Tilapia consumed all the feed offered during the dosing period. The mean daily delivered doses for tilapia in Tanks A and B were 19.42 and 19.82 mg FFC kg−1 BW d−1 respectively (>97% of target). The total delivered doses were 194.2 and 198.2 mg FFC kg−1 BW for tilapia in Tanks A and B respectively.
Florfenicol concentration in water
The mean accuracy of duplicate QC standards at four concentrations in each accepted analytical run was between 2.8% and 8.7% and precision between 1.9% and 9.2% CV. Florfenicol levels in RAS water samples taken before the dosing period (1 day before dosing and 1 h before the first FFC-medicated feed administration) were <LOQ. The florfenicol concentration in water increased during the dosing and post-dosing period to a maximum mean of 1400 ng mL−1 in samples taken concurrent with the 24-h fillet collection before decreasing to 847 ng mL−1 at 240 h post-dosing. There was minimal difference in FFC concentration between water samples taken from the clarifier and from the suspended solids filter (Fig. 3).
Florfenicol amine concentration in tissue
Recovery for all fortified [at ~0.5 and ~2.0 μg g−1 (22 each)] QC samples assayed with unknown samples ranged from 86.5% to 108.9% with no unfortified QC sample > LOQ. Five dilution QCs were prepared at ~30 μg g−1 (requiring dilution of 20× prior to analysis) because the maximum uncorrected fillet FFA concentration approached 30 μg g−1. The per cent recovery of those QCs ranged from 87.4% to 100.2% and met the per cent recovery acceptance criteria established during method validation (Gaikowski et al. 2010). System suitability and performance criteria were met for each sample set.
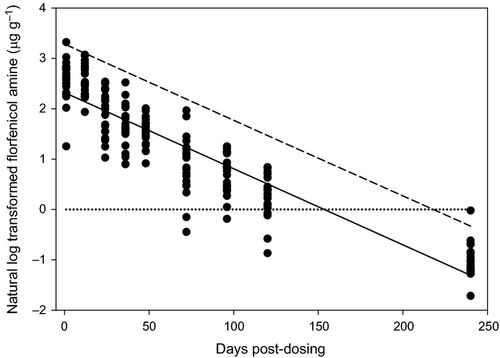
Fillet FFA concentrations are presented in Table 3. All control fish were <LOQ. Uncorrected fillet FFA concentrations determined from tilapia collected during the post-dosing period ranged from 0.18 μg g−1 to 27.8 μg g−1. Mean uncorrected FFA concentrations in tilapia fillet decreased during the post-dosing period from 13.77 μg g−1 at 1-h post-dosing to 0.39 μg g−1 at 240-h post-dosing (Table 3). The minimal decrease in FFA concentration in samples taken at the 1 and 12 h post-dosing periods suggests that FFC absorption from feed was still occurring.
Fillet FFA concentration data were fitted to log-linear regression (Table 4; Fig. 4) and one-compartment non-linear regression models (Table 4). Fitting the data to these models predicted a withdrawal period of 10 days and an estimated half-life (t1/2) of 1.33 days (Table 4).
| Log-linear regression | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model Ln FFA (μg g−1) = intercept (Ln FFA μg g−1) + slope (Ln FFA μg g−1 h−1) × hours post-dosing | ||||||||
| Intercept | Slope | Bartlett's | Shapiro-Wilk's | Log-linearity | Model fit | WTa (h) | ||
| r 2 | F-test | |||||||
| Uncorrected | 2.3215 | −0.0151 | P = 0.07 | P = 0.03 | P < 0.01 | 0.83 | F = 851.3, df = 1; P < 0.01 | 241 |
| Corrected | 2.4078 | −0.0151 | P = 0.07 | P = 0.03 | P < 0.01 | 0.83 | F = 850.4, df=1; P < 0.01 | 248 |
| One compartment non-linear regression | ||||||
|---|---|---|---|---|---|---|
Model FFA (μg g−1) = intercept (FFA μg g−1) ×  where day = days post-dosing where day = days post-dosing |
||||||
| Intercept | Slope | F-value | Degrees of freedom | P > F | Half-life (t1/2) | |
| Uncorrected | 12.1207 | −0.522 | 431.7 | 2 | <0.01 | 1.33 |
| Corrected | 13.2194 | −0.5222 | 418.17 | 2 | <0.01 | 1.33 |
- WT, withdrawal time.
Discussion
The depletion of FFA following administration of FFC-medicated feed has been well-documented in a variety of freshwater and marine fish, including tilapia (e.g. Pinault, Millot & Sanders 1997; European Agency for the Evaluation of Medicinal Products (EMEA) 1997; Wrzesinski, Crouch, Gaunt, Holifield, Betrand & Endris 2006; Bowser et al. 2009; Kosoff et al. 2009; Gaikowski et al. 2010). However, none of those studies evaluated FFC administration in fish held under conditions of water reuse or recirculation. Commercial RAS are often designed to achieve water exchange rates of 5% to 10% of total system volume each day (Masser, Rakocy & Losordo 1999). This reduced water exchange could alter depletion of FFC residues either by slowing elimination of FFC residues or by uptake of FFC residues from RAS water. Few residue depletion studies have been conducted in fish held in RAS and none to date with FFC. One such study evaluated the depletion of the antibiotic oxytetracycline from fish fillet and RAS water (Bebak-Williams, Bullock & Green 2002). The peak oxytetracycline concentrations in fish fillet reported by Bebak-Williams et al. (2002) were similar to those found when fish were fed oxytetracycline-medicated feed in a flow-through rearing system (i.e., no water reuse; e.g. Bjorklund & Bylund 1990; Bjorklund, Rabergh & Bylund 1991).
Differences in study design (e.g. feeding procedure, test temperature, feed rates, target dose) complicate direct comparison between FFA residue depletion studies conducted with tilapia fed FFC-medicated feed while being reared in flow-through rearing systems and this study. However, comparison of the half-life value (t1/2) in this study (1.33 days) and those reported in previous studies (Bowser et al. 2009; t1/2 = 2.2 days, Gaikowski et al. 2010; t1/2 = 2.32 days) suggest that substantial differences in the depletion of FFC residues from tilapia are not likely to occur solely due to differences in rearing systems, especially since the target dose level and the delivered doses were higher in this study than those used by Bowser et al. (2009) or Gaikowski et al. (2010).
Although no adverse effects of FFC were observed in effectiveness studies conducted in RAS (Gaunt et al. 2010), this is the first report of the measurement of FFC concentrations in water from a RAS following treatment of fish with FFC-medicated feed and related to the efficiency of biofilter nitrification. Although FFC concentration in RAS water increased from <LOQ to 1400 ng mL−1 at 1 day post-dosing, both the rate and magnitude of that increase were much less than the theoretical FFC water concentration (which was calculated assuming that all FFC in the medicated diet dissolved in the tank water). The substantial difference between the observed FFC water concentration and the theoretical FFC water concentration (Fig. 3) was expected and is obviously attributable to absorption, distribution, metabolism and excretion in the fish, removal of fish containing absorbed FFC, removal of fish faeces containing excreted FFC residues during tank cleaning and that only FFC was determined in water samples (not FFC metabolites like FFA and others). In a study of oxytetracycline administered to fish in a RAS, peak concentrations were achieved between dosing days 5 and 10 days and were reportedly much less than those expected to be discharged when fish were fed oxytetracycline-medicated feed in a flow-through rearing system (Bebak-Williams et al. 2002).
The effects on biofilter nitrification of some parasiticides and oxidizing agents administered via water in recirculating aquaculture systems have been assessed. Benzalkonium chloride (Schwartz, Bullock, Hankins, Summerfelt & Mathias 2000), chloramine-T (Schwartz et al. 2000), formalin (Keck & Blanc 2002), hydrogen peroxide (Schwartz et al. 2000; Moller, Arvin & Pedersen 2010) and peracetic acid (Pedersen, Pedersen, Nielsen & Nielsen 2009) all induced biofilter nitrification impairment at one or more treatment regimens. Collins, Gratzek, Dawe and Nemetz (1976) evaluated the effects of the antibiotics chloramphenicol, oxytetracycline, erythromycin thiocyanate, sulphamerizine and nifurpirinol on biofilter function after administration directly into water. Erythromycin thiocyanate (a single dose of 50 mg L−1) stopped nitrification for 14 days after a single administration whereas other antibiotics did not alter nitrification. Conversely, Klaver and Matthews (1994) reported that oxytetracycline (single doses of 12.5–75 mg L−1) inhibited nitrification in synthetic freshwater.
Therapeutic use of antibiotics administered in medicated feeds may similarly pose a risk of adverse effects on nitrifying bacteria in biofilters. Antibiotics administered in medicated feed would be expected to enter water in RAS as the parent drug leached from uneaten medicated feed and as antimicrobially active metabolites (and possibly the parent drug) following excretion from fish or leaching from faeces. Skjolstrup, McLean, Nielsen and Frier (2000) evaluated the effects of oxolinic acid administered in medicated feed on nitrification rates in a freshwater RAS – they found no impact of oxolinic acid on nitrification rate during the 7-day dosing period or during a 14-day post-dosing period. Although Bebak-Williams et al. (2002) focused on describing the oxytetracycline residues following administration of oxytetracycline-mediated feed in a freshwater RAS, they did not report any untoward effects of administration on biofilter performance. None of these studies measured the sensitivity of nitrifying bacteria Nitrosomonas sp. or Nitrobacter sp. to the antibiotics studied. Skjolstrup et al. (2000) was the only study available in which antibiotic concentrations were measured in RAS water and correlated with RAS nitrification rate.
The minimum inhibitory concentration (MIC) of FFC for Nitrosomonas sp. (65 μg mL−1) and Nitrobacter sp. (50 μg mL−1) determined as part of the environmental safety assessment process to address drug registration requirements of the US Food and Drug Administration (R.G. Endris, Merck Animal Health, personal communication) were >35-fold higher than the maximum FFC concentration in water determined in the present study (1430 ng mL−1). Although the direct effect of FFC on nitrifying bacteria present in the RAS biofilter in this study was not determined, the close correlation between (1) increasing nitrite levels and biofilm-associated restriction of RAS tank water supply to the biofilter during the dosing and post-dosing periods, (2) decreasing nitrite concentration after flushing biofilms from water supply lines to the biofilter during the dosing and post-dosing periods and (3) the ~35-fold lower observed FFC concentration relative to the lowest nitrifying bacteria MIC indicate that the changes in biofilter nitrogen oxidation efficiency observed (as determined by increasing nitrite levels) in the present study were not the result of FFC administration in medicated feed. These data support that administration of FFC-medicated feed to fish in a RAS at a dose of 15 mg FFC kg−1 BW d−1 for 10 consecutive days will not affect biofilter nitrification.
In conclusion, the FFC marker residue FFA depleted from the fillet of tilapia following administration of FFC-medicated feed at a mean daily dose of 19.62 mg FFC kg−1 BW d−1 for 10 days with a half-life of 1.33 days. The predicted withdrawal period at this exaggerated dose (~1.3× the 15 mg FFC kg−1 BW d−1 for 10 days dosing regimen on the approved drug label) was 10 days for all FFC residues to deplete to below the 1 μg g−1 withdrawal limit. Administration of FFC-medicated feed did not alter the capacity of the RAS biofilter to remove nitrogenous wastes.
Acknowledgments
The authors thank Ms. Theresa Schreier, Ms. Maren Tuttle-Lau, Mr. Mike Boogaard, Mr. Steve Redman and Mr. Nick Schloesser for their technical assistance in the conduct of this study. This study was fully funded by Merck Animal Health through a Cooperative Research and Development Agreement with the US Geological Survey. The data generated in this study were accepted by the US Food and Drug Administration and other international regulatory bodies to describe the depletion of FFC residues following administration in FFC-medicated feed to tilapia. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government.




