Assessment of the bacterial community diversity associated with the queen conch Strombus gigas (Linnaeus, 1758) from the Caribbean coast of Colombia using denaturing gradient gel electrophoresis and culturing
Abstract
The biotic diversity of Strombus gigas has not been thoroughly studied despite the status of the queen conch as an important ecological resource. The bacteria associated with the conch influence their host in several ways, including through the metabolism of nutrients, protection against invasive bacteria and the regulation of the physical conditions. In this study, conventional microbiological methods and molecular tools such as denaturing gradient gel electrophoresis (DGGE) were used to assess the composition of the bacterial communities associated with the queen conch (S. gigas) in wild and captive habitats in Colombia. A genetic analysis of the bacterial communities revealed a high level of diversity based on the large number of bands detected using DGGE. In addition, differences in bacterial community structure were found between the conchs in captivity and the wild populations. The dominant phylogenetic affiliations of the bacteria, as determined using 16S rRNA gene sequencing, were grouped into four classes, namely, Betaproteobacteria (16%), Gammaproteobacteria (70%), Firmicutes (7%) and Actinobacteria (2%). These groups are related to host defence processes and the decomposition of organic matter. The 16S rDNA sequence analysis of the cultured bacteria and the resulting DGGE profiles are useful tools for characterizing the diversity of the bacteria associated with the analyzed conchs.
Introduction
Microorganisms are dispersed throughout the oceans as free-living individuals or in complex associations with other marine organisms (Ashen & Goff 2000). The queen conch, Strombus gigas, belongs to the class Gastropoda and is one of the largest and most commercially important gastropod molluscs. Strombus gigas inhabits shallow and sandy sea beds and is distributed throughout the tropical Atlantic and Caribbean coasts of Central and South America (Warmke & Abbott 1961; Randall 1964; Brownell & Stevely 1981; Mccarthy 2007). This species is considered to be vulnerable because its population has declined. Overfishing, the loss of important breeding habitats (e.g. sea grass beds) and human activities such as urbanization, pollution and other destructive processes are the primary causes of population decline (Glazer & Quintero 1998). According to information from the Colombian Agricultural Institute (ICA), the export volumes of resources such as the Nassau grouper, the queen conch (Strombus gigas) and the Caribbean spiny lobster are currently approximately 200 tons per year; 90 per cent of those exports are sent to the US market, representing revenues for the Colombian economy in excess of 10 million dollars annually.
One alternative for restoring overfished populations of S. gigas is the establishment of marine protected areas (MPAs) (Anon 1999; Appeldoorn & Rodriguez 1994; Stoner 1996), which enable the preservation of schools that spawn at high densities and provide shelter for reproducing adults (Anon 1999). The aquaculture or semi-culture of species of interest (Brownell 1977; Creswell 1994) is emerging as a good management alternative for establishing whether repopulation is feasible. The study of all aspects of these alternatives, including strategies for the conservation of this species, is fundamental to their success (Pérez-Enriquez, Garcia-Rodriguez, Mendoza-Carrion & Padilla 2011). In addition to the ecology of the queen conch, such studies should incorporate the characteristics of the conch, its associated microbiota and how the microbiotic organisms could be directly related to the growth and development of the conch (Verschuere, Rombaut, Sorgeloos & Verstraete 2000). Strombus gigas acts as a host to marine microorganisms, and this role could be important in relation to improvements in the management strategies for queen conch in the Caribbean region.
Bacterial communities are an essential component of eukaryotic organisms and are involved in many processes including the metabolism of nutrients, protection against invasive bacteria and the regulation of the physical and physiological state of the organism (Davis & Stoner 1994; Aldana-Aranda & Patiño 1998; Shnit-Orland & Kushmaro 2009; Ahmadnia, Farhangi, Rafiee & Noori 2012). Defining the microbial diversity associated with an organism facilitates an understanding of the associations between the host and the microorganisms. The relationship between the diet of the host and the extracellular enzymes produced by bacteria within the host is well described in the literature (Tanu, Deobagkar, Khandeparker, Sreepada, Sanaye & Pawar 2011), as are the roles of microbes in biogeochemical cycles and the transformation of organic matter (Mohapatra, Bapuji & Sree 2003; Dang, Zhu, Wang & Li 2009).
Although the evaluation of bacterial population structures has been of interest to the scientific community (Ducklow, Boyle, Maugel, Strong & Mitchell 1979; Rodriguez, Hariharan & Nimrod 2011), little is known about the bacterial diversity associated with the sea conch. Bacterial genera such as Enterobacter, Klebsiella and Vibrio were identified in the conch when culture-dependent methods were used (Rodriguez et al. 2011). The bacterial populations associated with the queen conch have previously been determined using cultivation-dependent techniques, and the intergenic spacer region between the 16S and 23S rDNA genes (the internal transcribed spacer, or ITS) has been used to describe the variation in bacterial populations composed of Psychrobacter spp., Halomonas spp., Pseudoalteromonas spp., Vibrio spp. and Cobetia spp. (Acosta, Gómez, Romero, Cadavid & Moreno 2009). However, a striking characteristic of the indigenous bacteria in many environments is the lack of cultivability of the majority of living bacteria (Schloss & Handelsman 2005). Recently, the development of culture-independent molecular techniques based on the amplification of genes has allowed researchers to assess non-culturable populations. DNA fingerprinting techniques based on 16S rRNA gene analysis are currently revolutionizing the existing knowledge about microbial biodiversity in different environments (Kvennefors, Sampayo, Ridgway, Barnes & Hoegh-Guldberg 2010). To obtain additional information regarding the bacterial composition of the queen conch (S. gigas) from the Rosario Islands in the Colombian Caribbean, both conventional microbiological methods and culture-independent molecular tools were used, including denaturing gradient gel electrophoresis (DGGE), which is based on the fingerprints of the amplified 16S rDNA gene. These analyses were performed with a particular emphasis on the different bacterial populations of conchs in captivity compared with free-roaming or wild conchs.
This study is the first research work on bacterial diversity in S. gigas from the Caribbean coast of Colombia to use the molecular approach of DGGE, which permits the detection of dominant bacterial communities that could be of importance for the conch. DGGE complements the results described by Acosta et al. (2009) regarding an analysis of the 16S rRNA gene sequences of cultured bacteria. Strombus gigas is a candidate for culture (fish farming), but queen conch culture is still in its infancy due to problems such as feeding and seed production; there is also a paucity of studies of infectious diseases and immune status. A better knowledge of the bacterial population of the queen conch might be helpful for the formulation of optimal live feed for the growth and development of S. gigas, which is necessary for the management and implementation of conservation efforts aimed at this ‘at risk’ conch species.
Materials and methods
Sample selection and cultivation
The conch samples were collected in July and December 2007 for the study described by Acosta et al. (2009). Twenty healthy animals were collected in sterile plastic containers and transported to the laboratory on ice. The samples were processed as described by Acosta et al. (2009). Samples of the food being consumed by the sampled wild conches were also collected and transported on ice. Secretion samples were obtained from each conch by placing the conch on a sterile glass surface. Food samples in sterile 1-L containers were filtered, resuspended in 25 mL of sterile water and sent to the laboratory. Intestinal samples were obtained from the conchs by aseptically dissecting and carefully extracting the digestive tract. After dissection, the parts of the digestive tract including the stomach and anus were separated for analysis, and 0.5 g of tissue from the middle part of the intestine was washed with sterile water, fragmented and processed. The secretions (s), intestine (i) and food (algae of the genera Isocrysis and Chaetoceros)(a) samples of wild (W) and captive (C) conchs were preserved at −80°C for 1 year and subsequently processed at the Molecular and Cellular Biology Laboratory of the National University of Colombia (Universidad Nacional de Colombia). The culture-dependent samples were reactivated according to the protocol for resuscitation from a viable but non-culturable state (VNC) described by Whitesides and Oliver (1997) and Du, Chen, Zhang, Li and Li (2007). The samples were cultured in marine agar, and serial dilutions of each sample were spread on each culture medium and incubated at 20°C in an aerobic atmosphere. The colony-forming units (CFUs), which were counted as the cultivable fraction or streaks of the samples (F), were subsequently used for DNA extraction (Table 2).
In addition, approximately 60 randomly selected colonies were sub-cultured to ensure the purity of those that were isolated and used for DNA extraction. From these 60 colonies, 55 samples were selected for sequencing based on their distinctive features at the microscopic and macroscopic level.
Molecular analysis
PCR 16S rDNA DGGE
The DNA samples that were obtained by Acosta et al. (2009) were used to study the diversity and dynamics of the dominant bacterial communities. The total DNA samples obtained by extraction from the original biological material were termed the fraction of DNA revealed by the molecular approach (D), and the DNA obtained from the cultivable streaking fraction in marine agar was named the CFU DNA (F) (Table 2).
The DNA was extracted and purified as described by Acosta et al. (2009). The D and F DNA samples were subjected to 16S rRNA gene amplification between the V3 and V6 regions with primers specific to the conserved 907R and 341F-GC domains (Table 1) as previously described by Moreno, Moy, Daniels, Godfrey and Cabello (2006). One end of the 341-GC primer is composed of approximately 40 base pairs (bp) of guanine and cytosine deoxyribonucleotide phosphates used to modify melting behaviour by denaturing the fragment of interest, thereby improving the resolution of fragments of similar size, but with different GC contents. The PCR reaction was performed in a final volume of 30 μL of solution containing 1–4 μL of DNA (50 ng). The thermocycling conditions and the concentrations of the buffer, MgCl2, dNTPs and Taq DNA polymerase were performed as described previously Moreno et al. (2006).
| Name | Sequence (5′–3′) | Position | Reference |
|---|---|---|---|
| 27F | AGAGTTTGATCCTGGCTCAG | 27–46 | Delong (1992) |
| 1492R | GGTTACCCTGTTACGACTT | 1492–1510 | Delong (1992) |
| 341F | CCTACGGGAGGCAGCAG | 341–357 | Muyzer et al. (1993) |
| 341F-GC |
CGCCCGCCGCGCGCGGCGG GCGGGGGGGGCACGGGGGG CCTACGGGAGGCAGCAG |
341–357 | Muyzer et al. (1993) |
| 907R | CCCCGTCAATTCATTTGAGTTT | 907–928 | Yu and Morrison (2004) |
| 518F | CCAGCAGCCGCGGTAATACG | 518–537 | Lane (1991) |
| 800R | TACCAGGGTATCTAATCC | 800–818 | Lane (1991) |
The PCR products that were obtained using the 341F-GC and 907R primers were run on 6% (weight/volume) polyacrylamide gels in 1× TAE buffer (40 mM Tris base, 20 mM glacial acetic acid and 1 mM EDTA) with a denaturing gradient of 30–60% urea–formamide for 15 h at 85 V and a constant temperature of 60°C in a D-Code system (Bio-Rad, Hercules, CA, USA). The gels were stained with AgNO3 (Amresco, Solon, OH, USA) (Sanguinetti, Dias Neto & Simpson 1994) and analyzed using the program GelCompar II (Applied Maths, Austin, TX, USA) (Rademaker & Bruijn 2008). After the representative lanes from each DNA sample were aligned, a cluster analysis was performed using the Dice coefficient (Nei & Li 1979) and the unweighted pair-group method with arithmetic average (UPGMA) (Mohammadi & Prasanna 2003).
The presence and absence matrix was used to generate an analysis of similarity (ANOSIM) based on the Bray–Curtis index using PAST software version 2.0. The similarity analysis was used to examine the statistical significance of differences between the DGGE profiles (Hammer, Harper & Ryan 2001).
16S rDNA sequence analysis
The DNA bands with unique migration patterns based on the marker set and the most intense bands in each DGGE gel were excised in duplicate (where possible) and placed in sterile vials. The DNA of each of the bands was recovered through the ‘crush and soak’ elution method (Sambrook & Russell 2001) for DNA precipitation by replacing two volumes of ethanol at 4°C for each 0.6 volumes of isopropanol at 4°C, resulting in 10 μL of eluent DNA, of which 6 μL was used to carry out the re-amplification with the 341F and 907R primers. The amplification products were purified using an UltraClean PCR Clean-Up Kit (MO BIO Laboratories, Carlsbad, CA, USA). The amplicons were sent to Macrogen (Seoul, Korea) to be sequenced on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). The sequences that were thus obtained were edited using the BioEdit® program (Hall 1999), and the presence of chimeric sequences was evaluated with the CHIMERA CHECK software program (http://www.bioinformatics-toolkit.org). The edited sequences were compared with known sequences in the GenBank database using the basic local-alignment search tool (BLAST) (Altschul, Madden, Schäffer, Zhang, Zhang & Miller 1997) and the sequence match tool of the Ribosomal Database Project (RDP) website. The edited sequences were aligned using the ClustalW program (Thompson, Higgins & Gibson 1994). MEGA software version 5 (http://megasoftware.net/) was used to construct a phylogenetic tree via the neighbour-joining method (Saitou & Nei 1987) with 1000 bootstrap replicates. The evolutionary distances were calculated using the Jukes–Cantor method (Jukes & Cantor 1969).
DNA extraction from the bacterial isolates
The DNA of the isolated strains was obtained from the liquid cultures through phenol–chloroform and ethanol precipitation as previously described by Sambrook and Russell (2001). After DNA extraction, the samples were analyzed on a 1% (W/V) agarose gel stained with EZ-Vision (Amresco, Solon, OH, USA) according to the manufacturer's recommendations. The DNA bands were visualized by illumination with ultraviolet light and photographed using a UV transilluminator system (Biometra, Geottingen, Germany). The extracted DNA samples were stored at −20°C.
To identify the isolates, the 16S rRNA gene was amplified according to Espejo, Feijo, Romero and Vasquez (1998) with the universal primers Eubac27F and 1492R, as described by Delong (1992) (Table 1). The PCR products were analyzed using electrophoresis on a 1% (W/V) agarose gel stained with EZ-Vision.
Amplicons that were approximately 1465 bp long were purified and sequenced by Macrogen in both directions with the 518F, 907F, 800R and 1492R primers and an ABI PRISM 3100 Genetic Analyzer. Further analyses were carried out according to the methods and procedures described above for the sequences of the bands obtained from the DGGE gels. The sequences were deposited in the GenBank database with the accession numbers JN602212–JN602254.
Results
Analysis of the DGGE profiles
The assessment of the diversity of the bacterial populations that were associated with wild and captive conchs from the Colombian Caribbean was carried out using the DGGE technique, which enabled the differentiation of 16S rRNA gene fragments of approximately 585 bp (Fig. 1) with the assumption that each of the bands in the different patterns represents a group of bacteria. The results of the banding profiles, evaluated using the program GelCompar II, revealed the presence of several migration patterns. From this gel analysis, 47 unique and common DNA bands were selected based on the band intensity. The different secretion and intestine samples exhibited more complex patterns and more diverse bands compared with those of the food samples in the DGGE (Fig. 1). The results of the DNA bands sequences are provided in Table 2.
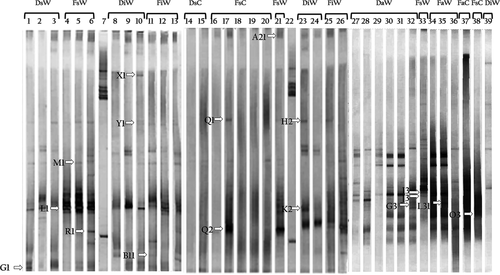
| Origin | DGGE band | Phylogenetic affiliation | Related sequence | GenBank accession No. | % of identity |
|---|---|---|---|---|---|
| FsW | L1 | γ-proteobacteria | Pseudoalteromona sp. (JF820747) | JQ410456 | 100 |
| R1 | β-proteobacteria | Janthinobacterium sp. (AB252072.1) | JQ410460 | 98 | |
| A21 | β-proteobacteria | Herbaspirillum sp. (AB545652.1) | JQ410463 | 99 | |
| I3 | Unknown | Uncultured bacterium (AM992750.1) | JQ410465 | 100 | |
| J3 | β-proteobacteria | Burkholderia phytofirmans (GQ181027.1) | JQ410466 | 99 | |
| DsW | G1 | β-proteobacteria | Pandoraea sp. (EF397586.1) | JQ410457 | 95 |
| FsC |
O3 Q1 Q2 |
γ-proteobacteria Unsuccessfully sequenced bands |
Acinetobacter sp. (GQ174293.1) | JQ410469 | 100 |
| FiW | B11 | Unknown | Uncultured bacterium (GQ057443.1) | JQ410461 | 99 |
| DiW | Y1 | β-proteobacteria | Uncultured bacterium (AY792250.1) | JQ410458 | 99 |
| X1 | β-proteobacteria | Burkholderia sp. (AF408997.1) | JQ410459 | 99 | |
| K2 | β-proteobacteria | Burkholderia sp. (HM063925.1) | JQ410462 | 99 | |
| H2 | β-proteobacteria | Burkholderia mallei (S55000.1) | JQ410464 | 97 | |
| FaW | L31 | γ-proteobacteria | Acinetobacter sp. (EU867305.1) | JQ410467 | 99 |
| DaW | G3 | β-proteobacteria | Pandoraea sp. (AB510957.1) | JQ410468 | 97 |
- DGGE, denaturing gradient gel electrophoresis.
- DNA was obtained from the DNA samples (D) or the cultivable fraction (F) that was derived from the secretions (s), intestines (i) or food (a) of wild (W) or captive (C) conchs.
The banding patterns obtained using DGGE were grouped into three clusters with the UPGMA method for the construction of molecular phylogenetic trees (Fig. 2).
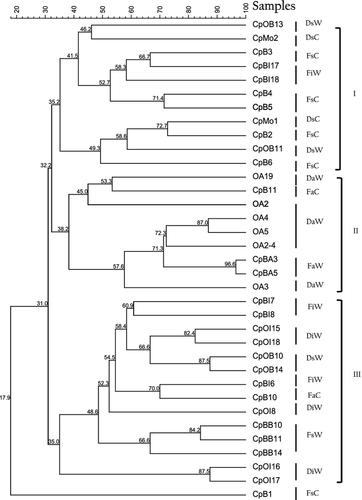
Cluster 1 corresponds to the banding pattern of the bacterial populations associated with the secretions of the conchs in captivity, cluster 2 is associated with the food samples, and cluster 3 is associated with the intestine and secretion samples of the wild conchs. The banding profiles of the food samples were more closely related to the samples of the secretions of conchs in captivity (>32% similarity) than to the samples of the secretion and intestines of the wild conchs (<31% similarity) (Fig. 2). According to the binary matrix of data on the presence (1) and absence (0) of bands, 55 different bands were detected on the gels. The samples associated with wild conchs and with captive conchs produced 271 and 76 bands respectively. A band-based binary presence/absence matrix was calculated by applying the Dice similarity coefficient and used for an analysis of similarity (ANOSIM) based on the Bray–Curtis coefficient, which enables significance testing of the data groups (Hammer et al. 2001). Significant differences were found between the samples associated with wild and captive conchs [r = 0.26 (Bray Curtis) and r = 0.25 (Jaccard), (P < 0.0021 in both cases)].
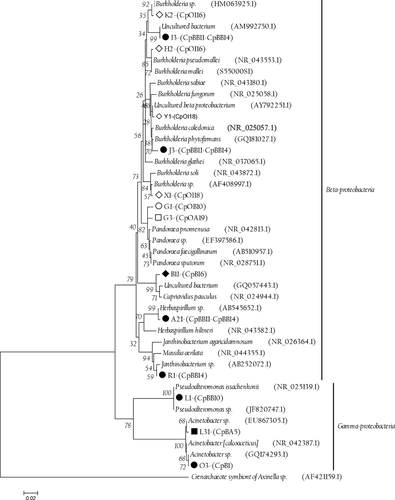
Furthermore, the results of the analysis of the sequences obtained from the re-amplified 16S rRNA bands revealed a high level of similarity with Gammaproteobacteria (20%) and Betaproteobacteria (60%) (Table 2). The results of the phylogenetic affiliations based on the sequence analysis of the excised bands from the DGGE gels are summarized in Table 2 and Fig. 3.
Isolation and identification of isolates
A total of 55 strains were selected and sent to be sequenced, including 31 strains sampled from the conch secretions, 15 from the intestine samples and nine from the food of captive conchs. However, only 41 strains could be molecularly identified based on the sequence of the 16S rRNA gene from position 27 to 1492 (corresponding to the position in Escherichia coli). All of the sequences had high similarity (99–100%) with reported sequences in the GenBank database, and the majority were similar to sequences from bacteria that had been obtained from experiments performed in marine environments rather than from cultivated sources (Table 3) (Xu, Wang, Wang & Xiao 2005; Yoon, Lee, Kang & Oh 2005; Yu, Li, Zeng & Chen 2009; Nithyanand, Manju & Karutha Pandian 2011).
| Origin | Strain from the queen conch | GenBank accession No. | Sequence of nearest 16S rDNA | Similarity (%) | Bacterial group |
|---|---|---|---|---|---|
|
DsW FsC FiW FsC FsW |
18CpOB10 19CpB3 21CpBI7 25CpB1 7CpBB10 10CpBB10 2CpBB11 5CpBB11 8CpBB11 2CpBB12 4CpBB13 5CpBB13 |
Uncultured Psychrobacter sp. FJ695576 |
99 | γ-proteobacteria | |
| DiW |
6CpOI15 13CpOI15 24CpOI15 28CpOI15 |
Uncultured Psychrobacter sp. FJ695589 | 99–100 | ||
| FaW |
1CpBA3TCBS 5CpBA3 1CpBA4 3CpBA4 |
||||
| FsW |
3CpBB11 1CpBB14 |
||||
| FsW |
3CpBB10 2CpBB14 6CpBB14 8CpBB14 |
Psychrobacter sp. DQ399761 | 99–100 | ||
| 1CpBB10 | JN602224 | Psychrobacter celer EF101550 | 99 | ||
| FsC |
1CpB4 3CpB4 10CpB4 |
Psychrobacter sp. GU570642 | 100 | ||
|
DiW FsC DiW |
5CpOI8 22CpB4 26CpOI8 |
Halomonas sp. AM403725 | 99–100 | ||
|
FaC DaW |
1CpB10 12CpOA5 |
Halomonas sp. AM403725 | 99–100 | ||
| DiW | 2CpOI6 | JN602249 | Halomonas sp. AB166968 | 100 | |
| DiW | 27CpOI17 | JN602236 | Exiguobacterium arabatum FM203124 | 100 | Firmicutes |
| FiW |
5CpBI9 3CpBI9TCBS |
Exiguobacterium sp. DQ407720 | 100 | ||
| DsW | 29CpOB11 | JN602242 | Oceanobacillus sp. DQ358670 | 100 | |
| DsW | 20CpOB13 | JN602241 | Micrococcus sp. GU213502 | 100 | Actinobacteria |
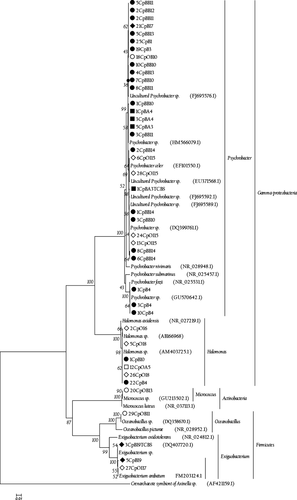
Based on the phylogeny of the related sequences from the BLAST search and the reference sequences obtained from the RDP, three bacterial groups were identified at the phylum level: Proteobacteria (89%), Firmicutes (9%) and Actinobacteria (2%), within which there were five different genera: Psychrobacter (74% of the total, 30 isolates), Halomonas (15%, six isolates), Exiguobacterium (7%, three isolates), Oceanobacillus (2%, one isolate) and Micrococcus (2%, one isolate) (Table 3 and Fig. 4).
Discussion
Little is known regarding the microbiota and symbiotic microorganisms hosted by the queen conch (Acosta et al. 2009). The bacterial microbiotas of several marine organisms have been studied, including those of algae, coral (Kvennefors et al. 2010), fish (Sun, Yang, Ling, Chang & Ye 2009) and conchs (Rodriguez et al. 2011), with the goal of understanding the effects of bacterial populations on the ecology, conservation, health and pathogen defence mechanisms of these hosts. A further goal has been to identify the variations in microbial diversity according to the type of inhabited ecosystem and other factors, for example the stimulation of epithelial proliferation (Rawls, Samuel & Gordon 2004).
In this study, the bacterial diversity associated with both wild and captive conchs from the Rosario Islands in Colombia was examined using both culture-dependent approaches (agar plating) and culture-independent techniques (DGGE and sequence analysis of the 16S rRNA gene). Variations among the bacterial populations were identified based on where the hosts were obtained (Fig. 2). When the diversities of the bacterial populations associated with the samples of wild and captive conchs from Rosario Island in the Colombian Caribbean were compared using the DGGE technique, the analysis revealed significant differences (75.45% average dissimilarity), suggesting that the molecular approach based on the analysis of DNA extracted directly from the sample combined with the culture-dependent approach appears to be an adequate strategy for determining the organisms of the microbial community.
When the DGGE profiles of the 16S rRNA gene sequences of the cultivable fraction were compared with the DGGE profiles of the fraction of DNA revealed by the molecular approach (D), differences in band intensity were apparent, with the most intense bands being found predominantly in the CFU gels (Fig. 2). These differences could have occurred because the DGGE technique can be affected by low concentrations of DNA of different species or the presence of high concentrations of competing DNA during PCR reactions (Ogier, Son, Gruss, Tailliez & Delacroix-Buchet 2002), and the relative abundances of the other members of a community can affect the detection of certain species (Kisand & Wikner 2003). However, the DGGE technique is able to detect a member even when it constituted only 1% of the population (Muyzer, Waal & Uitterlinden 1993; Muyzer & Smalla 1998); the analysis of the DGGE profiles revealed a diverse bacterial community in the secretion, intestinal tract and food samples, with important shifts in the bacterial community structure of wild vs. captive conchs (Fig. 1). The presence in both conch groups of bands from the cultivable fraction of DNA (F) (the B11 band of wild conchs, with an unknown phylogenetic affiliation, and the O3 band of captive conchs, identified as Acinetobacter) and from the fraction of DNA revealed by the molecular approach (D) (the X1 and H2 bands of wild conchs, identified as Burkholderia) indicates that the use of DGGE profiles can identify unique and diverse bacterial communities depending on where the hosts were obtained (Edenborn & Sexstone 2007).
The sequence data of the culture-dependent and independent samples were found to be phylogenetically related at the class level and belonged to the Gammaproteobacteria; however, the genera that were identified were different (Table 3), with the exception of the sequences corresponding to the genus Pseudoalteromonas, which was detected using both DGGE (L1 Band) and the isolated culture DI1Sg (GQ253505), as previously reported by Acosta et al. (2009). The 16S rRNA gene sequences of the excised bands from the DGGE gels were grouped into two classes: Betaproteobacteria and Gammaproteobacteria, with the latter being reported as the dominant bacterial group in bacterial communities associated with marine invertebrates (Wegley, Edwards, Rodriguez-Brito, Liu & Rohwer 2007; Kvennefors et al. 2010). Betaproteobacteria have also been previously described as common inhabitants of marine environments (Kirchman, Dittel, Malmstrom & Cottrell 2005; Zhang, Jiao, Cottrell & Kirchman 2006).
The band sequences that were associated with the intestine samples of the wild conchs (iW H2, K2 and X1) exhibited similar percentages (between 97% and 99%) of Burkholderia spp., an extremely heterogeneous bacterial genus that has been previously described as nitrogen-fixing (Kennedy, Choudhury & Kecskes 2004; Barua, Tripathi, Chakraborty, Ghosh & Chakrabarti 2012) and as an opportunistic pathogen (Onofre-Lemus, Hernández-Lucas, Girard & Caballero-Mellado 2009). The role of Burkholderia in aquatic organisms is still poorly understood. In a study of the intestinal microbiota of fish such as Epinephelus coioides, Sun et al. (2009) reported that the presence of an increased cell density of Burkholderia cepacia is associated with slow growth in juveniles. The sequence that was amplified in this study corresponds to the A21 band of the related Herbaspirillum species, which was isolated from an aquatic Aquaspirillum species and is also associated with nitrogen-fixing bacteria (Ding & Yokota 2004). The ecological impact of biotic nitrogen fixation in animals has been little studied in insects, but a nitrogen-poor diet can be compensated for by the presence of nitrogen-fixing bacteria in the intestines (Behar, Yuval & Jurkevitch 2005; Bothe, Ferguson & Newton 2007).
The sequence corresponding to L1 (sW), with a similarity percentage of 100%, belongs to the genus Pseudoalteromonas, which is frequently isolated from marine environments, corals, bryozoans and fish (Pukall, Kramer, Rohde & Stackebrandt 2002; Shnit-Orland & Kushmaro 2009; Svanevik & Lunestad 2011). This genus has been associated with the production of antibacterial substances (Chen, Lin, Chen, Wang & Sheu 2010) and metabolites that are active against a broad range of bacteria and fungi (Holmstrom & Kjelleberg 1999).
The sequences of the L31 (aW) and O3 (sC) bands have similarities of 99% and 100%, respectively, with Acinetobacter, which is associated with commensalism processes in marine invertebrate intestines and has been reported to belong to the normal microflora of other species of conchs (Ducklow et al. 1979).
Although the molecular approach provides accurate information about bacterial community composition, a comprehensive overview of queen conch microbiota can also be improved using culture-based methods. In this study, the cultivation methods that were used resulted in the isolation of strains belonging to taxonomic groups that previously could not have been grown, thus facilitating the discovery of a greater diversity of genera than had been described previously for the queen conch (Stevenson, Eichorst, Wertz, Schmidt & Breznak 2004; Davis, Joseph & Janssen 2005; Stott, Crowe, Mountain, Smirnova, Hou, Alam & Dunfield 2008; Acosta et al. 2009). Süβ, Engelen, Cypionka and Sass (2004) and Nithyanand et al. (2011) reported the isolation of γ-proteobacteria, Actinobacteria and Firmicutes, the same three bacterial phyla found in this study. The dominant bacterial genera were Psychrobacter (74%) and Halomonas (15%), which is a result comparable to the one obtained for marine sediments in southern Okinawa (Dang et al. 2009).
The greater band and isolate diversity of the bacterial populations from the wild conchs compared with the conchs in captivity are most likely due to the different environments in which the animals live (Kvennefors et al. 2010). The stress derived from high population densities and sub-optimal water conditions in the animal-breeding environment might sustain the growth of a microbial community that could endanger the health of conchs, such as the community dominated by Vibrio spp. reported by Acosta et al. 2009 or the Burkholderia spp. reported in this study. However, the 16S rDNA sequence analyses of the cultured bacteria and the DGGE profiles obtained in this study were useful tools for inferring the bacterial diversity that was present in the conch samples. The results here may not be representative of all captive and wild conchs; nevertheless, this study provides the first insight on the molecular analysis via DGGE of bacteria communities in S. gigas from the Caribbean coast of Colombia. Further studies of the interactions between the conch and its microbiota will support the development of knowledge about microbial diversity and the importance of microbes for host defence processes, the decomposition of organic matter and related biogeochemical cycles.
Acknowledgments
This research was funded by the National University of Colombia, Medellin through the 20101007734 Bicentennial DIME project.




