Review article: liver disease in adults with variants in the cholestasis-related genes ABCB11, ABCB4 and ATP8B1
Joint Senior Authorship: Deepak Joshi and Richard J. Thompson.
The Handling Editor for this article was Professor Gideon Hirschfield, and this uncommissioned review was accepted for publication after full peer-review.
Summary
Background
Children with intrahepatic cholestasis and genetic variants which result in the disruption of the formation and maintenance of bile (ABCB11, ABCB4 and ATP8B1) generally have a rapidly progressive clinical course. Adults with different phenotypes of cholestasis are increasingly being evaluated for variants in these genes associated with childhood diseases.
Aims
To review the literature with respect to the presence of variants in cholestasis-related genes in adults with various liver phenotypes, and provide clinical implications of the findings.
Methods
A search of the literature on variants in specific cholestasis-related genes in adults was conducted.
Results
The common variant p.Val444Ala in ABCB11 confers increased risk of drug-induced liver injury and intrahepatic cholestasis of pregnancy (ICP). Individuals with variants in ABCB4 are at risk of ICP and low phospholipid-associated cholelithiasis. Variants in ABCB4, and possibly ABCB11 and ATP8B1, can be identified in up to a third of patients with cryptogenic chronic cholestasis.
Conclusions
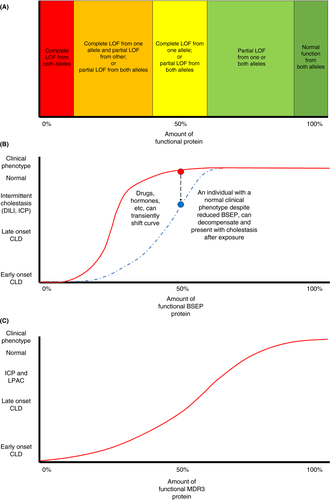
Individuals with variants in ABCB11 rarely develop cholestasis until BSEP function dips below a threshold, which is also affected by other factors (e.g., drugs, hormones). However, variants in ABCB4 and consequent reduction in MDR3 protein, have a more linear dose-response curve. In individuals with an ABCB11 variant, medications known to reduce BSEP activity should be used cautiously; they should be monitored during pregnancy for ICP; and first-degree relatives should be counselled and screened. No proven management strategy exists, although ursodeoxycholic acid may be beneficial. Further work is needed to define the genotype-phenotype correlation and natural history, and to evaluate the penetrance.
1 INTRODUCTION
Cholestatic liver diseases are a heterogenous group of conditions with an array of overlapping clinical manifestations. Early-onset disease is frequently genetic in origin. A particular focus on patients with progressive familial intrahepatic cholestasis (PFIC) has identified an increasing catalogue of variants in genes vital to the transport of biliary constituents and the stability of the canalicular membrane. PFIC patients present in childhood with intrahepatic cholestasis and frequently progress to end-stage liver disease before adulthood.1 The three major proteins affected in PFIC are the bile salt export pump (BSEP) encoded by ABCB11, the major phospholipid transporter (multidrug resistance protein 3, MDR3) encoded by ABCB4, and a protein contributing to membrane lipid composition (FIC1) encoded by ATP8B1 (Figure 1). The ABC transporters are a superfamily of transporters, mostly with two membrane spanning domains and two nucleotide binding sites, the latter of which are highly conserved through the family.2 ATP8B1 belongs to a subfamily of aminophospholipid-transporting P-type ATPases.3
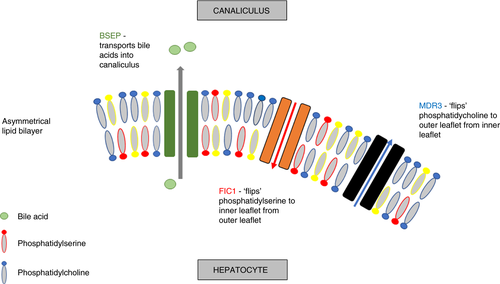
The consequences of a particular variant are only to some extent predictable. This information has been partly derived from in vitro analysis, and in silico prediction, but mainly from the behaviour of previous patients with disease.4-6 Severe deficiencies in any of these proteins, particularly where there is a complete loss of function from both alleles, is almost always associated with severe early-onset disease.7-9 The major PFICs follow a Mendelian inheritance in a recessive pattern, and can present with end-stage liver disease and liver cancer. Missense changes are associated with a spectrum of effects on proteins. Some missense changes may cause a complete loss of function and be just as severe as a non-sense variant; others are much better tolerated and may be of little or no functional consequence; while the majority represent a more intermediate loss of function. There can be a delay in onset of clinical symptoms of years, or even decades. In those with late-onset disease, most have a single variant, others have variants with milder reduction in protein function, or a combination of both.10 It is unlikely that these variants are behaving like true recessive Mendelian inheritance alleles and it is predicted that reduced protein levels manifest as a slower progression of liver disease or a predisposition to further hepatic insult.
A key aspect to the process of performing genetic testing is the clinical interpretation of detected variants. The American College of Medical Genetics and Genomics and the Association for Molecular Pathology published updated guidelines which are used as a framework to classify variants as: ‘pathogenic’, ‘likely pathogenic’, ‘uncertain significance’, ‘likely benign’ or ‘benign’.11 Multiple criteria are incorporated in the final interpretation of a variant, the end result is quite conservative in ascribing pathogenicity to a variant. The guidelines are intended for variants with a definitive role in a Mendelian disorder. In liver disease involving variants in cholestasis-related genes, the need for a second insult is often required and the degree of damage attributable to genetics is not always clear.4, 10 Variants in these genes which are categorised as ‘likely benign’ or ‘benign’ are not likely to be the sole cause of disease but may confer an increased risk to developing cholestasis. These latter two categories of variants are often not included in diagnostic reports.
This review summarises the published data on variants in ABCB11, ABCB4 and ATP8B1 identified in adults, in a range of cholestatic syndromes.
2 DRUG-INDUCED LIVER INJURY
Drug-induced liver injury (DILI) is a common cause of terminating new drugs during clinical trials and the leading cause for post-marketing withdrawals.12 The incidence of DILI in general populations is estimated at 13.9 per 100 000.13 Large studies assessing the outcomes of patients with DILI, identified mortality of up to 8%, and need for liver transplantation in 2%.14, 15 The development of DILI is unpredictable. The three main patterns of DILI are hepatocellular, cholestatic and mixed, which are based on the first laboratory tests available after the clinical event and involve the alanine aminotransferase (ALT), alkaline phosphatase (ALP) and ratios between the two values.16 The only clear genetic association with DILI is between flucloxacillin and HLA-B*5701.17 Several drugs are thought to cause cholestasis by inhibiting BSEP function, such as cyclosporine, bosentan and troglitazone.18, 19 In vitro work to determine the effect on BSEP in a large set of drugs, and correlate it with known DILI associations and taurocholate transport, identified a strong correlation between BSEP inhibitors and DILI.20 Over half the BSEP inhibitors were associated with severe DILI, in particular those which reduced taurocholate transport, while those BSEP inhibitors with less severe or no DILI had minimal effect on taurocholate transport.
A cohort of 36 patients with DILI (23 drug-induced cholestasis, 13 drug-induced hepatocellular injury) underwent genetic sequencing for known variants in ABCB11 and ABCB4, in comparison to healthy controls.21 Four variants specific for DILI were discovered: two in ABCB11 for drug-induced cholestasis (p.Asp676Tyr and p.Gly855Arg); one in ABCB4 for drug-induced cholestasis (p.Ile764Leu); one in ABCB4 for drug-induced hepatocellular injury (p.Leu1082Gln). In addition, the common ABCB11 variant p.Val444Ala, which is known to reduce protein levels by half compared to wild type, was observed more frequently in patients with drug-induced cholestasis than drug-induced hepatocellular injury and healthy controls. Homozygosity for p.Val444Ala was seen in 61% of drug-induced cholestasis, compared to 31% and 32% respectively in drug-induced hepatocellular injury and healthy controls. Functional analysis of the p.Gly855Arg ABCB11 variant identified 20% expression of reference BSEP, and the authors concluded that patients heterozygous for this variant have enough functional BSEP protein in normal healthy state, however, there is insufficient activity following drug or hormonal challenge, resulting in cholestasis. A similar causative role for the p.Val444Ala ABCB11 as a susceptibility factor, as a result of reduced hepatic BSEP expression compared to wild type, was also proposed.
The Spanish DILI Registry study of 188 patients (51 drug-induced cholestasis, 48 mixed liver injury, 89 drug-induced hepatocellular injury), sequenced patients and healthy controls for ABCB11 p.Val444Ala.22 In keeping with previous data, homozygotes for p.Val444Ala were more frequent in DILI than controls (OR 1.6, 1.1-2.4, P = 0.01). Causative drugs were checked for the presence of specific chemical moieties with BSEP-mediated taurocholate transport inhibiting properties. In the drugs which cause >50% BSEP inhibition, homozygous p.Val444Ala did not affect the DILI risk, but in drugs with <50% BSEP inhibition, homozygous p.Val444Ala was significantly associated with DILI (P = 0.01). The authors hypothesise that drugs which do not cause sufficient change in BSEP activity (ie <50% BSEP inhibition) require a contributing factor, in this case homozygous ABCB11 p.Val444Ala, to drop below the threshold of functional BSEP level, with resultant hepatotoxicity. The association within this cohort was with drug-induced hepatocellular injury not drug-induced cholestasis, however, it is worth considering that in the early stages of BSEP-mediated hepatotoxicity the elevated transaminases are a consequence of bile acid-related hepatocyte injury, which does not manifest with the classical cholestatic pattern (raised ALP) used in the definitions of DILI.
Further work from this group using the Spanish DILI Registry, performed extended genetic sequencing for common variants in ABCB1, ABCB4 and ABCC2, in 141 patients with DILI and compared these to healthy controls. Results detected no significant difference in common variants for these genes between the groups.23
Where functional BSEP work has been carried out, DILI is particularly evident in BSEP inhibiting drugs which reduce taurocholate transport. From the limited data available (Table 1) on DILI and genetic variants for proteins involved in biliary transport, the signal is of an association with common variants in ABCB11 gene. Reduced levels of BSEP are generally sufficient, but following a further insult with medications, intrahepatic cholestasis may occur. In individuals with variants this is most commonly seen with medications with a more modest reduction in BSEP activity (ie <50% BSEP inhibition). The biochemical picture of patients with BSEP related DILI does not usually fit the definition of drug-induced cholestasis, as the bile acid-induced hepatocyte injury predominantly presents with raised transaminases, and will not have a ratio of serum activity of ALT to ALP of 2 or less.16
| Author | Type of study | Number of patients | Genes tested | Association |
|---|---|---|---|---|
| Lang 200721 | Cohort of DILI compared to healthy controls | 36 (95 controls) | ABCB11 & ABCB4 |
Two ABCB11 variants and two ABCB4 variants in DILI cohort. ABCB11 p.Val444Ala common variant significantly associated with drug-induced cholestasis |
| Ulzurrun 201322 | Registry compared to healthy controls | 188 (219 controls) | ABCB11 p.Val444Ala | ABCB11 p.Val444Ala more in DILI than controls |
| Ulzurrun 201423 | Registry compared to healthy controls | 141 (161 controls) | ABCB4 common variants | No difference |
- Abbreviation: DILI, drug-induced liver injury.
3 INTRAHEPATIC CHOLESTASIS OF PREGNANCY
Intrahepatic cholestasis of pregnancy (ICP) affects 0.7% of pregnancies in the United Kingdom.24 The pathogenesis has been extensively studied with an apparent key role thought to be played by reproductive hormones and abnormal bile acid homoeostasis.25 Progesterone sulphates are elevated in patients with ICP, and from experimental work these have been shown to function as a farsenoid X receptor partial agonist, with aberrant gene expression including reduced BSEP expression.26 A similar mechanism of action has been demonstrated with 17β-estradiol.27 The genetic predisposition to ICP, and the causative or contributory role of variants in cholestasis genes, has been assessed by describing clinical events in patients and families with known ABCB4 variants, and by sequencing patients with ICP.
Investigation of relatives of a proband with PFIC, who was homozygous for a highly deleterious variant in ABCB4 (c.1712delT), and had undergone liver transplantation in childhood, was the first link between ABCB4 and ICP.28 Six females from the family had at least one episode of ICP, and the four in whom genetic data were available were all found to be heterozygous for the ABCB4 variant. Subsequent analysis of 31 patients with PFIC secondary to ABCB4 variants revealed three mothers with ICP, all of whom were heterozygous for ABCB4 variants (p.Arg652Gly, p.Arg957Ter, c.1744delT).9 In a family with multiple members with ABCB4 variant p.Arg788Trp and chronic liver disease, all nine female members who survived to adulthood suffered from ICP, including both homozygotes and heterozygotes.29 In five of them their first presentation of liver disease was with ICP. These findings suggest that there is a predisposition to ICP in patients with a family history of liver disease who are heterozygous for ABCB4 variants.
The results of data from patients with ICP being screened for variants are more variable. A cohort of patients with ICP is combined with those with oral contraceptive-induced cholestasis for analysis in a study testing for ABCB4 variants.30 Of this subgroup of 59 patients, 27% were heterozygous for variants in ABCB4. In small studies (21 and 33 patients respectively) with ICP patients sequenced for ABCB4 and ABCB11, there was an increase in genetic variation, particularly in ABCB4, compared to healthy controls.25 The variants were thought to play a causative role in a third of patients with ICP.31
High-throughput sequencing in 147 patients with ICP, for an extended panel of genes (including ABCB4, ABCB11, ATP8B1), detected 12 ABCB4 variants, 4 ABCB11 variants and 5 ATP8B1 variants.32 Two similar sized studies sequenced ATP8B1 in patients with ICP (176 and 182 patients) and identified variants in a minority of patients.33, 34 Although not a major gene contributing to occurrence of ICP, variants in ATP8B1 are likely to predispose to the development of cholestasis through a relative rise in biliary phospholipid.
A sequencing study that included 491 women with ICP, demonstrated that heterozygotes for the common ABCB11 p.Val444Ala variant were more likely to have ICP with an odds ratio of 1.7 (CI 1.4-2.1, P < 0.001) for ICP, and p.Val444Ala homozygous individuals were at highest risk compared to wild type, with OR 2.8 (CI 1.7-44, P < 0.0001).24 Although other ABCB11 variants are present in a minority of cases of ICP, the p.Val444Ala variant is a susceptibility locus for ICP and a significant predisposing factor for development of the condition. A genotyping study looking at the influence of common genetic variation on susceptibility to ICP in 563 cases and 642 controls used the HapMap dataset together with the Tagger algorithm (Haploview 4.1 build 22) to select 83 common variants around six candidate genes that included ABCB11, ABCB4 and ATP8B1.35 Six variants in ABCB11 (including p.Val444Ala) and six variants in ABCB4 were significantly associated with ICP.
A recent study of 310 women with severe early-onset ICP (defined by presentation before 33 weeks' gestation with serum bile acid concentrations ≥40 µmol/L) that utilised whole-genome sequencing, demonstrated that 20% had pathogenic variants in the ABCB4 or ABCB11.36
The data suggest that patients with ABCB4, ABCB11 and ATP8B1 variants, both homozygous and heterozygous, may be at an increased risk for ICP (Table 2). Considering the association with severe early-onset ICP, patients with known variants should receive specialist preconception care. However, these patients make up the minority of all patients with ICP, and the common ABCB11 p.Val444Ala variant is a more common risk factor. The reduced level of BSEP function in ABCB11 variants (including p.Val444Ala) may confer susceptibility to ICP.24 It is likely that women who are heterozygous for ABCB4 variants, or have common susceptibility variants, have a transient decompensation in protein function during pregnancy related to elevated concentrations of 17β-estradiol27 or progesterone sulphates,26 resulting in ICP.9 Similarly there may be abnormalities of canalicular membrane lipids during pregnancy in those with ATP8B1 variants.33
| Author | Type of study | Number of patients | Gene | Association |
|---|---|---|---|---|
| Jacquemin 199928 | Case study | 6 | ABCB4 | All tested were heterozygous for ABCB4 |
| Gotthardt 200829 | Case study | 9 | ABCB4 | All were heterozygous or homozygous for ABCB4 |
| Pasmant 201230 | Cohort of ICP combined with CIC | 59 | ABCB4 | 27% (combined data) heterozygous for ABCB4 |
| Anzivino 201331 | Cohort of ICP compared to healthy controls | 33 (100 controls) | ABCB4 & ABCB11 | 33% had a variant (5 ABCB4, 6 ABCB11), none in controls |
| Pauli-Magnus 200425 | Cohort of ICP compared to healthy controls | 21 (40 controls) | ABCB4 and ABCB11 | Genetic variation in ABCB4 and ABCB11 in ICP patients |
| Dixon 201732 | Cohort of ICP | 147 | ABCB11, ABCB4 and ATP8B1 | 14% had a variant (12 ABCB4, 4 ABCB11, 5 ATP8B1) |
| Mullenbach 200533 | Cohort of ICP compared to healthy controls | 182 (120 controls) | ATP8B1 | 3% had variant in ATP8B1, none in controls |
| Painter 200534 | Cohort of ICP compared to healthy controls | 176 (100 controls) | ATP8B1 | 3.5% had variant in ATP8B1, none in controls |
| Dixon 200924 | Cohort of ICP compared to healthy controls | 491 (261 controls) | ABCB11 |
1.4% had ABCB11 variant. p.Val444Ala common variant significant association |
| Dixon 201435 | Cohort of ICP compared to healthy controls | 563 (642 controls) | Common variants in ABCB11, ABCB4 and ATP8B1 | Association with common variants in ABCB11 and ABCB4 |
| Turro 202036 | Whole-genome sequencing of severe early-onset ICP | 310 | ABCB11 and ABCB4 | 20% had variants in ABCB11 or ABCB4 |
- Abbreviations: CIC, oral contraceptive-induced cholestasis; ICP, intrahepatic cholestasis of pregnancy.
4 CHRONIC LIVER DISEASE
In a proportion of patients with chronic cholestatic liver disease, the aetiology is not clear and does not fit clinically, serologically or radiologically with well recognised conditions such as primary biliary cholangitis or primary sclerosing cholangitis.37 Genetic testing in this cohort of unexplained chronic liver disease has been described in case reports incorporating family data, and screening studies of larger cohorts of patients with liver disease.
Case reports describing patients, a 47-year-old female and a 38-year-old male, who presented with adult onset advanced cholestatic liver disease of unknown cause, identified they were heterozygous for variants in ABCB4 (p.Gly535Asp and p.Val1068Glu).38, 39 The first patient underwent cholecystectomy aged 19, had pruritus during two pregnancies, and had a daughter who was also heterozygous for the same variant in ABCB4 and developed ICP. The second case had also previously undergone cholecystectomy, and his daughter and mother were both heterozygous for the same ABCB4 variant and had cholecystectomy aged 13 and 34 respectively. In both cases the aetiology of chronic liver disease was attributed to the ABCB4 variant, and their heterozygosity is felt to have resulted in a milder functional deficit in MDR3 and therefore presented with cirrhosis later in life.38 Another case report describes two brothers who presented with biliary cirrhosis in adulthood on the background of chronic cholestasis since adolescence, and were found to be compound heterozygous for variants in ABCB4 gene (p.Ser320Phe and c.2682+1G>A).40
A more extensive genotype and phenotype characterisation is available from a family of 11 siblings whose parents were 4th cousins, which highlights the difference in clinical presentation in homozygotes and heterozygotes.29 The proband was a 34-year-old female with idiopathic biliary cirrhosis requiring liver transplantation, who was homozygous for a missense variant in ABCB4 (p.Arg788Trp). Her mother suffered from ICP in all her pregnancies. Of her siblings, six were deemed severely affected (two childhood cirrhosis, four liver failure in adulthood), and five were mildly affected. The three severely affected who underwent sequencing were all also homozygous, while the mother and the mildly affected siblings were heterozygous, for ABCB4 variants.
A 20-year-old lady with persistent jaundice attributed to the oral contraceptive pill and had evidence of advanced fibrosis on liver biopsy, was found to be homozygous for a missense variant in ABCB11 (p.Ala523Gly).41 Her sister was also affected by episodic cholestasis and has the same variant in homozygous form. A 25 years old diagnosed with intrahepatic cholestasis and cirrhosis, which required liver transplantation, was found to be heterozygous for a missense variant in ATP8B1 (p.Arg600Trp) as well as homozygous for the common p.Val444Ala variant in ABCB11.42 A multi-genic cause for his presentation with advanced chronic liver disease in early adulthood was proposed.
In a study of 32 adults with a histological diagnosis of cholestatic liver disease and common causes of chronic liver disease excluded, genetic sequencing of ABCB4 gene was performed.43 Thirty-four per cent of this cohort was found to be heterozygous for disease-associated ABCB4 variants, defined as those that led to premature protein truncation, small deletions, insertions and missense variants. There were no differences in clinical or biochemical profile between those with and without variants, but there was a significantly higher fibrosis grade (Ishak score) in those with ABCB4 variants, with a third having F3 or F4 disease. The majority of patients with ABCB4 variants had a significant biochemical response to 15 mg/kg/day of ursodeoxycholic acid (UDCA). Similarly, when genetic sequencing of the ABCB4 gene was performed prospectively in 90 patients (80 with chronic cholestasis, 10 with prior history of cholestasis [ICP, juvenile cholestasis]) out of 2602 consecutive hepatobiliary referrals screened for chronic cholestasis; heterozygous variants in ABCB4 gene, predominantly missense variants, were identified in 20% of those tested, including those with primary biliary cholangitis or primary sclerosing cholangitis.44
In a series of six young adults (aged 17-22) with chronic liver disease, the patients and their first-degree relatives were sequenced for a number of genes (including ABCB11 and ATP8B1), and found two patients to be heterozygous for variants in ATP8B1 (p.Asn45Thr, p.Ile349Thr).45 In 33 patients with unexplained cholestasis an 8 gene panel was performed (including ABCB4, ABCB11 and ATP8B1), and 18% had a major variant in ABCB4 or ABCB11, while others had variants of unclear significance.46 A similar gene panel (including ABCB4, ABCB11 and ATP8B1) was performed on 48 patients with cryptogenic cholestasis, and likely pathogenic variants were seen in 21% of patients: 2 in ATP8B1 (p.Asp1219His, p.Pro23Leu); 2 in ABCB4 (p.Lys672Ter, p.Ala364Val); and 6 in ABCB11 (p.Tyr93Ser, p.Val597Leu, p.Arg1128Cys, p.Ala523Gly—reported previously,40 p.Ser1100Glnfs*38, p.Glu135Lys).47
Recent data from sequencing for variants in a combination of ATP8B1, ABCB11 and ABCB4 genes in 427 paediatric and adult patients with chronic cholestasis, included a large subgroup of adult patients (48 for ATP8B1; 67 for ABCB11; and 116 for ABCB4).48 A third of all patients carried at least one disease causing variant, and although the majority of variants were seen in children, 18% were diagnosed in adults with cholestasis. In those with adult onset disease ATP8B1 variants were identified in 6 patients, ABCB11 in 16 and ABCB4 in 15 patients. A significant proportion of adult patients without disease causing variants were found to have common variants in ATP8B1, ABCB11 and ABCB4.
As part of a large nationwide genome-wide association study in Iceland, analysis of common ABCB4 variants was performed and correlated with the presence of liver disease.49 Two rare variants, a missense variant and a frameshift insertion variant, were found to have a large effect on the presence of gallstone disease, cholestasis in pregnancy, cirrhosis, abnormal liver enzymes, liver and gallbladder cancer. Further sequencing was performed around the ABCB4 gene, and the authors did not feel additional variants were missed from the population.
Case and family studies of patients with idiopathic biliary cirrhosis have revealed a collection of patients with variants in ABCB4 (homozygous, heterozygous and compound heterozygous) with onset of disease in adulthood and various phenotypes of liver disease in first-degree relatives (Table 3). There are fewer data for ABCB11 and ATP8B1 in adults with chronic liver disease, but variants have been detected in a series of cases. The studies sequencing larger cohorts of adult patients with cholestatic liver disease have identified up to 34% as having heterozygous variants in ABCB4 gene, and there is increasing data on heterozygous variants in ATP8B1 and ABCB11 genes. The later onset of disease in these patients could be related to a milder functional deficit, either as a result of the variants being less damaging on a functional level, or because patients are heterozygotes. The only genome-wide association study in the field, suggests that common variants in ABCB4 are associated with a range of phenotypes of liver disease, including hepatobiliary malignancy. International guidelines recommend considering genetic testing in patients where common cholestatic syndromes have been excluded and it is clinically appropriate.37
| Author | Type of study | Number of patients | Gene | Association |
|---|---|---|---|---|
| Lucena 200338 | Case study | 2 | ABCB4 | Heterozygous ABCB4 (CLD and ICP), daughter heterozygous and ICP |
| Denk 201039 | Case study | 3 | ABCB4 | Heterozygous ABCB4 (CLD), daughter and mother heterozygous and had cholecystectomy <40 y of age |
| Oliveira 201640 | Case study | 2 | ABCB4 | Siblings both compound heterozygous ABCB4 (CLD) |
| Gotthardt 200829 | Case study | 12 | ABCB4 | Homozygous ABCB4 (CLD), siblings all homozygous or heterozygous (CLD, ICP), mother heterozygous (ICP) |
| Vitale 201641 | Case study | 2 | ABCB11 | Homozygous ABCB11 (CLD), sibling homozygous (episodic cholestasis) |
| Hsu 200942 | Case study | 1 | ATP8B1 and ABCB11 | Heterozygous ATP8B1 and p.Val444Ala ABCB11 (CLD) |
| Ziol 200843 | Cohort of chronic cholestasis | 32 | ABCB4 | 34% heterozygous for ABCB4 variants |
| Degiorgio 201644 | Cohort of chronic cholestasis | 90 | ABCB4 | 20% heterozygous for ABCB4 variants |
| Kulecka 201745 | Cohort of chronic liver disease | 6 | ATP8B1 and ABCB11 | Two heterozygous for ATP8B1 variants |
| Aamann 201846 | Cohort of cholestasis | 33 | ABCB4, ABCB11 and ATP8B1 | 18% had a major variant (5 ABCB4, 1 ABCB11) |
| Vitale 201847 | Cohort of cholestasis | 48 | ABCB4, ABCB11 and ATP8B1 | 21% heterozygous for variants (2 ABCB4, 6 ABCB11, 2 ATP8B1) |
| Droge 201748 | Cohort of cholestasis | 427 (including paediatric) | ABCB4, ABCB11 and ATP8B1 | 15 patients ABCB4 (13% of tested), 16 patients ABCB11 (22% of tested), 6 patients ATP8B1 (13% of tested) |
| Gudbjartsson 201549 | Genome-wide association study | ABCB4 common variants | Two ABCB4 common variants associated with gallstones, ICP, cirrhosis, liver cancer, gallbladder cancer |
- Abbreviations: CLD, chronic liver disease; ICP, intrahepatic cholestasis of pregnancy.
It is important to recognise that some of the patients identified with variants had a diagnosis of recognised liver disease syndrome. Testing should therefore be considered in these cases, especially in the presence of atypical features. It is unclear to what extent the presence of genetic variants is acting as disease modifiers in these patients.
5 LOW PHOSPHOLIPID-ASSOCIATED CHOLESTASIS
Although biliary cholelithiasis is a very common clinical condition, work has been undertaken in a subgroup of such patients evaluating the role of genetic variants. Genetic sequencing of ABCB4 to assess for an underlying genetic factor was undertaken in six adults with atypical presentations of cholelithiasis: including the presence of cholesterol gallstones and intrahepatic sludge; the recurrence of symptoms after cholecystectomy; and the prevention of recurrence with UDCA.50 They were aged 22-55 years, and included three females who had worsening of biliary symptoms during pregnancy or after oral contraception was used. All patients were found to have variants in ABCB4, including one patient who was homozygous (p.Ser320Phe, p.Thr175Val, p.Pro1161Ser, 1327insA) which was thought to underpin their clinical presentation with cholelithiasis. The group then characterised these patients as having a condition called low phospholipid-associated cholestasis (LPAC). They performed subsequent genetic analysis on 32 patients with LPAC, 28 with symptomatic gallstones alone and 33 controls without cholelithiasis.51 Of those with LPAC, 56% were heterozygous or homozygous for variants in ABCB4 gene, and no variants were detected in the control groups. They concluded that variants in ABCB4 were a significant risk factor for symptomatic and recurrent cholelithiasis in young adults. Work from a different group included 43 patients with LPAC as part of a larger study evaluating ABCB4, and 37% were heterozygous for ABCB4 variants.30
The largest analysis of ABCB4 in LPAC was from 156 consecutive patients, and 51% were heterozygous or homozygous for variants.52 Although there was no difference in clinical characteristics between those with and without variants in ABCB4, analysis of those with ABCB4 variants identified an earlier onset of symptoms in individuals with truncating variants to those with missense variants (26.5 vs 33.9 years). Interestingly of the patients with ABCB4 variants, two had biliary cirrhosis and one developed an intrahepatic cholangiocarcinoma.
These findings, however, were not reproduced in a study of 104 patients with symptomatic cholelithiasis under the age of 40 who underwent laparoscopic cholecystectomy and had ABCB4 sequencing.53 From this cohort less than 2% had variants in ABCB4, however, it must be noted that these patients did not necessarily fit the criteria for LPAC as outlined in the original study.
The total number of patients with LPAC who have been tested for variants in ABCB4 remains relatively small (Table 4). However, there does appear to be an association with ABCB4 variants in those with the whole clinical syndrome, which only makes up a minority of all patients with cholelithiasis. From the family studies of chronic liver disease in patients with ABCB4 variants, those with variants frequently had undergone cholecystectomy by early adulthood, adding further weight to the role of ABCB4 variants in early cholelithiasis.38, 39
| Author | Type of study | Number of patients | Gene | Association |
|---|---|---|---|---|
| Rosmorduc 200150 | Cohort of atypical cholelithiasis | 6 | ABCB4 | All ABCB4 variants (5 heterozygous, 1 homozygous) |
| Rosmorduc 200351 | Cohort of LPAC, compared to cholelithiasis and healthy controls | 32 | ABCB4 | 56% of LPAC were heterozygous or homozygous for ABCB4, no variants in control |
| Pasmant 201230 | Cohort study, subgroup with LPAC | 43 | ABCB4 | 37% ABCB4 variants (heterozygous) |
| Nakken 200953 | Cohort of cholelithiasis <40 y with cholecystectomy | 104 | ABCB4 | <2% ABCB4 variants |
| Poupon 201352 | Cohort of LPAC | 156 | ABCB4 | 51% of LPAC were heterozygous or homozygous for ABCB4 variants |
- Abbreviation: LPAC, low phospholipid-associated cholelithiasis.
6 PRACTICAL RECOMMENDATIONS
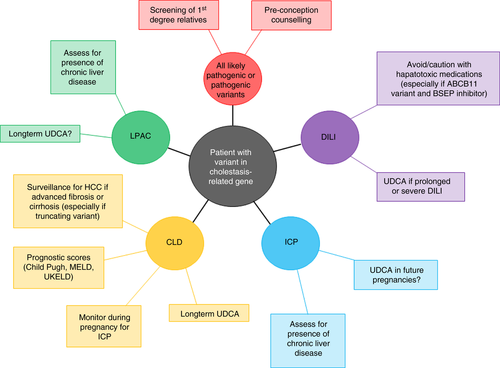
It is reasonable to perform genetic testing in adults with unexplained cholestasis and cholestatic chronic liver disease,37, 47 particularly in those with a family history of liver disease or ICP. Individuals who develop DILI, particularly related to medications known to reduce BSEP function (Table 5) or if it is severe DILI, should undergo testing, without too much focus on pattern of biochemical abnormality as bile acid-induced hepatocyte damage presents with raised transaminases. All individuals with ICP do not require testing, but those with other associated liver manifestations, severe ICP (onset before 33 weeks' gestation and serum bile acids >40 µmol/L) or with persistent cholestasis post-partum, could be referred for testing. Genetic testing for patients who fit the criteria for LPAC appears to have a high yield with ABCB4 variants. Although their clinical characteristics may not differ, there is a signal that those with variants need investigation for underlying chronic liver disease.
| Drugs |
|---|
| Non-steroidal anti-inflammatory |
| Amoxicillin/clavulanic acid |
| Piperacillin/tazobactam |
| Flucloxacillin |
| Oral contraceptive pill |
| Androgenic anabolic steroids |
| Fluvastatin |
This article concentrates on variants in the three genes (ABCB11, ABCB4 and ATP8B1) most commonly associated with early-onset cholestasis, which have also been most extensively described in adults. Clinical testing of cholestasis-related genes is mainly performed on gene panels which include a wide range of additional genes, that have been derived from childhood liver disease. These genes account for a smaller proportion of childhood liver disease, however, it is unclear if they are equally rare in adult liver disease. Although variants in these genes, such as TJP2, CFTR, NR1H4, MYO5B and USP53, may contribute to liver disease in adults, data are currently limited to TJP247 and NR1H454 in a small numbers of patients, and the strength of the associations is not clear. With increased testing of adults, further data on the prevalence of variants will become available, and when combined with accurate clinical phenotype data, will allow conclusions to be drawn on their causative role and contribution to cholestasis. We recommend that details on these variants are submitted to ClinVar (ncbi.nlm.nih.gov/clinvar/), which is a freely accessible public archive of variants with phenotype, interpretation and supporting evidence.55 When rare variants are identified in any of cholestasis-related genes, particularly if the variant is of uncertain significance, it is crucial that the clinical phenotype is explored thoroughly to see if it fits with the pathological pattern of disease. The final clinical interpretation should be in collaboration with geneticists and pathologists, and genetic input into multi-disciplinary meetings may become more commonplace.
For people with previous DILI and a known variant, medications which reduce BSEP activity should either be avoided or utilised with caution and closer monitoring (Table 5). Those with ABCB4 variants are at an increased risk of ICP, and should be counselled prior to conception and monitored closely during pregnancy for the development of ICP. Although there are no proven therapeutic interventions for patients with chronic liver disease and these variants, there may be a role for treatment with UDCA. In children with PFIC who received 20-30 mg/kg/day of UDCA there was a significant improvement in liver biochemistry and pruritus, and a suggestion it may prevent evolution to cirrhosis.56 A benefit from UDCA has been described in preventing symptom recurrence post-cholecystectomy in patients with LPAC, and a biochemical response to 15 mg/kg/day UDCA has been reported in a cohort of patients with ABCB4 variant-associated chronic liver disease.43 Although this is promising, further evaluation is needed to determine if this is replicated with histological and clinical endpoints.
Until further information on the natural history of patients with genetic variants is available it will prove difficult to understand the risk to patients of progression to chronic liver disease, or to offer specific prognosis in patients with chronic liver disease associated with these variants, outwith of standard liver prognostic models. In a similar vein their risk of hepatocellular carcinoma and cholangiocarcinoma development is unknown and therefore surveillance should be undertaken according to existing guidelines. These are important considerations prior to testing unaffected first-degree relatives, and it is advisable that where first-degree relatives undergo genetic screening they have access to a genetic counsellor.
7 CONCLUSIONS AND FUTURE DIRECTIONS
The findings from studies evaluating the presence of variants of genes associated with biliary homeostasis in adults have been summarised, and these encompass acute and chronic phenotypes of liver disease. In patients with acute, transient presentations of cholestasis, such as DILI and ICP, there appears to be an association with variants, especially common variants, in ABCB11. In health, these patients are likely to have reduced, but sufficient, BSEP levels for normal bile acid transport, which a threshold level of approximately 25% of functional BSEP protein (Figure 2). Following a second insult, from drugs or hormones, to an already genetically vulnerable liver, there may be a reduction in BSEP below this threshold level which results in cholestasis. Variants in ABCB4 have been described in patients with chronic liver disease presenting in adulthood. In these patients the variants may be associated with production of a higher levels of the MDR3 protein than seen in those with severe early-onset disease, as a result of a less damaging variant or being heterozygotes. There is, however, a sufficient reduction in MDR3 protein compared to healthy controls, which results in a persistent reduction in micelle formation, with associated bile acid toxicity and subsequent chronic cholangiopathy. The relationship between amount of functional MDR3 protein and clinical phenotype is likely to take a more linear dose-response relationship (Figure 2).
Although targeted therapies for the genetic defects or the associated specific liver phenotypes are not currently available, there are potentials for future development. The nature of BSEP deficiency secondary to ABCB11 variants, and the low level of corrections which could result in clinical improvement, means that it may be suited for novel therapies, including gene and protein manipulation.5 Improvements in function seen in patients with cystic fibrosis and specific gating variants in CFTR gene (also an ABC transporter) treated with Ivacaftor, a potentiator of CFTR, has seen encouraging early work in its use in ABCB4 variants, resulting in a significant in vitro increase in phosphatidylcholine secretion.2
More detailed natural history of adults with variants in these cholestasis-related genes is crucial to permit a more accurate genotype-phenotype correlation, and an individualised approach to these patients. Unanswered questions for clinicians and patients relate to the risk of progression of liver disease, the development of complications such as liver cancer, and the management of first-degree relatives. While this information is awaited, we recommend employing supportive management and use of UDCA as outlined in Figure 3.
ACKNOWLEDGEMENTS
Declaration of personal interests: RJT consults for: Albireo, Alnylam, EVOX Therapeutics, Generation Bio, Mirum Pharma, Retrophin, Qing Therapeutics and Sana Biotechnology. No other potential conflict of interest. No financial support with respect to this manuscript.
Funding: JSN: None; CW funded by: the National Institute of Health Research Biomedical Research Centre at Guy's and St Thomas' Foundation Trust and King's College London; DJ advisory fees: Intercept.
AUTHORSHIP
Guarantor of the article: JSN.
Author contributions: JSN wrote and edited the article. DJ, CW and RJT edited and provided expert opinion. All authors approved the final version of the article.




