Emerging Roles of Protein O-GlcNAcylation in Bone Remodeling: New Insights Into Osteoporosis
Funding: This work was supported by the Joint Funds of the Liaoning Provincial Natural Science Foundation (No. 2023-MSLH-400).
Jinpeng Wang, Site Xu and Yuchuan Xue contributed equally to this work.
ABSTRACT
Background
Bone is a dynamic tissue undergoing constant remodeling mediated by osteoblasts and osteoclasts. An imbalance between these cells can lead to reduced bone mass, disrupted microarchitecture, and ultimately osteoporosis. O-GlcNAcylation is a dynamic and reversible posttranslational modification where uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) is added or removed from serine/threonine residues of proteins by OGT and OGA, respectively. Emerging evidence indicates that appropriate O-GlcNAcylation is essential for bone remodeling, although its specific effects remain controversial.
Aims
This review aims to summarize the process of O-GlcNAcylation and critically evaluate its specific effects on osteoblast-mediated and osteoclast-mediated bone remodeling.
Materials & Methods
Based on a comprehensive analysis of published scientific literature, we synthesized the current evidence regarding the role of O-GlcNAcylation in bone cell differentiation and function, and its association with osteoporosis.
Results
Our analysis reveals that cellular demands for O-GlcNAcylation vary during osteoblastic and osteoclastic differentiation. Moderate O-GlcNAcylation is essential for osteoblast differentiation, whereas dynamic alterations in O-GlcNAcylation are crucial for osteoclast differentiation. Furthermore, elevated O-GlcNAcylation levels are consistently observed in both primary and secondary osteoporosis cases, suggesting a potential pathogenic role in the dysregulation of bone remodeling.
Discussion
These findings indicate that the effects of O-GlcNAcylation are cell type- and differentiation stage-dependent in bone. The observed elevation of O-GlcNAcylation in osteoporosis underscores its potential contribution to the dysregulation of bone remodeling pathways.
Conclusion
This review provides novel mechanistic insights into osteoporosis pathogenesis via dysregulation of the O-GlcNAcylation post-translational modification. Understanding these mechanisms will facilitate the development of novel therapeutic strategies targeting O-GlcNAcylation to restore balanced bone remodeling.
1 Introduction
Post-translational modifications (PTMs) serve as key regulators of signal transduction pathways, enabling rapid cellular responses to environmental stimuli through precise and dynamic control of protein function [1]. Among these PTMs, O-GlcNAcylation is a reversible and dynamic process involving the addition and removal of UDP-GlcNAc to serine/threonine residues on cytosolic and nuclear proteins, catalyzed by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), respectively [2, 3]. UDP-GlcNAc, the only substrate for O-GlcNAcylation, is the end product of the hexosamine biosynthetic pathway (HBP), which integrates multiple metabolic inputs including glucose, amino acids, fatty acids, and nucleotide metabolism [4]. Hence, O-GlcNAcylation is sensitive to nutrient availability and is regarded as the nutrient sensor [5]. Nutrient availability critically regulates cellular adaptation during development by driving rapid metabolic reprogramming to support essential physiological activities, particularly cell differentiation, thereby highlighting the pivotal role of O-GlcNAcylation in determining cell fate [6]. Indeed, O-GlcNAcylation modulates diverse cellular activities including transcriptional regulation, protein synthesis, signal transduction, and metabolism pathways [7, 8]. Dysregulation of protein O-GlcNAcylation has been implicated in the pathogenesis of various chronic and age-related disorders [8-10].
Bone remodeling maintains bone homeostasis throughout life by continuously replacing aged and impaired bone tissue with fresh bone to preserve bone mass and strength [11]. Bone remodeling is coordinately regulated by osteoblasts and osteoclasts, which are responsible for bone formation and resorption, respectively [12]. Emerging evidence has revealed that O-GlcNAcylation plays a crucial role in the regulation of bone remodeling. Global O-GlcNAcylation levels guide the differentiation of mesenchymal stem cells into different directions, including adipogenic, chondrogenic, and osteogenic lineages [13]. Osteogenic differentiation is associated with increased O-GlcNAcylation, which could be further enhanced through OGA inhibition [14-16]. Meanwhile, osteoclast differentiation requires dynamic O-GlcNAcylation alterations, characterized by initial upregulation followed by subsequent downregulation to facilitate terminal maturation [17]. Dysregulated O-GlcNAcylation could disrupt bone homeostasis and thereby result in various bone metabolism diseases, particularly different types of osteoporosis [18]. Therefore, O-GlcNAcylation performs complex roles in the regulation of bone remodeling.
Given the pivotal role of O-GlcNAcylation in bone remodeling, elucidating the distinct regulatory mechanisms of O-GlcNAcylation in osteoblasts and osteoclasts could advance our understanding of metabolic bone disease pathogenesis. In this review, we demonstrate that osteoblasts, osteoclasts, and macrophages exhibit a reliance on distinct O-GlcNAcylation features. We further describe the current understanding of O-GlcNAcylation in osteoporosis, suggesting that dysregulated bone O-GlcNAcylation may serve as a critical mechanism underlying osteoporosis development.
2 O-GlcNAcylation Homeostasis
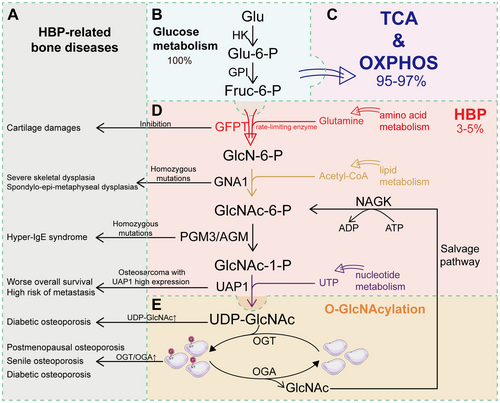
O-GlcNAcylation is highly enriched in nuclear pore complexes [19, 20], nuclear envelopes, cytoskeletal proteins [21], as well as the Golgi apparatus and endoplasmic reticulum (ER) [22, 23]. Consequently, O-GlcNAcylation is tightly involved in diverse biological processes, such as transcription, cell differentiation, and cellular metabolism [24]. Unlike most other PTMs, O-GlcNAcylation is uniquely regulated by a single pair of enzymes for the addition and removal of O-GlcNAc: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), respectively [7] (Figure 1). The cyclic process of removing and adding O-GlcNAc, known as O-GlcNAc homeostasis, is characterized by its high degree of dynamism [25]. Genetic ablation of either OGT or OGA in mice could result in embryonic lethality or perinatal death, underscoring the essential role of O-GlcNAcylation in the processes of development and survival [26, 27].
3 OGT and OGA: Key Regulators of O-GlcNAcylation
The removal and addition of O-GlcNAc is highly dynamic. Alterations in hormone levels, nutrient intake, or environmental conditions could trigger rapid changes in the overall cellular O-GlcNAcylation [28-30]. Cells actively regulate and maintain a specific level of O-GlcNAcylation, indicating that the preservation of O-GlcNAc homeostasis is crucial for optimal cellular function. Prolonged disruptions in O-GlcNAc homeostasis could modify the expression of OGT and OGA to restore O-GlcNAc homeostasis [8]. Therefore, there appears to be a highly sophisticated regulatory system within cells for maintaining O-GlcNAcylation homeostasis [5]. Given that O-GlcNAc homeostasis is controlled only by OGT and OGA, the precise mechanisms by which these enzymes achieve substrate specificity remain largely elusive. It is suggested that OGT predominantly identifies substrates through its tandem tetratricopeptide repeats (TPRs), and concurrently, OGT assembles into dynamic holoenzymes with various protein partners, which in turn enhance the process of substrate recognition [31]. Numerous proteins are identified as the adaptor protein for OGT substrate recognition, such as MAPK and HCF-1 [30, 31]. Meanwhile, OGA has also been identified as an adaptor protein for OGT, with the two enzymes capable of interacting to constitute an “O-GlcNAczyme” complex [32]. However, the mechanisms by which OGA recognizes its substrates remain poorly understood due to the absence of a crystal structure for OGA [33].
Additionally, both OGT and OGA are O-GlcNAcylated, which implies that they could be regulated through autoregulation and mutual regulation at the PTM level [34, 35]. The expression of OGT and OGA is sensitive to cellular O-GlcNAcylation levels [36]. Pharmacologically elevating O-GlcNAc by inhibiting OGA leads to a decrease in OGT protein expression and an increase in OGA protein expression [37]. Furthermore, reduced OGT activity leads to decreased global O-GlcNAcylation, which in turn induces rapid downregulation of OGA protein expression [38]. OGA could also upregulate OGT expression by activating the transcription factor CCAAT/enhancer-binding protein β (C/EBP-β), while knockout of OGT could result in a rapid decrease in OGA expression, revealing a bidirectional regulatory circuit that maintains O-GlcNAc homeostasis via coordination of OGT and OGA [36, 39].
4 O-GlcNAcylation: Nutrient Sensor
UDP-GlcNAc, the substrate of O-GlcNAcylation, is synthesized de novo from glucose mainly via the hexosamine biosynthesis pathway (HBP) and salvage pathways mediated by N-acetyl-D-glucosamine kinase (NAGK) [4, 40] (Figure 1). During the process of HBP, glucose is utilized in combination with glutamine, acetyl-CoA, and UTP derived from amino acid, lipid, and nucleotide metabolic pathways, respectively [41, 42]. O-GlcNAcylation is highly sensitive to the HBP, indicating that HBP flux is also involved in the regulation of O-GlcNAcylation.
4.1 O-GlcNAcylation Serves as a Nutrient Sensor due to HBP Flux
HBP is indeed a relatively minor branch of glycolysis, but it is important for the biosynthesis of UDP-GlcNAc [43, 44]. In HBP, glucose taken up by cells or fructose-6-phosphate produced through glycolysis undergoes a serial action of five enzymatic reactions to ultimately produce UDP-GlcNAc [45]. Glucose is first converted into glucose-6-phosphate (G-6P) by hexokinase (HK), and then into fructose-6-phosphate (F-6P) mediated by phosphoglucose isomerase (PGI), which is the same as the first two steps of glycolysis [46]. Instead of further entering glycolysis to generate pyruvate, a small amount of F-6P undergoes a reversible reaction with glutamine from amino acid metabolism to generate glucosamine-6-phosphate (GlcN-6P), catalyzed by glutamine–fructose-6-phosphate aminotransferase (GFPT) [47-49]. In the presence of acetyl-CoA, the product of lipid metabolism, GlcN-6P is then acetylated by glucosamine-6-phosphate acetyltransferase (GNAT) to produce N-acetylglucosamine-6-phosphate (GlcNAc-6P), which is then converted to N-acetylglucosamine-1-phosphate (GlcNAc-1P) by N-acetylglucosamine phosphoglucomutase (AGM). Finally, GlcNAc-1P is converted to UDP-GlcNAc catalyzed by UDP-GlcNAc pyrophosphorylase (UAP1), with UDP provided from UTP during nucleotide metabolism [50]. UDP-GlcNAc serves as a crucial substrate for O-GlcNAcylation mediated by OGT and OGA, as is mentioned above. Therefore, with the integration of nutrients derived from amino acid, lipid, and nucleotide metabolic pathways, the HBP flux could reflect the nutrient availability and also allows O-GlcNAcylation to serve as a nutrient and stress sensor [8, 48, 51]. Furthermore, considering that only a minor fraction of glucose, ranging from 3% to 5%, is typically diverted to the HBP flux, even a slight increase in the glucose supply to the HBP could equate to a significant percentage change for this pathway, further enabling HBP to be the effective cellular nutrient sensor [52] (Figure 1).
Indeed, global levels of O-GlcNAcylation are demonstrated to be increased in response to the increased availability of nutrients within the HBP flux [53]. It was observed that hyperglycemia is always associated with increased levels of O-GlcNAcylation in various tissues [54]. Low concentrations of glucosamine can significantly enhance O-GlcNAcylation, thereby also highlighting the crucial role of HBP flux as a key determinant in the regulation of O-GlcNAcylation [55]. Furthermore, it was noted in myotubes that saturated fatty acids, which are sources of acetyl-CoA, could upregulate the expression of GFAT and subsequently increase the intracellular levels of UDP-GlcNAc [56]. Direct infusion of free fatty acids could also increase UDP-GlcNAc levels in skeletal muscle [57]. Therefore, O-GlcNAcylation is tightly regulated by various nutrients in the HBP flux, which thereby results in its high sensitivity to nutrient availability and multiple forms of other cellular stress [8] (Figure 1).
4.2 Alterations of O-GlcNAcylation Result in Pathological Conditions
Given that O-GlcNAcylation acts as a nutrient sensor, physiological variations in nutrient availability can alter O-GlcNAcylation levels within an optimal zone, but sustained nutrient deprivation or excess could disrupt O-GlcNAc homeostasis and result in progressive defects [5]. Alterations in O-GlcNAcylation levels have been largely observed in tumors, which could result in excessive O-GlcNAcylation of tumor-associated proteins and activate diverse metabolic pathways associated with cancer development [41, 58-60]. Furthermore, alteration in nutrient availability is involved in various age-related diseases, such as diabetes, Alzheimer's disease, and osteoporosis [15, 61, 62]. Abnormal HBP flux could also contribute to various bone-related diseases, such as cartilage damage and skeletal dysplasia, further highlighting the critical role of O-GlcNAcylation in maintaining bone remodeling (Table 1).
| Key enzyme | Functions | Abnormalities | Outcomes | References |
|---|---|---|---|---|
| Glutamine–fructose-6-phosphate aminotransferase 1 (GFPT1) |
Controls the flux of glucose into HBP Fruc-6-P → GlcN-6-P |
Inhibition of GFAT-1 via azaserine (AZA) | Cartilage damages | [63] |
| Glucosamine 6-phosphate N-acetyltransferase (GNA1) | GlcN-6-P → GlcNAc-6-P | Homozygous mutations in GNA1 |
Severe skeletal dysplasia Spondylo-epi-metaphyseal dysplasias (characterized by short stature and abnormal modeling of the spine and long bones) |
[64-66] |
| Phosphoacetylglucosamine mutase (AGM) | GlcNAc-6-P → GlcNAc-1-P | Homozygous mutations in PGM3 | Hyper-IgE syndrome (characterized by severe skeletal dysplasia with radiographic pattern of Desbuquois dysplasia) | [67-69] |
| UDP-N-acetylhexosamine pyrophosphorylase (UAP1) | GlcNAc-1-P → UDP-GlcNAc | Osteosarcoma patients with high expression of UAP1 | Worse overall survival and high risk of metastasis | [70] |
| O-linked N-acetylglucosamine transferase (OGT) and O-GlcNAcase (OGA) | Addition/removal of UDP-GlcNAc to serine/threonine residues | Increased OGT/OGA ration in skeletal muscle | Postmenopausal osteoporosis | [71] |
5 O-GlcNAcylation in Osteoporosis
Osteoporosis is one of the most common age-related diseases characterized by the disruption of bone remodeling. Bone remodeling is driven by the balance of bone formation mediated via osteoblasts and bone resorption mediated via osteoclasts [72]. Osteoblast maturation involves a multistep differentiation process originating from mesenchymal stem cells, while osteoclasts are multinucleated cells that also require differentiation from mononuclear precursors [73, 74]. Cell differentiation is governed by transcriptional networks that integrate nutrient and metabolic signals, indicating that HBP flux and O-GlcNAcylation play essential roles in bone remodeling by modulating the differentiation of osteoblasts and osteoclasts.
5.1 Metabolic Alterations in Osteoporosis
As described above, HBP integrates multiple metabolic pathways, including those involving lipids, amino acids, and nucleotides, and dysregulation in these pathways is commonly associated with osteoporosis. It was demonstrated by Li et al. that dysregulation of lipid, amino acid, and energy metabolism pathways is implicated in osteoporosis pathogenesis and may serve as potential early diagnostic biomarkers [75]. Emerging evidence has revealed a bidirectional crosstalk between lipid homeostasis and skeletal metabolism [76, 77]. Various types of fatty acids are noted to regulate osteoblastic and osteoclastic differentiation [78-80]. It was also reported in multiple clinical studies that abnormal lipid-related metabolites were observed in the serum and urine samples of osteoporosis patients [81-83]. Furthermore, it was also suggested in multiple studies that patients with osteoporosis exhibited various kinds of amino acid abnormalities, such as valine, tryptophan, and glycine [84-86]. Nucleotides and their metabolites are also important for bone health, while the dysfunction of nucleotide metabolism was also observed in the mouse model of postmenopausal osteoporosis [87]. Thus, the major metabolic pathways involved in HBP flux exhibited abnormalities in osteoporosis, demonstrating that HBP and O-GlcNAcylation are essential for bone remodeling.
5.2 Moderate O-GlcNAcylation Is Required for Osteoblastic Differentiation
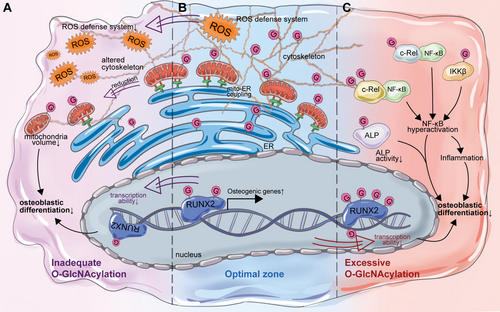
As mentioned above, O-GlcNAcylation is highly enriched in proteins of the nucleus, cytoskeleton, and various organelles such as the ER and Golgi apparatus. O-GlcNAcylation is also crucial for maintaining the normal function of the aforementioned organelles in osteoblasts. It was demonstrated by Weng et al. that O-GlcNAcylation primarily targeted mitochondria and cytoskeleton in osteoblasts, while knockout of OGT in osteoblasts could inhibit osteoblast differentiation by inducing morphological changes, such as altered morphologies of the cytoskeleton, decreased mitochondria-endoplasmic reticulum coupling, and volume of endoplasmic reticulum [88]. Indeed, the levels of O-GlcNAcylation increase during the differentiation of osteoblasts, while enhancing O-GlcNAcylation by inhibiting OGA activity could further promote osteoblastic differentiation [14]. RUNX2 is the principal transcriptional regulator of osteoblast differentiation [89]. RUNX2 was noted to be O-GlcNAcylated, resulting in the upregulation of its target gene osteocalcin (OCN), which is the late marker of osteogenesis [14, 90]. It was further demonstrated that RUNX2 is O-GlcNAcylated in the N-terminal activation domain and the C-terminal proline/serine/threonine-rich (PST) region that regulates RUNX2 activity and its interaction with transcriptional co-regulators [15]. Furthermore, enhanced O-GlcNAcylation of RUNX2 also upregulates alkaline phosphatase (ALP) expression, an early marker for osteoblast differentiation [15]. Furthermore, O-GlcNAcylation is involved in the insulin-induced hypertrophic chondrocyte differentiation, enabling both chondrogenesis and osteogenesis [91]. It was observed in cartilage endplate stem cells that increased O-GlcNAcylation levels could promote osteogenic differentiation via O-GlcNAcylation of RUNX2 and SOX9, suggesting the critical role of O-GlcNAcylation in osteoblastic differentiation and bone formation [92].
O-GlcNAcylation also functions as the cellular antioxidant defense system [93]. Oxidative stress serves as a key pathological mediator of bone loss by impairing osteoblast differentiation [94, 95]. High levels of reactive oxygen species (ROS) could inhibit the differentiation of osteoblasts and thereby suppress bone formation [96, 97]. The global level of O-GlcNAcylation is significantly increased due to oxidative stress [29, 98]. The initial ROS stimulus induces elevated O-GlcNAcylation, which subsequently initiates intracellular signaling cascades that establish a sustained oxidative defense system, ultimately resulting in a significant reduction in ROS regeneration [99]. It was also noted in osteoblasts that increased levels of O-GlcNAcylation were in concordance with a decrease in ROS, which was regulated by OGT and OGA [100]. Antioxidant treatment could increase OGT expression and global O-GlcNAcylation levels, while antioxidant withdrawal significantly elevated OGA activity [100]. Therefore, the elevated O-GlcNAcylation induced by increased OGT activity is also essential for osteoblast adaptation to oxidative stress.
Indeed, O-GlcNAc homeostasis serves as a crucial guardian against cellular stress. Global levels of O-GlcNAcylation are transiently elevated under moderate stress, but sustained excessive O-GlcNAcylation beyond the optimal zone could potentially lead to adverse cellular outcomes [5]. For example, the level of O-GlcNAcylation was significantly increased in diabetes-associated periodontitis [101]. Excessive O-GlcNAcylation could inhibit osteoblast differentiation via modifying the Ser-513 residue of ALP, leading to decreased ALP activity [101]. In addition, it was also observed in osteoblasts treated with high glucose, glucosamine, or N-acetylglucosamine that excessive O-GlcNAcylation of RUNX2 could in turn decrease its transcriptional activity and levels of osteogenic markers, which could also be rescued by reducing O-GlcNAcylation [102].
Furthermore, high glucose condition is usually accompanied by high levels of O-GlcNAcylation and low-grade chronic inflammation, indicating a potential association between O-GlcNAcylation and inflammation [103, 104]. Numerous studies have suggested that inflammation can inhibit osteogenic differentiation potential [105, 106]. Hence, inflammation induced by excessive O-GlcNAcylation could also be involved in the inhibition of osteoblast differentiation. NF-κB is a glucose-responsive transcription factor that is involved in the regulation of various biological processes, especially inflammation [107, 108]. The NF-κB complex is composed of homodimers or heterodimers of Rel and NF-κB proteins, including NF-κB1, NF-κB2, RelA, RelB, and c-Rel [109]. Activation of NF-κB is tightly regulated by HBP flux and OGT [110]. It was noted that RelA was O-GlcNAcylated on Thr-352, which thereby increased the transcriptional activity of NF-κB [111]. RelA was also O-GlcNAcylated on Thr-305, facilitating its acetylation at the adjacent Lys310, which is required for the complete NF-κB transcriptional activation [112, 113]. In addition, the Ser-350 site of c-Rel was also observed to be O-GlcNAcylated, contributing to the activation of NF-κB [114]. Meanwhile, high glucose induced the O-GlcNAcylation of IKKβ via OGT, upregulated the catalytic activities of IKKβ, and contributed to the activation of NF-κB, demonstrating that O-GlcNAcylation could contribute to activate NF-κB in multiple ways [115]. Of note, NF-κB was regarded as the endogenous inhibitor of osteoblasts early in 2009 [116]. Numerous studies have reported in vitro and in vivo that the activation of NF-κB can inhibit osteoblastic differentiation, while its inhibition can promote osteoblastic differentiation [117-120]. Hence, hyperactivation of NF-κB due to its excessive O-GlcNAcylation might also contribute to decreased osteoblastic differentiation and subsequently inhibit bone formation under hyperglycemic conditions.
Therefore, the basal or a moderate increase in cellular O-GlcNAcylation is required for osteogenic differentiation through the promotion of RUNX2 transcriptional activity and the improvement of adaptive responses to oxidative stress in osteoblasts. However, excessive global O-GlcNAcylation in osteoblasts may in turn suppress the differentiation of osteoblasts via excessive O-GlcNAcylation of osteoblastic markers and hyperactivation of NF-κB (Figure 2).
5.3 Dynamic Alteration in O-GlcNAcylation Is Required for Osteoclast Differentiation
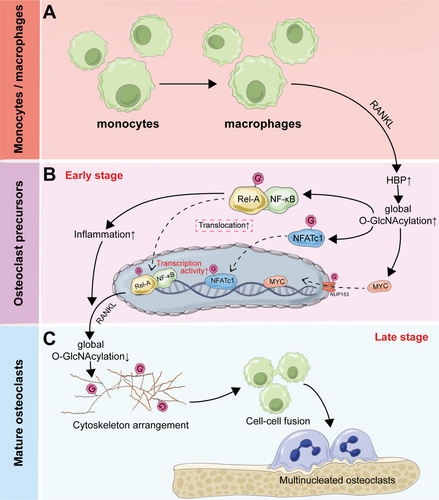
Osteoclasts, the primary cells responsible for bone resorption, are multinucleated cells that differentiate from mononuclear cell precursors of monocyte/macrophage lineage [121, 122]. Macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) are required for osteoclast differentiation, which is further amplified under inflammatory conditions [123, 124]. As described, O-GlcNAcylation of NF-κB promotes its activation, driving inflammatory responses that are essential for osteoclast differentiation. Several studies have also demonstrated that O-GlcNAcylation could induce inflammatory responses in macrophages, which were inhibited by decreased cellular O-GlcNAcylation [114, 125, 126]. Therefore, O-GlcNAcylation is also involved in the differentiation of osteoclasts, thereby regulating the process of bone remodeling.
Indeed, HBP flux and O-GlcNAcylation are involved in the bone marrow-derived osteoclast differentiation. During RANKL-mediated osteoclast differentiation, the HBP pathway is activated, which enhances O-GlcNAcylation of both NF-κB RelA and the master osteoclastogenic regulator NFATc1, thereby enabling their nuclear translocation and subsequent activation of osteoclast-specific transcriptional programs [127]. It was also observed in osteoclast-specific OGT-deficient mice that decreased O-GlcNAcylation could contribute to downregulation of osteoclastic markers (NFATc1, αvintegrin, and cathepsin K), increased bone density, and attenuated inflammation-induced bone loss, demonstrating the essential role of O-GlcNAcylation in osteoclast-mediated bone resorption [128].
However, similarly with osteoblasts, the role of O-GlcNAcylation in the differentiation of osteoclasts is not simply positively regulated. It was noted by Takeuchi et al. that increased O-GlcNAcylation in Raw264.7 cells by treatment with OGA inhibitor, PUGNAc, or glucosamine could inhibit the differentiation of osteoclasts [129-131]. It was also observed in the mouse model of postmenopausal osteoporosis that intake of GlcN and GlcNAc could inhibit the differentiation of osteoclasts and suppress bone loss [132]. The discrepancies observed in these studies were attributed to variations in cell types, but it seems unreasonable because RAW264.7 cells were frequently used in numerous studies to explore the mechanisms of osteoclastic differentiation [133-135]. It has been observed that the O-GlcNAcylation of NF-κB can either enhance or suppress the transcription of target genes, which is dependent on the cellular context and the type of O-GlcNAcylation-inducing conditions [136-138]. Until recently, Li et al. have demonstrated the necessity of dynamic O-GlcNAcylation during osteoclastogenesis, revealing that increased O-GlcNAcylation promotes osteoclast differentiation during the early stages, while its downregulation is required for osteoclast maturation [17]. Of note, differentiation and maturation of osteoclasts require the proliferation and differentiation of osteoclast progenitors into mononuclear preosteoclasts and the fusion of preosteoclasts into multinucleated osteoclasts. O-GlcNAcylation of nucleoporin 153 (NUP153) was significantly increased during early osteoclastic differentiation. NUP153 is a nuclear pore component that is known to directly bind with transcription factors to control their translocation [139]. O-GlcNAcylated NUP153 upregulates the nuclear transport of MYC, a key transcription factor of osteoclastogenesis, and thereby promotes osteoclastic differentiation [17, 140]. In addition, the inflammatory condition induced by the initial increase in O-GlcNAcylation levels is also essential for the early differentiation of osteoclasts. Meanwhile, as mentioned above, O-GlcNAcylation is essential in maintaining the morphology of the cytoskeleton in osteoblasts [88]. Similarly, downregulation of O-GlcNAcylation in later stages of osteoclastic differentiation stimulates rearrangement of the cytoskeleton and enhances cell–cell fusion to promote osteoclast maturation, which better explains the above contradiction and also the complex effects of O-GlcNAcylation [17] (Figure 3).
5.4 Macrophage Polarization: Coordinating the Differentiation of Osteoblasts and Osteoclasts
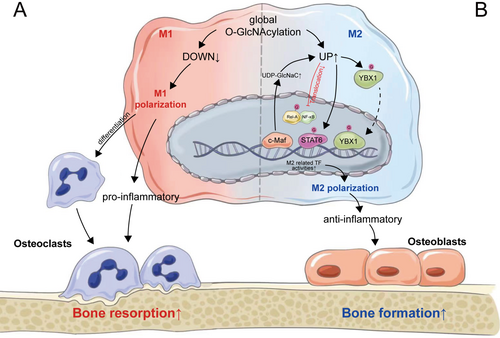
Bone remodeling is driven by a balance of cells that degrade and produce bone [72]. Apart from the direct regulation of osteoblast and osteoclast differentiation during bone remodeling, their activities are also modulated by external environmental factors and interactions with other cells in the bone microenvironment [141]. As previously indicated, the inflammatory condition, which is essential for the early differentiation of osteoclasts, could also inhibit osteoblast differentiation, thereby revealing the intimate association between inflammation and the activities of both osteoblasts and osteoclasts. Macrophages, the precursors of osteoclasts, have elaborate strategies for the regulation of the inflammatory response [142]. As a highly plastic and dynamic cell population, macrophages exhibit a remarkable capacity to modify their phenotype, enabling them to alter their functions to adapt to various biological functions and pathophysiological processes, which is known as macrophage polarization [143]. Macrophages exist in either a dynamic proinflammatory (M1) or anti-inflammatory (M2) polarization state that is dependent on the extracellular environment [144]. M1 macrophages produce pro-inflammatory cytokines such as TNF-α and IL-1β that contribute to increased osteoclast differentiation and decreased osteoblast differentiation [145, 146]. Meanwhile, M2 macrophages could secrete anti-inflammatory cytokines, thereby facilitating bone formation and inhibiting bone resorption [146, 147]. The imbalance of M1/M2 macrophages can shift bone remodeling towards bone resorption rather than formation, which accelerates bone loss and progressively exacerbates osteoporosis [148, 149].
It was also observed that glucose uptake, glycolysis, and UDP-GlcNAc biosynthesis were altered during macrophage polarization [150]. O-GlcNAcylation is temporarily downregulated during M1 polarization and transiently upregulated at the onset of M2 polarization, suggesting that O-GlcNAcylation might be more essential for M2 polarization [151]. Indeed, the HBP flux exerts a direct, specific, and critical role during M2 polarization. M2 polarization is characterized by the significant increase in the two metabo-transcriptional modules, including the UDP-GlcNAc biosynthesis module and the glutamine/glutamate-associated module [152]. OGT is regarded as the novel mediator of M2 polarization in human macrophages and also the suppressor of the M1 phenotype [153]. Increased O-GlcNAcylation levels via GlcN treatment could also increase the expression of M2 markers and reduce the production of inflammatory cytokines [154]. Meanwhile, M2-specific defects were observed with the inhibition of HBP flux via glucosamine [152]. It was further noted that increased O-GlcNAcylation could inhibit the activation of NF-κB signaling via suppressing the translocation of Rel-A into the nucleus and thereby inhibit M1 polarization and promote M2 polarization [155]. It is interesting here that the activation of NF-κB is inhibited by increased O-GlcNAcylation, which contradicts the process in the early stage of osteoclastic differentiation. This could be interpreted as variations in cell types and the extent of increased O-GlcNAcylation, which necessitates additional validation and exploration in subsequent research. Furthermore, inhibiting OGA could also elevate the accumulation of M2-like macrophages via increased O-GlcNAcylation of STAT6 [156]. Conversely, O-GlcNAc signaling is downregulated during macrophage proinflammatory activation, while OGT knockout in macrophages could result in M1 polarization [151].
Apart from direct regulation of O-GlcNAcylation via OGT or OGA, HBP flux is also involved in the regulation of macrophage depolarization. C-Maf is the key M2-related transcription factor governing various M2-related genes such as IL-10 and IL-12 [157, 158]. In addition, C-Maf is also recognized for its regulation of UDP-GlcNAc production and subsequent glycosylation, which is critical for M2 macrophage polarization [159]. Enhanced glucose flow through the HBP due to hyperglycemia could skew macrophage polarization to an M2-like phenotype [61]. Furthermore, overexpression of GFAT, the key enzyme of HBP flux, could enhance glutamine utilization to increase YBX1 O-GlcNAcylation and nuclear translocation, and thereby promote macrophage M2 polarization, demonstrating the critical role of HBP flux in regulating macrophage polarization [160].
Therefore, O-GlcNAcylation is critical in M2 macrophage polarization mediated by OGT, OGA, and also HBP flux. Increased M2 macrophages could secrete anti-inflammatory cytokines to reduce the inflammatory condition in the bone microenvironment, thereby facilitating osteoblastic differentiation and inhibiting osteoclastic differentiation. In addition, M1 macrophages also directly act as the precursors of osteoclasts [149]. Continuous decreases in M1 macrophages due to permanent O-GlcNAcylation could result in the deficiency of osteoclast precursors and thereby inhibit the late differentiation of osteoclasts, which also elucidated the essentiality of decreased O-GlcNAcylation levels during late osteoclastic differentiation, as previously described. However, the dynamic alterations and potential optimal range of O-GlcNAcylation during macrophage M2 polarization, similar to osteoblasts and osteoclasts, are less studied and require more attention in future studies (Figure 4).
5.5 O-GlcNAcylation in Other Cells in the Bone Microenvironment
Apart from osteoblasts, osteoclasts, and macrophages, O-GlcNAcylation in other cells is also essential for the maintenance of the bone microenvironment. Bone marrow mesenchymal stromal cell (BMSC) is a heterogeneous group of multipotent stem cells that has the potential to differentiate into osteogenic and adipogenic lineages [161]. Intracellular O-GlcNAcylation modification was identified as a posttranslational switch that dictates the differentiation fate and niche function of BMSCs [162]. O-GlcNAcylation modifications could promote osteogenic differentiation of BMSCs by modifying RUNX2 and inhibit the adipogenic specification by modifying C/EBPβ, while ablating OGT in BMSCs could result in impaired bone formation [162]. A recent study by Zhang et al. further demonstrated that O-GlcNAcylation of leptin could inhibit cellular senescence and promote osteogenic differentiation in mesenchymal stem cells [163]. It was also noted in Gl1+ mesenchymal stromal cells that the absence of OGT could inhibit bone formation and regeneration following fractures due to decreased O-GlcNAcylation [164]. In addition, chondrocyte differentiation is also dependent on O-GlcNAcylation, which is essential for skeletal growth [91]. However, excessive O-GlcNAcylation could result in hypertrophic differentiation of chondrocytes and lead to the progression of osteoarthritis [165, 166]. Therefore, O-GlcNAcylation modifications occurring in other cells in the bone microenvironment also exhibit complex regulation of bone metabolism (Figure 5).
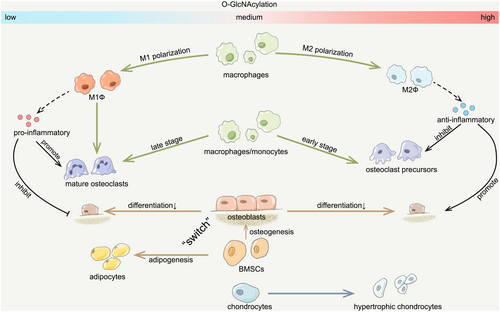
6 Aberrant O-GlcNAcylation: Novel Pathogenesis of Osteoporosis
Thus, O-GlcNAcylation plays a critical and intricate role that is indispensable to the process of bone remodeling. Appropriate O-GlcNAcylation is essential for osteoblast-mediated bone formation, while both excessive and insufficient O-GlcNAcylation could inhibit osteoblast differentiation and thereby suppress bone formation. Meanwhile, dynamic changes in O-GlcNAcylation are required for osteoclast differentiation, featuring an increase during the early phase and a subsequent decrease as the process matures. Moreover, enhanced O-GlcNAcylation has the potential to foster M2 polarization in macrophages and thereby shift the bone remodeling process towards bone formation, further underscoring the complex effects of O-GlcNAcylation in bone remodeling. Therefore, dysregulation of O-GlcNAcylation homeostasis could potentially interrupt the bone remodeling process, ultimately contributing to osteoporosis development (Figure 6).
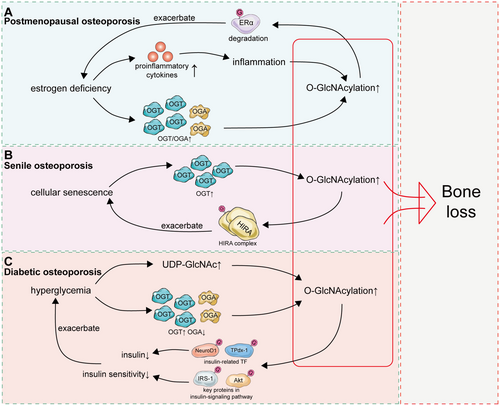
6.1 Postmenopausal Osteoporosis: Continuous Low-Graded Inflammation and Oxidative Stress
Osteoporosis is classified into primary osteoporosis and secondary osteoporosis, while primary osteoporosis is further divided into postmenopausal osteoporosis and senile osteoporosis [167, 168]. Estrogen deficiency is the predominant factor contributing to the development of osteoporosis in postmenopausal women [169]. It was observed in ovariectomized (OVX) mice, a common animal model for postmenopausal osteoporosis, that estrogen deficiency could contribute to increased levels of glycosylation [170]. The OGT/OGA ratio was furthermore noted to be increased in the skeletal muscle of early postmenopausal women which was restored following hormone replacement therapy [71]. Hence, a sustained increase in O-GlcNAcylation levels due to estrogen deficiency in postmenopausal women could potentially inhibit osteoblast differentiation and ultimately contribute to the development of postmenopausal osteoporosis. In addition, it was also noted that increased O-GlcNAcylation could inhibit the expression of estrogen receptor α (ERα), which might exacerbate bone loss due to estrogen deficiency in postmenopausal women [171, 172].
Beyond the direct influence on OGT/OGA, estrogen deficiency can also exert an indirect effect on the global levels of O-GlcNAcylation. Due to estrogen deficiency, blood levels of proinflammatory cytokines increase significantly in postmenopausal women, resulting in systemic chronic low-grade inflammation accompanied by oxidative stress [173-175]. As mentioned above, transient inflammation and oxidative stress could increase global levels of O-GlcNAcylation, which contribute to cell adaptation to external stimuli [176, 177]. However, sustained inflammation and oxidative stress in postmenopausal women could in turn disrupt the optimal zone of O-GlcNAcylation (Figure 6).
6.2 Senile Osteoporosis: Increased O-GlcNAcylation due to Cellular Senescence
Senile osteoporosis is also a common type of primary osteoporosis [167]. The most notable characteristic of cellular senescence is altered cellular responsiveness, such as metabolic responses to nutrient supply and utilization, stress responses against a variety of toxic stresses, and mitogenic responses [178]. Given that O-GlcNAcylation serves as an important nutrient sensor and cellular antioxidant defense system, O-GlcNAcylation is also tightly involved in the process of cellular senescence. Aberrant O-GlcNAcylation has been observed in several age-related diseases [179, 180]. Numerous studies have indicated that global O-GlcNAcylation levels are increased in aged tissues, elucidating the role of aging on O-GlcNAcylation independent of estrogen deficiency [93, 181-184]. Studies have demonstrated that elevated OGT expression during cellular senescence could impact the nucleosome assembly of the histone cell cycle regulator (HIRA) complex via O-GlcNAcylation and thereby promote senescence, while inhibiting OGT could attenuate cellular senescence [185]. Moreover, an elevation of O-GlcNAcylation was also observed in the testis of aged mice, while decreasing O-GlcNAcylation in aged testes could partially rescue the age-related impairment [183]. Hence, increased O-GlcNAcylation due to dysregulation of OGT and OGA could be a key factor contributing to bone loss in senile osteoporosis.
However, although global O-GlcNAcylation in various tissues was noted to be upregulated in aged tissues, not all proteins were more O-GlcNAcylated in senescent cells. During senescence, O-GlcNAcylation of SP1, the housekeeping transcription factor for nucleocytoplasmic trafficking (NCT) genes, was noted to be decreased, resulting in defective NCT functions and nuclear barrier formation [186]. It was also noted by White et al. that age-related loss of O-GlcNAcylation in neural stem cells could decrease neurogenesis and impair associated cognitive function. Therefore, more studies should focus on the O-GlcNAcylation levels of proteins related to bone remodeling to elucidate the mechanistic relationship between O-GlcNAcylation and senile osteoporosis (Figure 6).
6.3 Diabetic Osteoporosis: Prolonged Hyperglycemic Status
Diabetes mellitus is a well-known cause of secondary osteoporosis [187]. Hyperglycemia is the main pathology of diabetic osteoporosis, which is distinct from postmenopausal or senile osteoporosis [188, 189]. Hyperglycemia has been implicated in the pathogenesis of diabetic osteoporosis via inhibiting osteoblastic differentiation and promoting bone resorption, while hyperglycemia could also stimulate oxidative stress in bone tissues and thereby accelerate bone loss [190-192]. It was observed that hyperglycemia increases global O-GlcNAcylation of proteins in diabetes, while a reduction in blood glucose could reduce the normal levels of O-GlcNAcylation, demonstrating the critical effects of hyperglycemia on O-GlcNAcylation [179, 193]. It was further indicated by Banerjee et al. that diabetes could result in increased OGT and a concomitant decrease in OGA, which subsequently accounted for the hyperglycemia-related increase in O-GlcNAcylation [194]. Moreover, high blood glucose could raise levels of UDP-GlcNAc, the direct substrate of O-GlcNAcylation, thereby promoting O-GlcNAcylation [195].
Meanwhile, increased O-GlcNAcylation could further elevate blood glucose and exacerbate hyperglycemia. Decreased insulin production and insulin resistance are the major characteristics of type I and type II diabetes, respectively [196]. Protein O-GlcNAcylation and OGT expression are notably elevated in the β-cells of normal mouse pancreas relative to adjacent cells, suggesting a crucial role for O-GlcNAc in insulin expression [197]. The activities of TPdx-1 and NeuroD1, transcription factors responsible for insulin transcription, are suppressed by increased O-GlcNAcylation in response to hyperglycemia, thereby reducing insulin production [198, 199]. In addition, hyperglycemia-induced excessive O-GlcNAcylation of insulin-signaling pathway proteins, such as IRS-1 and Akt, could result in a blunting of the phosphorylation status of IRS-1 and Akt, thereby leading to desensitization of insulin signaling [200, 201]. Therefore, hyperglycemia provides UDP-GlcNAc and upregulates OGT and OGA, contributing to enhanced O-GlcNAcylation, which subsequently diminishes insulin production and induces insulin resistance and further exacerbates hyperglycemia. These findings establish O-GlcNAcylation as a key regulator of diabetic pathogenesis and its skeletal manifestations (notably osteoporosis); further mechanistic studies are required to fully elucidate its pathophysiological contributions. Current evidence confirms that O-GlcNAcylation plays a pivotal regulatory role in diabetes pathogenesis-related complications such as diabetic osteoporosis, but more studies still need to be conducted for further verification (Figure 5).
Overall, the global level of O-GlcNAcylation was noted to be increased in most kinds of osteoporosis, including postmenopausal osteoporosis, senile osteoporosis, and diabetic osteoporosis. Excessive O-GlcNAcylation could impact the normal function of osteoblasts, osteoclasts, and macrophages, as mentioned above, and thereby accelerate bone loss, indicating the crucial role of O-GlcNAcylation in the regulation of bone remodeling (Figure 6).
7 Discussion
At the nexus of glucose, amino acid, fatty acid, and nucleotide metabolism, O-GlcNAcylation is regarded as the main cellular nutrient sensor. The global level of O-GlcNAcylation is delicately regulated by OGT and OGA to satisfy the requirements of different cell types and tissues. Because of this, the levels of O-GlcNAcylation can rapidly change in response to fluctuations in nutrient availability and then return to a baseline level quickly after stimulus removal [5]. However, the long-stained nutrient alterations that exceed the cellular capacity for regulation could result in excessive O-GlcNAcylation and impairment in the management of nutrients, thereby contributing to various diseases [166].
Bone remodeling, mediated by osteoblasts and osteoclasts, is essential for the maintenance of bone homeostasis. The differentiation of osteoblasts and osteoclasts requires optimal and dynamic levels of O-GlcNAcylation, while both overly high and low levels could hinder the normal process of differentiation. In osteoblasts, O-GlcNAcylation is needed for the transcriptional activity of RUNX2, the key osteogenesis transcription factor, and the cellular adaptive responses to oxidative stress, but excessive global O-GlcNAcylation could, in turn, suppress the transcriptional activity of RUNX2 and inhibit osteoblastic differentiation. Different from osteoblasts, the differentiation of osteoclasts requires a fluctuation in global O-GlcNAcylation. The inflammatory response triggered by the initial rise in O-GlcNAcylation levels is crucial for the early differentiation of osteoclasts. Subsequently, the reduction of O-GlcNAcylation during later stages facilitates the rearrangement of the cytoskeleton and augments cell–cell fusion, thereby promoting the maturation of osteoclasts. Notably, however, osteoblasts and osteoclasts are both the pillars of bone [202]. The differentiation of osteoblasts and osteoclasts occurs simultaneously in bone tissues. It presently remains unknown how the global levels of O-GlcNAcylation are precisely regulated to satisfy the drastically different needs of cells in the bone microenvironment concurrently. Moreover, the cross-talk between osteoblasts and osteoclasts is also critical to maintaining the equilibrium governed by bone resorption and bone formation [203]. It is currently unclear whether the intercellular communication between osteoblasts and osteoclasts could regulate the O-GlcNAcylation processes of each other.
Serving as the key nutrient sensor, O-GlcNAcylation levels are finely tuned within an optimal range in response to physiological nutrient fluctuations. Numerous studies have shown that global O-GlcNAcylation levels are increased in various kinds of osteoporosis, highlighting the crucial role of O-GlcNAcylation in the regulation of bone remodeling. In this review, we focus on the diverse role of O-GlcNAcylation in osteoblasts, osteoclasts, macrophages, and other cells in the bone microenvironment under physiological and pathological conditions (Figure 5). A small but growing number of studies have regarded O-GlcNAcylation modification as a new potential therapeutic target for bone-related diseases. GlcNAc and PUGNAc (an O-GlcNAcase inhibitor) were found to exert significant chondroprotective effects in cartilage trauma models [63]. In vitro studies suggested that OSMI, a small-molecule inhibitor of OGT, could inhibit bone resorption with no obvious differences from zoledronic acid, a commonly used medication for osteoporosis treatment [127]. However, it was also noted that GlcNAc could suppress osteoclast differentiation in part through the promotion of O-GlcNAcylation [130]. The contradictory results may be explained by cell-type-specific variations in O-GlcNAcylation requirements within the bone microenvironment. Future studies should focus on elucidating the cell-type-specific mechanisms of O-GlcNAcylation regulation in the bone microenvironment and subsequently develop cell-selective O-GlcNAcylation modulators, which could contribute to a deeper understanding of osteoporosis pathogenesis and provide theoretical foundations for novel targeted therapeutic strategies.
Author Contributions
Jinpeng Wang: conceptualization, data curation, investigation, visualization, writing – original draft. Site Xu: conceptualization, data curation, visualization, writing – original draft. Yuchuan Xue: conceptualization, data curation, visualization, writing – review and editing. Kaicheng Wen: supervision, resources. Mingzhe Sun: conceptualization, project administration, supervision. Lin Tao: conceptualization, data curation, project administration, supervision, visualization, writing – review and editing.
Acknowledgments
We are deeply grateful to the Joint Funds of the Liaoning Provincial Natural Science Foundation (Grant No. 2023-MSLH-400) for their support in facilitating this review.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.




