Editorial: Balancing the Yin and Yang of Alcohol-Associated Liver Disease—Integrating Pathophysiology, Liver-Directed Therapy and Addiction Management
Funding: The authors received no specific funding for this work.
Alcohol-associated liver disease (ALD) is a major cause of morbidity and mortality, yet treatment remains limited to supportive care and alcohol abstinence [1]. The mechanisms linking excessive alcohol consumption to liver damage are not fully understood, but interleukin-17A (IL-17A) levels are elevated in heavy drinkers and further increased in ALD patients, suggesting its role in alcohol use and liver injury [2]. Experimental models have shown that IL-17A blockade, whether genetically or pharmacologically, reduces alcohol intake and prevents liver and brain damage [2]. Guselkumab, a monoclonal antibody targeting the p19 subunit of IL-23, inhibits IL-23 signalling and Th17 cell development that drives IL-17A production. It is currently approved in the United States for treating plaque psoriasis, psoriatic arthritis, and ulcerative colitis but presents a mechanistic rationale for therapeutic investigation in ALD [3-5].
In the current trial by Díaz et al., guselkumab was tested in three dose groups: 30, 70 and 100 mg in patients with early-stage ALD, defined by increased liver fat content (MRI-PDFF ≥ 8% or CAP ≥ 300 dB/m) and AST/ALT levels below 200 U/L. [6] The drug was well tolerated, with no dose-limiting toxicities or serious adverse events [6]. In higher-dose groups, biomarkers of inflammation, including IL-17, IL-23, IL-1β and TNF-α, were reduced, and self-reported alcohol consumption decreased [6]. This study highlights the potential of IL-17A blockade for ALD, but several key issues remain to be addressed.
The open-label design limits the ability to attribute observed changes, and the absence of a control group makes it difficult to compare guselkumab's effectiveness to standard treatments or other interventions. Furthermore, while this study focused on early-stage ALD, the real unmet need lies in patients with more severe forms of the disease, such as alcohol-associated hepatitis or advanced cirrhosis. These patients are at higher risk for liver failure, complications, and mortality, requiring more effective interventions [7]. By focusing on early-stage ALD, this study does not address the challenges of treating more advanced disease with higher disease burden. Despite these limitations, the study's results are promising, particularly in reducing alcohol consumption. These findings align with previous animal studies, which showed reductions in alcohol intake with IL-17A inhibition [2]. While human data is still preliminary, the decrease in self-reported alcohol consumption suggests that guselkumab may have a similar effect in humans, providing a foundation for further investigation.
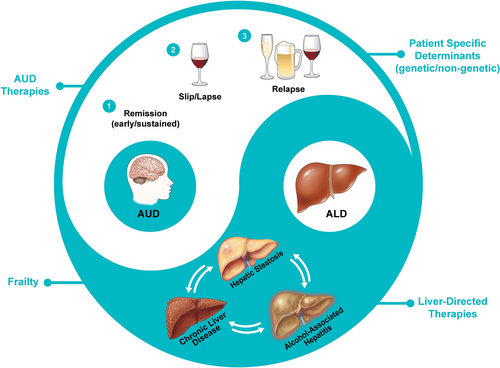
Guselkumab shows promise in reducing alcohol consumption and inflammation, offering an exciting therapeutic avenue for ALD. This study establishes the safety profile and suggests the potential to address both alcohol use disorder (AUD) and ALD simultaneously, targeting two key aspects of the disease with one treatment. However, more controlled studies using a double-blind, placebo-controlled study design are needed to confirm its efficacy and safety, particularly in patients with advanced ALD. Treating ALD requires a comprehensive approach that targets both AUD and liver damage (Figure 1) [6, 8-10]. Combining IL-17A blockade with behavioural therapies for alcohol abstinence embodies a Yin–Yang approach to ALD management, underscoring the need for further research into integrated treatment strategies to improve outcomes.
Author Contributions
Raj Vuppalanchi: conceptualization, writing – original draft, visualization, writing – review and editing. Suthat Liangpunsakul: conceptualization, writing – original draft, writing – review and editing.
Conflicts of Interest
Dr. Vuppalanchi conducts research supported by Lilly, Galectin Therapeutics, Takeda, AstraZeneca, Zydus Pharmaceuticals, Gilead and GSK, but these activities are unrelated to this paper. Dr. Liangpunsakul discloses consulting roles with Durect Corporation, Surrozen and Korro Bio.
Linked Content
This article is linked to Diaz et al papers. To view these articles, visit https://doi.org/10.1111/apt.70026 and https://doi.org/10.1111/apt.70064.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




