Mitochondrial Dysfunction and Defects in Mitochondrial Adaptation to Exercise Training in the Muscle of Patients With COPD: Disease Versus Disuse
Funding: This work was supported by Fonds de dotation groupe Adène partially.
Maurice Hayot and Pascal Pomiès contributed equally to this work.
ABSTRACT
Aim
Chronic obstructive pulmonary disease (COPD) is frequently associated with skeletal muscle dysfunction, having a considerable impact on exercise tolerance and patient prognosis. Mitochondria play a role in skeletal muscle weakness and exercise intolerance in COPD, but the majority of studies on mitochondrial function are biased by the fact that physical activity is greater in healthy subjects than in patients. Furthermore, exercise training (ET) has been proposed as a therapeutic strategy to prevent skeletal muscle dysfunction in COPD, but very few results are available on mitochondrial adaptation in response to ET.
Methods
Skeletal muscle mitochondrial function and the potential efficacy of ET on this function were compared between 12 patients with COPD and 21 healthy subjects with similar low levels of physical activity. Various markers of mitochondrial respiration, oxidative stress, biogenesis, and dynamics were assessed.
Results
Lower oxidative phosphorylation (OxPhos; p < 0.001) and increased nonphosphorylating respiration (p = 0.025) and mitochondrial oxidative damage (lipid peroxidation (p = 0.014) and protein carbonylation (p = 0.020)) were observed in patients. While ET increased OxPhos efficiency (p = 0.011) and reduced nonphosphorylating respiration (p < 0.001) and lipid peroxidation (p < 0.001) in patients' muscle mitochondria, it fails to improve maximal respiration (p = 0.835) and expression of the antioxidant enzyme MnSOD (p = 0.606), mitochondrial transcription factor TFAM (p = 0.246), and mitochondrial complexes I, III, and IV (p = 0.816, p = 0.664, p = 0.888, respectively) as observed in healthy subjects.
Conclusion
The mitochondrial dysfunction and the defects in mitochondrial adaptation to ET that we observe in the muscle of patients with COPD are intrinsic to the disease and do not arise from muscle disuse.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is frequently associated with skeletal muscle dysfunction, having a considerable impact on exercise tolerance, health-related quality of life, and patient prognosis [1]. Alterations in skeletal muscle mitochondria potentially contribute to the exercise intolerance in patients with COPD [2], as these organelles are involved in aerobic muscle performance. In addition, mitochondria are one of the main sources of reactive oxygen species (ROS) that affect various cellular signaling pathways and may therefore play a role in skeletal muscle impairment in COPD [3].
In patients with COPD, the energetic status of skeletal muscle remains controversial. Indeed, studies have shown that mitochondrial respiration is impaired in the skeletal muscles of patients, irrespective of the amount of mitochondria [4, 5], and that this alteration is accompanied by greater mitochondrial oxidative stress that could contribute to muscle degradation [4]. Other studies have suggested that altered oxidative capacity is due to lower mitochondrial volume density [6, 7] and reduced expression of a key transcription factor for mitochondrial biogenesis, PGC-1α [8]. Moreover, while mitochondrial dynamics play an important role in mitochondrial function, results on the fusion/fission ratio are scarce in COPD muscle and suggest that mitochondrial fission is very weakly impaired in patients [9]. The extent to which these alterations are pathological or adaptive to the mitochondrial functional phenotype induced by physical inactivity is currently unclear [10]. More importantly, as mitochondrial function is strongly influenced by physical activity level [11, 12], it is essential to control for this dimension in patients with COPD to ensure a valid comparison with healthy subjects. To date, only one study has assessed mitochondrial activity in the muscles of patients with COPD compared to healthy subjects with similar levels of physical activity [13]. The authors showed decreased mitochondrial density, impaired mitochondrial respiration, and increased oxidative stress in patients, indicating that these mitochondrial alterations are intrinsic to the disease [13].
In skeletal muscle mitochondria from healthy subjects, exercise training (ET) increases antioxidant enzyme activity, biogenesis, and fusion, and reduces oxidative stress [14]. This is why ET has been proposed as a therapeutic strategy to increase muscle oxidative capacity and thus prevent skeletal muscle atrophy in chronic disease [1]. Surprisingly, in COPD, there is a contrast between the abundant literature on the effects of ET on muscle structure, mass, typology, strength, and endurance, and the small amount of data available on its impact on mitochondrial activity, biogenesis, and dynamics. To our knowledge, while endurance exercise has been shown to improve skeletal muscle oxidative capacity in patients with COPD [15], only four studies have explored mitochondrial adaptation in response to ET. It has been shown that classical concentric ergometer training increased mitochondrial biogenesis and respiration, while eccentric ergometer training had no impact on mitochondrial adaptation in patients with COPD [16]. Mitochondrial respiration was also improved after ET in patients with COPD [17, 18], while endurance or resistance training increased the content of the mitochondrial antioxidant enzyme MnSOD [19]. Nevertheless, none of these studies standardized physical activity between healthy subjects and patients with COPD, which makes the published data dependent on the greater initial physical activity usually observed in healthy subjects.
The dysfunctional mitochondrial phenotype and defects in mitochondrial adaptation to ET in COPD have been observed in patients without, more often than not, considering their initial physical activity level lower than that of healthy subjects. To eliminate this bias, we therefore compared patients with COPD to healthy subjects with similar measured activity levels. Our hypothesis was that the mitochondrial functional alterations and ET adaptation defects observed in patients would be independent of physical activity and therefore intrinsic to the pathology. Our first aim was to compare mitochondrial activity, oxidative stress, biogenesis, and dynamics in quadriceps muscle between patients with COPD and sedentary healthy subjects (SHS) with similar levels of physical activity. Next, to assess the adaptation of mitochondrial function to ET in patients, we compared the response of skeletal muscle mitochondria to an ET program between patients and SHS with similar low levels of physical activity.
2 Results
2.1 Clinical and Functional Characteristics of the Studied Groups
2.1.1 Before Training
Of the 25 patients with COPD and 26 SHS initially selected, 12 patients and 21 SHS were included in the study because some patients did not meet the inclusion criteria or refused to participate. The patients, with moderately impaired lung function (forced expiratory volume in 1 s (FEV1): 55.4% predicted; Table 1), showed significant reductions in peak oxygen uptake (V̇O2peak; p = 0.003), oxygen consumption at ventilatory threshold (V̇O2VT; p = 0.012), 6-min walking distance (6MWD; p < 0.001) and maximal voluntary contraction (MVC) of quadriceps (p = 0.040), compared to SHS. However, the two groups showed no statistical difference in terms of physical activity level (accelerometer: p = 0.484; VOORIPS questionnaire: p = 0.111) and cumulated time of physical activity during the past 15 years (QUANTAP: p = 0.560). The baseline characteristics of the two groups are shown in Table 1.
| SHS | COPD | p | |
|---|---|---|---|
| Subjects, n | 21 | 12 | |
| Age (year) | 61.6 ± 6.0 | 58.3 ± 5.5 | 0.461 |
| Gender (female/male) | 11/10 | 1/11 | — |
| FEV1 (% pred) | 104.2 ± 11.5 | 55.4 ± 16.5 | < 0.001 |
| FVC (% pred) | 109.7 ± 12.5 | 107.1 ± 11.9 | 0.552 |
| FEV1/FVC (% pred) | 75.6 ± 4.7 | 44.2 ± 9.4 | < 0.001 |
| PaO2 rest (mmHg) | — | 70.8 ± 9.0 | — |
| PaCO2 rest (mmHg) | — | 31.5 ± 4.1 | — |
| Accelerometer (AU) | 137.1 ± 12.2 | 121.9 ± 16.5 | 0.484 |
| VOORIPS | 4.1 (3.2–6.0) | 6.5 (3.5–8.2) | 0.111 |
| QUANTAP past 15 years | 8328 (9754–15 992) | 11 525 (4012–23 141) | 0.560 |
| VO2peak (ml/min/kg) | 25.9 ± 6.2 | 19.3 ± 4.1 | 0.003 |
| VO2peak (% pred) | 108.5 ± 14.5 | 68.3 ± 15.9 | < 0.001 |
| V̇O2VT (ml/min/kg) | 15.6 ± 3.4 | 12.8 ± 2.0 | 0.012 |
| V̇O2VT (% pred) | 65.1 ± 9.5 | 45.2 ± 6.2 | < 0.001 |
| 6MWD (m) | 617.8 ± 62.9 | 535.5 ± 63.1 | < 0.001 |
| MRC | — | 1.9 ± 1.3 | — |
| BMI (kg/m2) | 25.9 ± 3.0 | 25.2 ± 3.3 | 0.921 |
| MVC (kg) | 22.6 ± 8.7 | 16.8 ± 4.2 | 0.040 |
| Tlim (s) | 496.7 ± 360.7 | 347.5 ± 295.1 | 0.209 |
- Note: Data are presented as mean ± SD or median (interquartile range).
- Abbreviations: % pred: percentage of the predicted value; 6MWD: 6-min walking distance; AU: activity unit; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; MRC: Medical Research Council dyspnea test; MVC: maximal voluntary contraction of quadriceps; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension; Tlim: time to exercise limitation of quadriceps; VO2peak: peak exercise oxygen uptake; V̇O2VT: oxygen uptake at ventilatory threshold.
2.1.2 Effects of Exercise Training
The rehabilitation program of moderate intensity induced significant improvement of V̇O2peak (p < 0.001), 6MWD (p < 0.001), MVC (p < 0.001), and endurance time (Tlim; p < 0.001) of the quadriceps in SHS, and only of 6MWD (p < 0.001) and MVC (p = 0.035) in patients with COPD (Table 2). However, these four functional parameters, V̇O2peak, 6MWD, MVC, and Tlim, were significantly lower in patients than in SHS (Table 2; group effects: p < 0.001, p = 0.002, p = 0.034, p = 0.049, respectively).
| SHS | COPD | p | |||||
|---|---|---|---|---|---|---|---|
| Before training | After training | Before training | After training | Group effect | Exercise effect | Gr × Ex interaction | |
| V̇O2peak (ml/min/kg) | 25.9 ± 6.2 | 28.5 ± 6.3### | 19.3 ± 4.1** | 20.4 ± 4.4 | < 0.001 | < 0.001 | 0.070 |
| +10.0% | +5.7% | ||||||
| 6MWD (m) | 617.8 ± 62.9 | 652.8 ± 53.1### | 535.5 ± 63.1** | 580.7 ± 69.1### | 0.002 | < 0.001 | 0.161 |
| +5.7% | +8.4% | ||||||
| MVC (kg) | 22.6 ± 8.7 | 25.7 ± 10.5### | 16.8 ± 4.2* | 18.5 ± 5.6# | 0.034 | < 0.001 | 0.149 |
| +13.6% | +10.0% | ||||||
| Tlim (s) | 496.7 ± 360.7 | 773.5 ± 394.1### | 347.5 ± 295.1 | 380.5 ± 191.4 | 0.049 | < 0.001 | 0.068 |
| +55.7% | +9.5% | ||||||
- Note: Data are presented as Mean ± SD. The % of change between before and after training in SHS and patients with COPD is indicated. *p < 0.05, **p < 0.01 between SHS and patients with COPD before training; #p < 0.05, ###p < 0.001 between before and after training in SHS and patients.
- Abbreviations: 6MWD: 6-min walking distance; MVC: maximal voluntary contraction of quadriceps; Tlim: time to exercise limitation of quadriceps; V̇O2peak: peak exercise oxygen uptake.
2.2 Mitochondrial Respiration
2.2.1 Before Training
Mitochondrial respiration activity was recorded in permeabilized fibers from SHS and patients with COPD. The maximum oxygen consumption was similar between SHS and patients regardless of the oxidized substrate (Vmax PC: p = 0.484; Vmax Pyr: p = 0.877; VRot-Succ: p = 0.080; VAnt-TMPD + Asc: p = 0.880; Figure 1A–D). However, the nonphosphorylating respiration was significantly higher in patients with COPD than in controls (VOligo: p = 0.025; Figure 1E). In addition, mitochondrial ATP synthesis was significantly higher in SHS than in patients (VATP: p = 0.005; Figure 1F). The ratio between ATP synthesis and oxygen consumption (i.e., oxidative phosphorylation efficiency) was significantly lower in patients with COPD than in SHS (ATP/O: p < 0.001; Figure 1G).
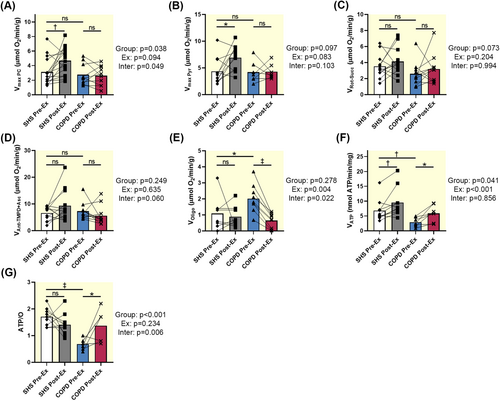
Expression of mitochondrial complexes I, II, III, IV, and V was similar between SHS and patients with COPD before training (p = 0.456, p = 0.877, p = 0.959, p = 0.876, p = 0.877, respectively; Figure 2A–E).

2.2.2 Effects of Exercise Training
Mitochondrial respiration with palmitoyl-carnitine and pyruvate as substrates was improved in SHS (Vmax PC: p = 0.005; Vmax Pyr: p = 0.011; Figure 1A,B) relative to the baseline values, whereas it remained unchanged in patients with COPD (Vmax PC: p = 0.835, Vmax Pyr: p = 0.940; Figure 1A,B). However, training had no effect on the mitochondrial respiration of complex II (VRot-Succ: p = 0.352 for SHS, p = 0.377 for COPD; Figure 1C) and complex IV (VAnt-TMPD + Asc: p = 0.088 for SHS, p = 0.308 for COPD; Figure 1D) in both groups. In contrast, the nonphosphorylating respiration was significantly decreased only in patients with COPD (VOligo: p < 0.001; Figure 1E), to values similar to those of SHS. In addition, the ATP synthesis rate and the ratio between ATP synthesis and oxygen consumption increased in patients with COPD after training (p = 0.012, p = 0.011, respectively; Figure 1F,G), while in SHS the increase is only for the ATP synthesis rate (p = 0.007; Figure 1F).
Endurance training increased the expression of mitochondrial respiratory complexes I, III, and IV in SHS (p = 0.035, p = 0.031, p = 0.041, respectively; Figure 2A,C,D), while it had no effect on the expression of these different complexes in patients (p = 0.816, p = 0.664, p = 0.888, respectively; Figure 2A,C,D).
2.3 Mitochondrial Oxidative Stress
2.3.1 Before Training
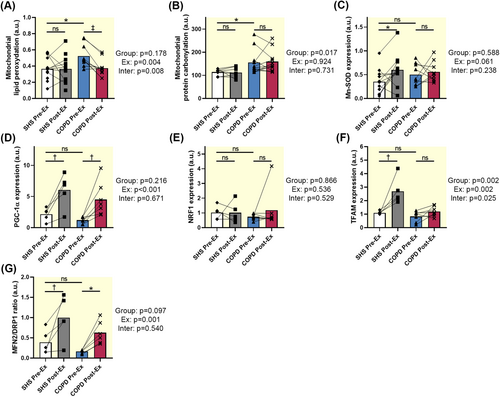
At baseline, mitochondrial lipid peroxidation and protein carbonylation were significantly higher in patients with COPD than in SHS (p = 0.014, p = 0.020, respectively; Figure 3A,B). Expression of the mitochondrial anti-oxidant enzyme MnSOD was similar in SHS and patients (p = 0.194; Figure 3C).
2.3.2 Effects of Exercise Training
Mitochondrial lipid peroxidation decreased after training only in patients with COPD (SHS: p = 0.849, COPD: p < 0.001; Interaction effect: p = 0.008; Figure 3A). Exercise training did not modify mitochondrial protein carbonylation in both groups (SHS: p = 0.870, COPD: p = 0.735; Figure 3B). MnSOD expression was increased after exercise training in SHS (p = 0.028; Figure 3C) but not in patients with COPD (p = 0.606; Figure 3C).
2.4 Mitochondrial Biogenesis
2.4.1 Before Training
We found no significant difference in the expression of PGC-1α, NRF1, and TFAM, three key proteins involved in mitochondrial biogenesis, between SHS and patients with COPD (p = 0.116, p = 0.432, p = 0.343, respectively; Figure 3D–F).
2.4.2 Effects of Exercise Training
PGC-1α expression was significantly increased in SHS and patients with COPD after exercise training (SHS: p = 0.004, COPD: p = 0.004; Figure 3D), while NRF1 expression remained unchanged in both groups (SHS: p = 0.994, COPD: p = 0.340; Figure 3E). Nevertheless, TFAM expression was significantly increased only in SHS after exercise training (SHS: p = 0.001; COPD: p = 0.246; interaction effect: p = 0.025; Figure 3F).
2.5 Mitochondrial Dynamics
2.5.1 Before Training
The fusion/fission ratio, represented by the MFN2/DRP1 ratio, was similar in both groups (p = 0.227; Figure 3G).
2.5.2 Effects of Exercise Training
After training, the MFN2/DRP1 ratio increased significantly in SHS and patients with COPD (SHS: p = 0.006, COPD: p = 0.016; exercise effect: p = 0.001; Figure 3G) due to an increase in MFN2 expression in both groups (data not shown).
3 Discussion
The first aim of our study was to compare mitochondrial function in skeletal muscle between SHS and patients with COPD with a similar low activity level. Our data showed that, although the expression of the various mitochondrial complexes was not altered, the efficiency of oxidative phosphorylation (OxPhos) was lower in patients with COPD. In addition, nonphosphorylating respiration and mitochondrial oxidative damage (lipid peroxidation and protein carbonylation) were increased in patients. The second aim of the study was to assess the mitochondrial response to moderate ET in the muscle of these patients. We observed that exercise increased OxPhos efficiency and reduced nonphosphorylating respiration in patients with COPD. ET also reduced lipid peroxidation, but not protein carbonylation, in patients' muscle mitochondria. Nevertheless, ET increased maximal respiration and expression of the antioxidant enzyme MnSOD, the mitochondrial transcription factor TFAM, and mitochondrial complexes I, III, and IV in SHS but not in patients with COPD.
3.1 At Baseline
In our study, the mitochondrial respiration rates were similar in both SHS and patient groups (Figure 1A–D). Interestingly, it has been reported that in the peripheral muscle of patients with COPD, mitochondrial respiratory function is impaired, solely due to a reduction in the number of mitochondria [7]. However, in our study, we found no significant difference between the two groups in the expression of mitochondrial complexes (Figure 2A–E). Our results are also controversial compared with findings that reported decreased PGC1α and TFAM levels in the skeletal muscle of patients with COPD [8]. This discrepancy may be explained by the fact that physical activity levels were not matched between patients and SHS in the latter study, with reduced physical activity in patients. This is contrary to our protocol, where physical activity was quantified with three different methods in order to be matched between patients and SHS. Matching physical activity levels is important in studies of muscle mitochondrial function, as physical activity and exercise capacity are positively correlated with muscle PGC1α mRNA levels in healthy subjects [20].
The nonphosphorylating respiration was higher in patients with COPD than in SHS (Figure 1E), reflecting a proton leak or electron slip [21]. In addition, ATP synthesis was significantly lower in the muscle fibers from patients (Figure 1F), consistent with previous measurements of metabolites in muscle samples that showed lower ATP production in patients with COPD [4]. OxPhos efficiency was lower in patients than in SHS (Figure 1G), indicating that the reduction in OxPhos efficiency in the muscle fibers from patients was primarily due to a reduction in the rate of ATP synthesis. These results suggest a dissociation between oxygen consumption and ATP production in the muscle of patients, indicative of mitochondrial inefficiency and potential uncoupling. The unchanged expression of mitochondrial complexes between the two groups further supports the notion that the functional deficits observed are not due to a reduction in mitochondrial machinery but rather to functional impairments.
In our study, higher mitochondrial protein carbonylation and lipid peroxidation in patients with COPD (Figure 3A,B) reflect an increase in the production of mitochondrial ROS observed previously by different teams [4, 22]. Numerous studies have shown a direct relationship between increased activity and expression of uncoupling proteins and the reduction of ROS production by mitochondria via activation of a proton leak mechanism [23]. It is thus possible that, in response to increased mitochondrial ROS production due to functional defects in the respiratory chain, mitochondrial uncoupling is activated to counteract elevated oxidative stress [23]. Another possibility would be that functional failure of the respiratory chain leads to electron slip and potential uncoupling, which would increase oxidative stress and thus mitochondrial oxidative damage [23].
Given the similar levels of inactivity between patients with COPD and SHS, the increased mitochondrial uncoupling, decreased OxPhos efficiency, and increased mitochondrial damage in the patients' muscles seem to stem from the disease itself and not from the low physical activity usually observed in COPD. These results confirm findings showing a decrease in mitochondrial density and respiration and an increase in oxidative stress in the muscle of patients with similar levels of physical activity to healthy subjects [13].
3.2 Effects of Exercise Training
Few studies have compared the mitochondrial response to ET in the skeletal muscle of patients with COPD versus healthy subjects [16-19]. Our work is the first to investigate this mitochondrial response in patients versus SHS with the same low level of activity, in order to exclude a bias that would arise from greater basal physical activity for healthy subjects.
Our ET program, which was conducted at moderate intensity and during a limited number of sessions, meets the requirements of a rehabilitation program for chronic respiratory diseases [24] in order to encourage and promote more sustainable adherence to healthy behaviors, particularly physical activity. With this type of ET, it is not surprising to see a modest improvement in exercise tolerance in patients with COPD, limited to a slight but significant increase in 6MWD, while the increase in V̇O2peak did not reach significance. Similar results were observed in a previous study conducted by our group in patients who underwent a similar 4-week rehabilitation program [25]. Furthermore, the significant increase in MVC in the COPD group, although slight, could reflect the potential benefits of this type of ET on peripheral muscles. The improvement was more complete in SHS in terms of tolerance to maximal and submaximal exercise and endurance, as previously described in sedentary subjects without ventilatory or cardiovascular limitations [25]. Furthermore, recent findings suggest that mitochondrial adaptations to ET are blunted in COPD [26]. Their study showed that, despite undergoing aerobic training, patients with COPD demonstrated a markedly attenuated mitochondrial response compared to healthy adults, both in terms of functional and structural indicators. It thus appears that the lack of V̇O2peak progression in our COPD group after training probably reflects a combination of persistent ventilatory and peripheral limitations, but also poor mitochondrial adaptation.
In this study, ET increased maximal respiration (Figure 1A,B), enhanced ATP synthesis (Figure 1F), and upregulated expression of mitochondrial complexes I, III, and IV (Figure 2A,C,D) in SHS. These adaptations are consistent with the well-established benefits of ET on mitochondrial biogenesis and oxidative capacity in healthy subjects [14]. In contrast, the response to ET in patients with COPD was more limited: while there was a reduction in nonphosphorylating respiration (Figure 1E) and an increase in ATP synthesis and OxPhos efficiency (Figure 1F,G), maximal respiration (Figure 1A,B) and mitochondrial complex expression (Figure 2A–E) remained unchanged. These results are in contradiction with studies showing an increase in maximal mitochondrial respiration in the muscle of patients with COPD following ET [16-18]. Although the beneficial effects of physical activity on mitochondrial function are indisputable, it has been shown that, depending on the type of physical activity offered to patients with COPD (endurance or resistance, eccentric or concentric, single-joint or whole-body exercises), mixed benefits can be achieved [27]. However, in the case of our pulmonary rehabilitation program, combining endurance and muscle-strengthening exercises, the results suggest that while ET can partially restore mitochondrial efficiency in COPD, it cannot completely reverse underlying mitochondrial dysfunction specific to COPD.
ET reduced mitochondrial lipid peroxidation in the muscle of patients with COPD (Figure 3A), a finding that underscores the potential of exercise to mitigate oxidative damage. However, the lack of improvement in mitochondrial protein carbonylation (Figure 3B) and the failure to increase MnSOD expression post-training (Figure 3C) suggest that the antioxidant response in COPD muscle remains inadequate to fully counteract the elevated oxidative stress generated by ET, in agreement with previous studies in vivo [28, 29] and in vitro [30]. Moreover, our work, and that of other teams [4, 31], suggest that mitochondrial impairment observed at rest and after ET in patients with COPD reflects the action of systemic factors regardless of initial physical activity level. The most likely candidate is the low-grade inflammation observed in COPD, where inflammatory cytokines are known to lead to the production of ROS in peripheral muscles [32], more specifically in the mitochondria. Such oxidative stress could lead to intrinsic damage to the respiratory chain and mitochondrial DNA, resulting in proton leak and mitochondrial uncoupling and consequent exercise intolerance.
By examining several regulators of mitochondrial biogenesis and dynamics, we showed that ET induced a differential response in SHS and patients with COPD: while both groups exhibited increased PGC1α expression and no change in NRF1 content (Figure 3D,E), only SHS showed an increase in TFAM (Figure 3F), a key protein involved in mitochondrial DNA replication and transcription. To our knowledge, only one publication has studied mitochondrial biogenesis in the muscle of patients with COPD after training, and their study focuses only on PGC1α, whose expression increases significantly in patients after ET [16]. Mitochondrial dynamics, as indicated by the fusion/fission ratio, improved in both groups following ET (Figure 3G), suggesting that ET can enhance mitochondrial quality control mechanisms even in COPD, potentially aiding in the removal of damaged mitochondria and the maintenance of mitochondrial function, such as respiration [33, 34]. Our results therefore suggest that although mitochondrial dynamics are unaffected in the muscle of patients with COPD following ET, the blunted TFAM response in patients could reflect a compromised capacity for mitochondrial biogenesis, potentially due to elevated oxidative stress and defects in the antioxidant system in patients' muscle (results in this manuscript and [28, 29]). These results are in line with those showing that high systemic inflammation and oxidative stress induce a defect in mitochondrial biogenesis, via altered expression of the transcription factors PGC1α and TFAM, in COPD muscles [10, 35].
3.3 Limitations
An important limitation in our study stems from the gender imbalance between the SHS group (11 females/10 males) and the COPD group (1 female/11 males), which could affect the results obtained on mitochondrial respiration. Nevertheless, two studies have shown that when mitochondrial respiration of complexes I to IV and mitochondrial ATP production are compared between women and men, no gender difference is observed [36, 37]. A similar result is also obtained when we compare Vmax Pyr between the 11 females and 10 males in the control group of our study (data not shown), indicating that the male/female imbalance does not impact our results. Another limitation of this study is the small number of samples used to assess the expression of markers of mitochondrial biogenesis and dynamics: five for SHS and six for patients. Further studies, with a larger number of samples, will therefore be necessary to validate our results. Nevertheless, this is the first study to evaluate the expression of the markers PGC1α, NRF1, TFAM, and the MFN2/NRF1 ratio in the muscle of patients with COPD before and after ET.
4 Materials and Methods
4.1 Study Subjects
Patients with COPD, defined by dyspnea, chronic cough or sputum production and/or a history of exposure to risk factors for the disease, and post-bronchodilator FEV1/forced vital capacity (FVC) < 70%, were included in this study. Exclusion criteria included other respiratory diagnoses, decompensated comorbidity, exacerbation in the last 6 months, and previous participation in an ET program. The SHS were disease-free and engaged in less than 150 min of moderate to vigorous physical activity per week, which is the recommended threshold for health improvement, as previously described [25]. All SHS and patients performed the tests in the Department of Clinical Physiology, University Hospital of Montpellier (Montpellier, France), or the Pulmonary Rehabilitation Center “La Solane” (Osseja, France). Informed written consent was obtained from all subjects, and the research protocol was approved by the institutional ethics committee of the Montpellier University Hospitals (2008-EESSS-V2 and 2009-04-BPCO-V2) and conducted in accordance with the Helsinki Declaration and the European guidelines for good clinical practice.
4.2 Physical Activity
Physical activity was assessed with three methods: VOORIPS questionnaire, a triaxial accelerometer (Tritrac RT3 Research Tracker; Stayhealthy, Monrovia, CA) for seven consecutive days, and validated for patients with COPD [38], and the “Quantification de l'Activité Physique” (QUANTAP). QUANTAP is a computer-assisted tool designed to determine physical activity over a lifetime in four dimensions (sports at school, leisure sports, occupation, and daily activities), validated for patients with COPD by our team [39].
4.3 Functional Evaluations
Spirometry was performed in accordance with the recommendations detailed in the European and American Thoracic Society Guidelines in order to determine FEV1, FVC, and the FEV1/FVC ratio. The results of the pulmonary function tests were compared to the normal values reported previously [40]. In addition, a 6-min walking test was performed in a 30-m corridor. The 6MWD was compared with the reference values [41]. Participants performed an incremental test on an electrically braked cycle ergometer (Ergoselect 200P; Ergolyne, Bitz, Germany) to determine V̇O2 peak and V̇O2VT [42]. The quadriceps strength and endurance were measured by isometric MVC and Tlim as previously described [25].
4.4 Exercise Training
SHS and patients with COPD underwent 6 weeks of training as described previously [25]. Briefly, training consisted of 20 sessions of endurance exercise conducted three or four times per week for 6 weeks. Each session lasted 45 min, and in one out of two sessions, it was followed by 30 min of strength-building exercise performed at 40% of the one-repetition maximum. An experienced clinician supervised all sessions, and the training intensity was progressively increased during the training protocol. Details are given in Supporting Information.
4.5 Muscle Biopsies
A muscle biopsy of the lateral part of the vastus lateralis was performed under local anesthesia using the Bergstrom needle biopsy technique [43]. Specimens were divided into two portions. The fragment for in situ mitochondrial respiration studies was immediately placed in an ice-cold isolation solution as previously described [44]. The portion for biochemical and molecular studies was flash frozen in liquid nitrogen and stored at −80°C.
4.6 Mitochondrial Respiration
Dissection and permeabilization of fiber bundles with saponin were performed as described previously [44]. They were then stored on ice in respiration medium (EGTA 0.5 mM, MgCl2 3 mM, taurine 20 mM, KH2PO4 10 mM, HEPES 20 mM, BSA 1 g/L, potassium-lactobionate 60 mM, mannitol 110 mM, dithiothreitol 0.3 mM, pH 7.1) until determination of mitochondrial respiration activity [44]. The rates of oxygen consumption were assessed with an Oroboros system (Oroboros Instruments, Innsbruck, Austria) and were expressed as μmoles of O2 per minute per gram of dry weight (μmolO2/min/g), or per unit of citrate synthase (CS) activity (μmolO2/min/U of CS activity) to normalize for differences in mitochondrial content between individuals [7]. Palmitoyl-carnitine and pyruvate were used as substrates to assess maximal mitochondrial respiration rate. The use of rotenone (complex I blocking effect) and succinate (complex II substrate) allowed evaluation of mitochondrial respiration via complex II (VRot-Succ). Antimycin A (complex III blocking effect) and TMPD + ascorbate (complex IV substrate) were used to study respiration via complex IV (VAnt-TMPD + Asc). Oligomycin, with its complex V blocking effect, was used to assess nonphosphorylating respiration (VOligo). In addition, we evaluated the rate of mitochondrial ATP synthesis in skinned fibers using the ATP bioluminescence assay kit (Merck, Darmstadt, Germany) as described in the manufacturer's protocol. ATP synthesis rate was expressed in nmoles of ATP produced per minute and per milligram of fiber (dry weight). The efficiency of oxidative phosphorylation was described by the ratio between the ATP synthesis rate and the oxygen consumption rate and was expressed as ATP/O.
4.7 Extraction of Mitochondrial Proteins
Mitochondrial proteins were extracted from a portion of the muscle biopsies as described previously [45]. Briefly, muscle biopsy samples were homogenized using an Ultra-Turrax tissue homogenizer (Prolabo, France) in ATP medium (100 mM KCL, 50 mM Tris, 5 mM MgSO4, 1 mM EDTA, 1 mM ATP, 0.5% BSA, pH 7.4). Muscle homogenates were centrifuged at 300 g for 5 min. The supernatants were centrifuged for 10 min at 4000 g. Pellets were resuspended in isolation medium (100 mM KCL, 50 mM Tris, 5 mM MgSO4, 1 mM EDTA, pH 7.4) and centrifuged for 10 min at 7000 g. The pellets were then resuspended in isolation medium before the protein concentration was determined with the Lowry method.
4.8 Mitochondrial Oxidative Stress
Oxidation of mitochondrial proteins was evaluated by measuring protein carbonyl formation with the OxyBlot Protein Oxidation Detection Kit (Merck, Darmstadt, Germany). 4-hydroxy-2-nonenal (4-HNE) protein adducts were measured on mitochondrial proteins using a rabbit polyclonal anti-HNE antibody (Abcam, Cambridge, UK) for assessment of lipid peroxidation. Expression of the anti-oxidant enzyme MnSOD was assessed by immunoblotting with a rabbit polyclonal anti-MnSOD antibody (Enzo Life Sciences, Lyon, France).
4.9 Western Immunoblotting
Mitochondrial extracts were separated by SDS-PAGE and transferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA). Proteins of interest were revealed by specific antibodies, followed by enhanced chemiluminescence. β-actin and GAPDH were used as loading controls. Scanned radiographs were quantified using the ImageJ software.
4.10 Real-Time Quantitative PCR
Quantification of PGC-1α, NRF1, TFAM, MFN2, and DRP1 mRNA expression in quadriceps muscles was performed in triplicate with real-time RT-qPCR. Total RNA was purified using the RNeasy Fibrous Tissue Mini Kit (QIAGEN, Hilden, Germany). The concentration of RNA was determined using a spectrophotometer, and quality was verified by agarose gel electrophoresis. RNA (100 ng per sample) was reverse transcribed with the Verso cDNA Synthesis Kit (Fisher Scientific, Illkirch, France) according to the manufacturer's instructions. qPCR was performed using the LightCycler 480 system (Roche, Basel, Switzerland), Platinum Taq DNA polymerase (Fisher Scientific), and SYBR Green 1 Master Mix (Roche). Forward and reverse primers (Table S1) were each designed in a different exon of the target sequence, eliminating the possibility of amplifying genomic DNA. Ct values of the target gene were normalized to the Ct values of the housekeeping gene hydroxymethylbilane synthase (HMBS). The expression level of each transcript was determined using the 2−∆∆Ct method.
4.11 Data Analysis
The sample size for the patients with COPD group was determined with a power of 90% and a two-sided 0.05 significance level, on the assumption that the exercise training induced an increase of the maximum oxygen consumption of 2 μmol/min/g and a standard deviation of 2. Data in the text, tables, and figures are presented as mean ± SD unless specified otherwise. Distribution normality was tested by the Kolmogorov–Smirnov test. The various parameters presented in Table 1 for healthy subjects and patients with COPD (baseline characteristics) were compared using a Mann–Whitney test. Each subject was evaluated twice, before and after exercise training. Thus, repeated-measure two-way ANOVA followed by a Fisher's LSD multiple comparison test was used to compare values between before and after training for healthy subjects and patients with COPD, for the various studied variables. A p-value of 0.05 was considered statistically significant. Data were analyzed using R.2.13.0 software or GraphPad Prism 8.
5 Conclusion
Our study is the first to compare muscle mitochondrial function and response to ET between patients with COPD and healthy subjects with a similar low activity level. Under these conditions, we observed a mitochondrial dysfunction and defects in mitochondrial adaptation to ET in the muscle of patients with COPD, indicating that these alterations are intrinsic to the disease and do not stem from muscle disuse. This result favors intrinsic disease-specific factors, while excluding an external factor such as disuse, in the onset of mitochondrial alteration and thus exercise intolerance in patients. The nature of these intrinsic pathophysiological mechanisms is still being investigated, with a focus on systemic inflammation-dependent alterations in mitochondrial respiratory chain function and/or biogenesis, with a view to developing targeted treatment strategies.
Author Contributions
Aldjia Abdellaoui: conceptualization, methodology, investigation, validation, formal analysis, writing – original draft, writing – review and editing. Farés Gouzi: conceptualization, methodology, investigation, validation, formal analysis, writing – review and editing. Cécile Notarnicola: methodology, validation, writing – review and editing, formal analysis. Annick Bourret: methodology, validation, writing – review and editing, formal analysis. Amandine Suc: methodology, validation, writing – review and editing, formal analysis. Dalila Laoudj-Chenivesse: methodology, validation, writing – review and editing, formal analysis. Nelly Héraud: methodology, validation, writing – review and editing, formal analysis. Jacques Mercier: conceptualization, methodology, investigation, validation, writing – review and editing. Christian Préfaut: conceptualization, investigation, methodology, validation, writing – review and editing. Maurice Hayot: conceptualization, methodology, investigation, validation, writing – review and editing, formal analysis. Pascal Pomiès: conceptualization, investigation, methodology, validation, writing – review and editing, formal analysis.
Acknowledgments
The team would like to thank Brigitte Picard (UMR Herbivores, Saint-Genès-Champanelle, France) for her scientific expertise.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




