Activation of the Carotid Sinus Nerve After Acute Myocardial Infarction in a Cardiorenal Syndrome Type 1 Model in Sprague–Dawley Rats
Funding: This Research was funded by FAPESP (São Paulo Research Foundation, Process # 2020/06043–7, and F.B. by Process #2021/03764–8), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Process 423999/2021–4), the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)—Brasil—PROEX for R.O.C—Finance Code 001.
ABSTRACT
Aim
To evaluate the effect of carotid sinus nerve stimulation (CSNS) in the progression of cardiorenal syndrome type 1 (CRS1), 3 days after acute myocardial infarction (AMI).
Methods
Male rats were divided into four groups. CSNS was applied daily for 10 min over 3 days. Cardiac, renal, and inflammatory parameters characterized the CRS1 and the electroceutical effects of CSNS.
Results
CSNS reduced the ischemic zone compared to the AMI group not exposed to CSNS (32.7% ± 2.2% vs. 8.0% ± 1.8%). Heart rate (bpm) was increased in the AMI group, showing 440 ± 7.6 at 48 h and 428 ± 1.0 at 60 h post-AMI. Additionally, arterial pressure (mmHg) was increased in the AMI group at 48 h, as follows: mean: 98 ± 1.7, diastolic: 89 ± 2.1, and systolic: 122 ± 5.3. In contrast, the CSNS + AMI group showed significant reductions of these parameters: mean: 79 ± 2.0, diastolic, 66 ± 1.7, and systolic: 99 ± 2.7. Renal injury was confirmed by increased apoptosis in the AMI group. A significant increase in TNF-α was observed in both heart and kidneys (pg/mg of tissue) in the AMI group and reduced IL-6 and IL-1β levels in the CSNS + AMI group, indicating an attenuation of the inflammatory responses by CSNS.
Conclusions
This study demonstrates early cardiac and renal dysfunction in CRS1 following AMI, associated with elevated inflammatory markers (TNF-α, IL-6, and IL-1β) and renal apoptosis. Therefore, CSNS appears to be a promising electroceutical approach for CRS1. Besides, on the basis of previous studies from our laboratory, CSNS involves stimulation of the baroreflex, activating the parasympathetic and inhibiting the sympathetic nervous system.
1 Introduction
Cardiorenal syndrome (CRS) encompasses a spectrum of disorders involving the interplay between cardiac and renal dysfunction. Acute or chronic impairment in one organ can lead to a cascade of pathological changes in the other, characterized by a bidirectional interaction [1]. This complex interaction operates through multiple interfaces involved in initiating and progressing acute heart and kidney damage. Multiple physiopathological processes are involved in this complex scenario, including hemodynamic alterations, endothelial damage, cell death, inflammatory cascades, oxidative stress, neutrophil migration, leukocyte traffic, and caspase-mediated apoptosis, as well as extracellular vesicles [2]. Together, these mechanisms shape the clinical phenotypes of CRS and highlight its systemic nature [3].
Among the various types of CRS, the acute cardiorenal syndrome type 1 (CRS1) is the most prevalent, accounting for nearly half of all cases [1]. Recent studies estimate that 25% to 33% of patients hospitalized with acute decompensated heart failure (ADHF) exhibit CRS1 [1], emphasizing its clinical importance. Despite its high prevalence, the early phases of CRS1—especially those involving acute clinical manifestations—remain poorly understood. Early identification of this stage is critical for timely therapeutic intervention, since delayed management can exacerbate the progression to chronic kidney and heart failure, significantly increasing cardiovascular mortality rates [1].
The pathophysiology of CRS is driven by dysregulation of neurohumoral systems, including renin-angiotensin-aldosterone system (RAAS), the autonomic nervous system (ANS), and atrial natriuretic peptides (ANP) [4]. Dysregulation of these systems can amplify the risk of CRS1 after acute myocardial infarction (AMI) by triggering hemodynamic imbalances, inflammatory cascades, and oxidative stress [1, 3].
Inflammation is a central contributor to the onset and progression of CRS1 [3]. Experimental studies have demonstrated that acute kidney injury (AKI) develops within days following AMI because of systemic inflammation. In a rodent model, AMI induced by coronary artery ligation [5] has been associated with increased apoptotic cells, elevated neutrophil gelatinase-associated lipocalin (NGAL), cardiac hypertrophy, renal fibrosis, and heightened pro-inflammatory cytokines (e.g., IL-6, TNF-α), mediated by nuclear factor kappa B (NFκB) activation [6-8].
The ANS also exerts a pivotal influence on CRS1 progression. In the context of AMI, intensified sympathetic activity exacerbates cardiac remodeling and inflammation, characterized by neutrophil infiltration and M1 macrophage activation. These immune cells release pro-inflammatory cytokines, prolonging ventricular dysfunction and increasing the risk of heart failure [9, 10]. Maladaptive autonomic responses, particularly impaired parasympathetic modulation, disrupt cardiovascular and renal homeostasis by altering neuroimmune interactions, contributing to systemic inflammation and organ dysfunction, i.e., [11], mechanisms that can be associated with the development and progression of cardiorenal syndrome.
Physiological reflexes, such as the baroreflex and chemoreflex, regulate autonomic activity in response to hemodynamic changes, and recent advancements have explored therapeutic applications targeting these reflex pathways [12]. The baroreflex ensures moment—to-moment blood pressure regulation through arterial baroreceptors, enhancing parasympathetic activity and suppressing sympathetic drive when stimulated [13]. Conversely, the chemoreflex maintains cardiorespiratory balance by modulating ANS activity in response to fluctuations in blood gas concentrations, simultaneously activating both sympathetic and parasympathetic pathways [14].
Studies have shown that CSNS activates both baroreflex and chemoreflex mechanisms, effectively reducing pro-inflammatory cytokines and attenuating tissue injury in inflammatory models [15]. Notably, CSNS has shown protective effects in mitigating alveolar bone loss and systemic inflammation in experimental Periodontitis, suggesting forthcoming applicability in inflammatory diseases [15].
Early detection and treatment of CRS1 require a deeper understanding of the initial pathological changes driving its progression. Therapeutic approaches during these early phases may prevent irreversible injury, improving the physiopathological outcomes. Dual modulation of the ANS (Baro- and Chemoreflex) via CSNS holds considerable promise as a novel electroceutical possibility, addressing both inflammatory and hemodynamic derangements inherent to CRS1. These aspects underscore the need for continued research into the neuroimmune mechanisms governing CRS1, particularly its acute manifestations.
Additionally, the current study assessed the potential protective effects of CSNS in mitigating ischemic damage and preventing further progression of myocardial and renal injury. This procedure was based on previous observations of our laboratory that this electroceutical approach applied to the experimental model of Periodontitis in rats attenuated bone loss and reduced the gingival and plasma pro-inflammatory cytokines [16] and delayed the onset of hypertension in spontaneously hypertensive rats (SHR) exposed to periodontitis [15].
Therefore, this study aimed to investigate the early progression of CRS1 3 days after AMI by analyzing in rats the renal and cardiac function, as well as their inflammatory responses. The focus was to identify early structural and biochemical alterations that could predict CRS1 development. Additionally, the current study assessed the potential protective effects of CSNS in mitigating ischemic damage and preventing further progression of myocardial and renal injury. By elucidating these mechanisms, the current study provides valuable insights into the early mechanisms of cardiorenal pathophysiology and the potential of carotid sinus nerve (CSN) modulation as an electroceutical strategy.
2 Materials and Methods
2.1 Animals Studies
Forty-five male Sprague–Dawley rats (250 g-300 g) were used in this study. The rats were provided by the Central Animal House at the Ribeirão Preto Campus of the University of São Paulo (USP).
Prior to surgical preparation, all animals were housed in individual cages in the vivarium of the Department of Physiology, Ribeirão Preto Medical School (FMRP-USP). Housing conditions included a controlled temperature of 24°C and a 12-h light–dark cycle, with unrestricted access to food and water. All experimental procedures were approved by the Animal Use Ethics Committee (CEUA) of FMRP-USP (local approval), under Protocol No. 177/2019, and were conducted by the Guide for the Care and Use of Laboratory Animals (NIH). Furthermore, all submitted material conforms to good publishing practice in physiology, as outlined in the Acta Physiologica guidelines [17].
- Control: Sham surgery for both CSNS and ligation of the anterior descending branch of the left coronary artery.
- AMI: Sham surgery for CSNS and ligation of the anterior descending branch of the left coronary artery.
- CSN-Stimulated: CSNS and sham surgery ligation of the anterior descending branch of the left coronary artery.
- CSN-Stimulated + AMI: CSNs and ligation of the anterior descending branch of the left coronary artery.
2.2 Surgical Procedures
All surgical procedures were conducted under aseptic conditions, and all implanted materials were thoroughly sterilized before use. Upon completion of the surgical procedures, analgesic was administered (Tramadol Hydrochloride, 2 mg/kg, s.c.) to minimize pain and a prophylactic dose of penicillin (benzylpenicillin—30 000 IUs plus streptomycin—16 mg; Pentabiótico Veterinário, Fort Dodge Saúde Animal Ltda, Campinas, Brazil) via intra-muscular injection to prevent infections.
2.2.1 Standardization of the Telemetry Model in the Cardiorenal Syndrome Type 1
The acquisition of hemodynamic parameters using telemetry was standardized for the CRS1 model. Under inhalatory anesthesia (3% isoflurane and 0.3 L/min oxygen after intratracheal intubation), rats underwent a pressure telemeter (TRM54PP; ADInstruments, Bella Vista, Australia) implantation in the aorta, utilizing a Millar Mikro-Tip catheter (SPR869; ADInstruments, Bella Vista, Australia) for high-fidelity arterial pressure signal recording. This procedure was performed 5 days before starting the experimental protocols. After implantation, wireless data transmission was verified using SmartPads, which transmitted data and energy to the implanted telemeters.
2.2.2 Implantation of the Electrodes
Rats were anesthetized with a mixture of Ketamine and Xylazine (50 mg/kg and 10 mg/kg, i.p.) before isolating the left CSN for electrode placement. Bipolar stainless-steel electrodes (0.008 in. bare, 0.011 in. Teflon coated; A-M Systems, Sequim, WA, USA), as previously described [18, 19], were positioned around the left CSN, covered with silicone elastomer (Kwik-Sil silicone elastomer; World Precision Instruments, Sarasota, Florida, USA), and the electrode wires were subcutaneously conducted and exteriorized in the interscapular region. Sham-operated rats underwent similar procedures without electrode placement. The electrode wires were connected to an electrical stimulator (S48 Square Pulse Stimulator; Grass Products/Natus Neurology Incorporated, Middleton, WI, USA), also connected to the digital recording system.
2.2.3 Induction of AMI
After electrode and pressure telemeter implantation, orotracheal cannulas were inserted, and the animals were mechanically ventilated using a rodent ventilator (Harvard Apparatus, Model 680). To induce AMI, the heart was exposed via the third left intercostal space, and the left anterior descending coronary artery was identified and ligated using 4–0 polyester sutures (Ethicon, Somerville, USA) [5]. The thorax was closed, and tissues were sutured. In the sham-operated groups, the heart was exposed following the same surgical procedure, but the coronary artery was not ligated.
2.3 Experimental Protocol
- Day-5: Telemeter implantation was performed for pulsatile arterial pressure recordings.
- Day 0: Animals underwent electrode implantation in CSN and AMI induction surgery.
-
Day 1 and 2:
- ○ All animals were placed on SmartPad devices for baseline hemodynamic recordings (30 min–detailed in section 2.6 and Figure 1 (item 2).
- ○ Only CSN-Stimulated and CSN-Stimulated + AMI groups received daily CSNS (3 V; 1 ms; 30 Hz; 10 min), followed by 30 min of post-stimulus recording. To reduce acute stress interference, hemodynamic data considered for analysis were obtained 2 h after the stimulation.
- ○ All animals were acclimatized to metabolic cages for 24 h prior to the final 24-h urine collection, in order to minimize stress-related alterations in renal function, as previously recommended [20].
-
Day 3:
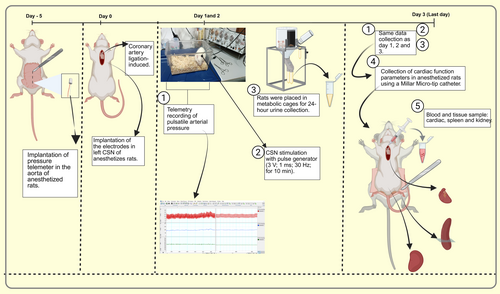
2.4 Blood and Tissue Collection
After recording the cardiac function parameters, blood was collected from the right carotid artery using a syringe, taking advantage of the incision made for catheter placement. The blood sample was centrifuged (3500 rpm, 4°C) for 10 min; plasma was collected and stored at −80°C for analysis of renal function and cytokine quantification.
The chest cavity was opened, and the heart was quickly excised and cannulated via the aorta using an 18-gauge gavage needle. The heart was perfused with ice-cold PBS and arrested in diastole by immersion in a saturated potassium chloride solution (2 mol/L). Subsequently, the heart was transversely sectioned 1 mm below the suture into two parts, perpendicular to the long axis of the left ventricle (LV). The first section was immediately fixed in 10% (w/v) paraformaldehyde for no more than 24 h and then transferred to 70% (v/v) ethanol for histological analysis. This section wascryosectioned at a thickness of 5 μm to assess the myocardial ischemic zone and myocyte apoptosis.
The second section of the heart was dried, weighed, quickly frozen in liquid nitrogen, and stored at −80°C for cytokine quantification.
The kidneys were perfused with ice-cold PBS, then dried and transversely sectioned at the medial region, and processed as follows: one part was immediately fixed in 10% (w/v) paraformaldehyde for up to 24 h, then transferred to 70% (v/v) ethanol—for histological preparation. The remaining renal tissue was similarly sectioned, weighed, frozen in liquid nitrogen, and stored at −80°C for subsequent cytokine analysis.
The spleen was divided into thirds, with one-third weighed, frozen in liquid nitrogen, and stored at-80°C for later cytokine analysis.
2.5 Myocardial Ischemic Zone
The ischemic zone was identified as a central area of tissue necrosis surrounded by viable cells with reduced levels of oxygenation, known as the risk area [21]. To assess collagen deposition, a key feature of myocardial ischemia, Picrosirius Red (PSR) staining was used for tissue analysis. The infarct size was quantified as the percentage of ischemic zone within the whole LV area. The ischemic zone [22, 23] was quantified by assessing the areas of collagen deposition, which were more intensely stained with PSR, within the LV and septum using the ImageJ program (NIH). PSR staining was validated compared with triphenyltetrazolium chloride (TTC). Although TTC failed to reveal infarcted areas 3 post-infarction PSR staining successfully identified early ischemic injury through collagen deposition. This method was adapted for the myocardial infarction model to characterize CRS1 during the 3-day post-infarction period. Tissue sections (5 μm) were digitized at 40× magnification, and all analyses were conducted blindly.
2.6 Cardiac Function
After recording the hemodynamic parameters, rats were anesthetized with Ketamine (50 mg/kg, i.p.) and Xylazine (10 mg/kg, i.p.). Cardiac function was obtained in the anesthetized rats by catheterization of the right carotid artery using a Millar Micro-tip catheter (Millar ADInstruments, model SPR-320; Bella Vista, Australia) [24]. The signal was then transmitted to an IBM/PC computer (Core 2 Duo, 2.2 GHz, 4 GB RAM) connected to an analog-digital interface (PowerLab, ADInstruments, Bella Vista, Australia). The following data were collected: change in ventricular pressure over one cardiac cycle (max-min P), HR, maximum (+dP/dT max), and minimum (−dP/dT min) dP/dT rates, left ventricular end-diastolic pressure (LVEDP), and the contractility index (CI).
2.7 Hemodynamic Parameters
Hemodynamic parameters obtained via telemetry were standardized for the CRS1 model. Pulsatile arterial pressure was recorded using SmartPads 24 h after AMI surgery. Data were obtained and analyzed at 24, 48, and 60 h across the four experimental groups. The telemetry system (gold standard) transmitted the recordings, which were amplified (ML224) and processed on an (IBM/PC Core 2 Duo computer) connected to a PowerLab analog-to-digital interface (ADInstruments, Bella Vista, Australia). Arterial pressure parameters (systolic, diastolic, and mean arterial pressure [MAP]) and HR were analyzed using LabChart 8.0 software (ADInstruments, Bella Vista, Australia).
2.8 Renal Function
Blood and urine samples were collected from the four groups over the previous 24 h to assess renal function. Quantitative analyses included osmolality, sodium excretion fraction and creatinine levels (plasma and urine), enabling the estimation of the glomerular filtration rate (GFR) [25].
Urinary and plasma osmolality were measured using an Osmometer (Fiske OS Osmometer, Norwood, MS, USA) on the basis of the freezing point, with the results expressed in mOsm/kg of water.
Sodium (Na+) concentrations in urine and plasma were determined using an electrochemical analyzer (Model 9180, Roche, Austria), and reported in mEq.
Creatinine levels in plasma and urine were quantified via a colorimetric assay using picric acid (Kit Labtest Diagnóstica S. A.).
2.9 Renal Injury
2.9.1 Determination of Kidney Injury Using Vimentin and ED-1 Markers
Immunohistochemistry was performed on kidney sections to assess renal injuries. Vimentin served as a marker for acute renal tubular injury because of its strong association with renal tubule degeneration. For tissue preparation, paraffin-embedded kidney sections from experimental groups were deparaffinized using three consecutive xylene baths, followed by rehydration through a series of alcohol baths with decreasing concentrations. Endogenous peroxidase activity was blocked by incubating the slices in a solution containing 10% (w/v) sodium azide and 30% (v/v) hydrogen peroxide for 10 min. After washing with PBS, sections were incubated with primary antibodies: anti-ED1 for macrophages (MCA341R, Bio-Rad, UK) or anti-vimentin (MUB1902P, Vector Laboratories, USA) for tubular cell injury. Following further PBS washes, a biotinylated secondary antibody (BA-9400-1.5, Vector Laboratories, USA) was applied, and color development was performed using DAB solution with nickel chloride and H2O2. Counterstaining was performed with methyl green or hematoxylin, depending on the primary antibody used.
2.9.2 Quantification of Apoptosis-Positive Cells by Terminal dUTP Nick-End Labeling (TUNEL)
Slides Were Prepared Using 7 μm-thick Sections From The Tunel Apoptosis Detection Kit, Following The Specified Protocol (apoptosis Detection Kit, Chemicon #s7107). In The Renal Cortex Area, Seven Fields Were Selected At 40× Magnification, And The Quantification Of Tunel-positive Cells For Apoptosis, Identified By Their Brown Staining, Was Performed.
2.10 Quantification of Cytokines
The quantification of the pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) was conducted using an assay kit in plasma and homogenized heart, kidney, and spleen tissues. Enzyme-linked immunosorbent assay (ELISA) using Duo Set kits from R&D Systems (Minneapolis, MN, USA) was utilized according to the manufacturer's instructions, as detailed in previous studies [26].
2.11 Statistical Analysis
The results were analyzed using Prism 8.0.2 software. Normality of each variable was assessed with the Kolmogorov–Smirnov test. As most variables exhibited a normal distribution, one-way analysis of variance (ANOVA) was used, followed by the Benjamini, Krieger, and Yekutieli test for post hoc comparisons. Parametric results are presented as mean ± standard error of the mean. Non-parametric data—including CI, CD, SD, and markers of renal apoptosis—were analyzed using the Kruskal–Wallis test and are presented as median (25th and 75th percentiles). Statistical significance was set at p < 0.05.
The figures were organized and created with the assistance of Biorender.
3 Results
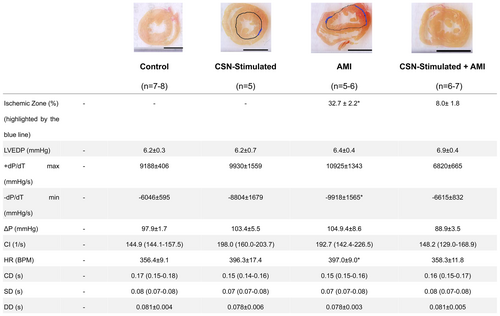
3.1 Myocardial Ischemic Zone, Cardiac Function, and Hemodynamic
Figure 2 shows that the AMI produced a remarkable ischemic zone (32.7% ± 2.2%) in the heart, illustrated by the highlighted blue line, whereas the CSNS attenuated significantly this effect, as illustrated by the reduced blue line (8.0% ± 1.8%) as well. Of note, CSNS exerted a protective effect related to AMI of the order of 75%. Besides, considering the other parameters also affected significantly by the AMI, i.e., HR (397 ± 9.0 vs. 358 ± 11.8 BPM) and −dP/dTmin (10 925 ± 1343 9 vs. 6615 ± 832 mmHg/s), the CSNS promoted a protective effect related to the AMI. Figure 2 shows that the remaining parameters, i.e., +dP/dTmax, (mmHg/s), LVEDP (mmHg), AP (mmHg), CI (l/s) CD (s), SD (s), DD (s) remained similar to the Control group after the AMI. Overall, the significant changes highlighted above were not sufficient to disturb the cardiac function, indicating its preservation after AMI within a very short period (3 days), despite the minor structural alterations. Worth mentioning, the preservation of the cardiac function after this short period (3 days) after AMI, emphasizes the beneficial effect of CSNS applied right after an AMI.
With respect to the hemodynamic results (Figure 3A) obtained at 24 h, a decrease in MAP was observed only in the CSN-Stimulated + AMI group (82.6 ± 3.2 mmHg) compared to the Control group (99.6 ± 3.6 mmHg); at 48 h, the AMI group showed an increase (97.6 ± 1.7 mmHg) relative to the Control group (89.8 ± 2.1 mmHg), whereas the CSN-Stimulated + AMI group exhibited a decrease (79.3 ± 2.0 mmHg) when compared to both the Control and AMI groups (Figure 3B). This tendency persisted and a similar result was observed at 60 h (Figure 3C). In contrast, the CSN-Stimulated group maintained MAP values similar to the Control group across all time intervals (90.1 ± 3.6 mmHg at 24 h, 90.9 ± 1.8 mmHg at 48 h, and 91.6 ± 2.2 mmHg at 60 h).
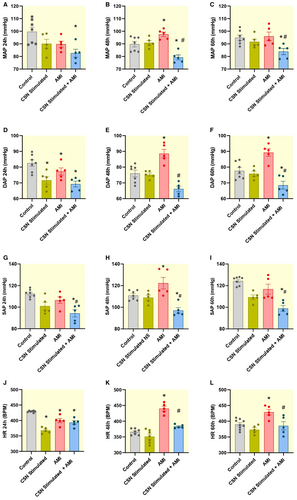
The DAP at 24 h from CSN-Stimulated + AMI (69.3 ± 1.8 mmHg), AMI (77.3 ± 2.2 mmHg) and CSN-Stimulated (71.6 ± 2.5 mmHg) groups showed a decrease compared to the Control group (82.6 ± 2.0 mmHg), as observed in Figure 3D. At 48 h, this parameter from the AMI group (88.6 ± 2.7 mmHg) showed an increase when compared to the Control group (76.0 ± 2.1 mmHg), whereas that from the CSN-Stimulated + AMI group (66.2 ± 1.7 mmHg) showed a reduction when compared to the Control and AMI groups (Figure 3E). These differences were maintained at 60 h (Figure 3F).
The SAP from the CSN-Stimulated + AMI group (94.2 ± 3.4 mmHg) showed a decrease when compared to the Control group (112.5 ± 1.5 mmHg), as observed in Figure 3G. In addition, this parameter from the CSN-Stimulated + AMI group also exhibited a reduction compared to the AMI group (106.4 ± 3.0 mmHg). At 48 h, the AMI group (122.3 ± 5.3 mmHg) displayed an increase as shown in Figure 3H, whereas the CSN-Stimulated + AMI group (97.1 ± 2.6 mmHg) showed a reduction of this parameter when compared to the Control group (110.9 ± 1.6 mmHg). At 60 h, according to Figure 3I, the CSN-Stimulated + AMI group (99.0 ± 2.7 mmHg) presented a lower SAP compared to both the Control (124.4 ± 1.5 mmHg) and AMI group (116.7 ± 4.6 mmHg). At the 24-h (101.0 ± 3.5 mmHg) and 60-h (109.3 ± 2.2 mmHg) intervals, the CSN-Stimulated group showed lower SAP than the Control group.
Concerning the HR, the CSN-Stimulated + AMI (393.2 ± 6.0 bpm), the AMI (399.5 ± 6.7 bpm) and the CSN-Stimulated groups (367.8 ± 4.2 bpm) showed a decrease in this parameter compared to the Control group (429.0 ± 1.0 bpm), as observed in Figure 3J. At 48 h, the AMI group (440.0 ± 7.6 bpm) was the only one to show an increased HR compared to both the Control (366.1 ± 3.7 bpm) and CSN-Stimulated + AMI groups (383.3 ± 2.6 bpm), as observed in Figure 3K. Similar results to the 48-h interval were observed in the 60 h interval (Figure 3M). Of note, the CSN-Stimulated group exhibited similar values compared to the Control group concerning the following parameters: MAP, DAP, SAP, and HR, at 48 h and 60 h. However, a remarkable reduction in DAP and HR was observed in the CSN-Stimulated group during the first 24 h following AMI, indicating a distinct autonomic response in this group.
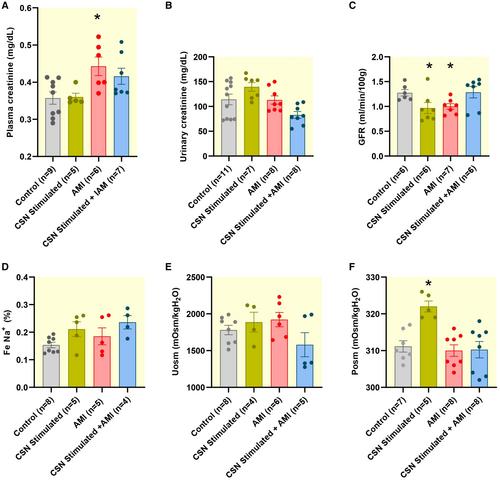
3.2 Increased Plasma Creatinine and Decreased GFR in Infarcted Animals Suggesting Renal Dysfunction
Plasma creatinine levels were increased in the AMI group (0.44 ± 0.0 mg/dL) compared to the Control group (0.36 ± 0.0 mg/dL), representing a 23.99% rise (Figure 4A). The CSN-Stimulated group exhibited a plasma creatinine level of 0.36 ± 0.0 mg/dL, whereas the CSN-Stimulated + AMI group showed a level of 0.41 ± 0.0 mg/dL, indicating similar values for these groups when compared to the Control group (Figure 4A). Additionally, as shown in Figure 4C, the GFR was 21.2% lower in the AMI group (1.0 ± 0.0 mL/min/100 g) and 23.6% lower in the CSN-Stimulated group (0.97 ± 0.1 mL/min/100 g) than in the Control group (1.27 ± 0.1 mL/min/100 g). The GFR from the CSN-Stimulated + AMI group (1.28 ± 0.1 mL/min/100 g) was similar to that from the Control group (1.27 ± 0.0 mL/min/100 g). An unexpected higher plasma osmolality was observed in the CSN-Stimulated group (Figure 4F). However, no significant changes were found concerning the other renal functional parameters, including urinary creatinine (Figure 4B), fractional sodium excretion (Figure 4D), and urinary osmolality (Figure 4E).
3.3 Renal Injury: Vimentin, Macrophages, and Apoptotic Cells
Histological and immunohistochemical analysis revealed a noticeable trend toward increased areas marked by Vimentin in the AMI, CSN-Stimulated, CSN-Stimulated + AMI groups, compared to the Control group (Figure 5A). However, although a slightly higher level was observed, it did not reach statistical significance. The histological analysis revealed similarities across all groups: Control group (Figure 5B), AMI group (Figure 5C), CSN-Stimulated group (Figure 5D), and CSN-Stimulated + AMI group (Figure 5E), with comparable levels of Vimentin-positive areas. The results for Vimentin staining were as follows: Control group, 0.35 ± 0.10; CSN-Stimulated group, 0.61 ± 0.09; AMI group, 0.50 ± 0.10; and CSN-Stimulated + AMI group, 0.51 ± 0.08. When quantifying ED-1 positive cells (macrophages) per 0.245 mm2 area (Figure 4F), only the CSN-Stimulated group presented a significantly higher level than in the Control group. The values for Control were 2.6 ± 0.2 (n = 5), CSN-Stimulated 4.2 ± 0.3 (n = 5), AMI 3.1 ± 0.3 (n = 5), and CSN-Stimulated + AMI 3.1 ± 0.4 (n = 5).
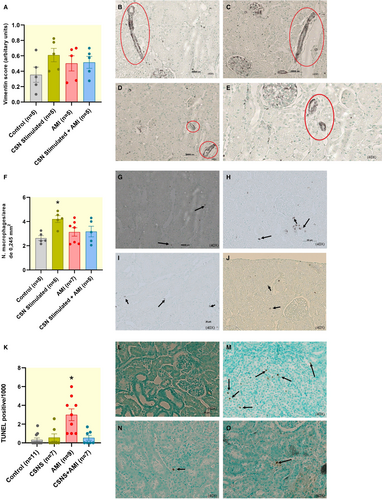
The AMI group (3.0 [1.0–4.5], Figure 5M) presented a significantly higher number of TUNEL-positive cardiomyocytes per 1000 myocytes than in the other three groups, as shown in Figure 5K. No significant differences were observed among the Control group (0.0 [0.0–0.8], Figure 5L), the CSN-Stimulated group (0.0 [0.0–1.4], Figure 5N), and the CSN-Stimulated + AMI group (0.0 [0.0–1.1], Figure 5O).
3.4 CSNS Decreased IL-6 and IL-1β Levels in the Heart After AMI
The levels of TNF-α in the heart were significantly increased in the AMI group and the CSN-Stimulated + AMI group compared to the Control group (Figure 6A), whereas the CSN-Stimulated group showed similar values compared to the Control group. In the kidney, a greater increase was observed in TNF-α in the AMI group compared to the Control group (Figure 6B). Concerning the CSN-Stimulated group and the CSN-Stimulated + AMI group, the results were not significantly different from the Control group, even though they tended to present higher levels (Figure 5B). In the spleen, TNF-α levels showed no statistical significance in the animals subjected to AMI alone compared to Control (Figure 6C). No difference was observed in the other groups either (Figure 6C). Concerning IL-6 and IL-1β levels in the heart, a reduction was observed in these cytokines in the CSN-Stimulated + AMI group compared to the Control group (Figure 6D,E, respectively). No significant changes were detected in the other groups, that is, CSN-Stimulated and AMI groups, compared to the Control group (Figure 6D,E). No expression of IL-1β and IL-6 was observed in the kidney or spleen. Similarly, no cytokine expression was observed in plasma.

4 Discussion
The current study evaluated the effects of CSNS on the early progression of CRS1 after AMI, focusing on cardiac and renal functions and inflammatory responses. The results showed that CSNS reduced the infarct area, improved hemodynamic parameters, and attenuated inflammation. In addition, CSNS reduced renal apoptosis, indicating a protective effect on the organ. This study highlights the importance of understanding the early stages that precede CRS1, enabling preventive therapies, and confirming CSNS as a promising electroceutical approach for controlling the progression of the disease.
The study successfully employed the AMI model, demonstrating its relevance for investigating early post-infarction changes. PSR staining was pivotal for precise assessment of myocardial ischemia, highlighting areas of collagen deposition critical for understanding pathological remodeling. The results revealed a reduction in the infarct size because of the CSNS, underlining its role in mitigating ischemic progression and myocardial injury. Hemodynamic analyses confirmed minimal impairment in the early stages of ischemia, aligning with previous findings that suggest a compensatory sympathetic activation [27]. This response is characterized by an increase in blood pressure (systolic, diastolic, and mean arterial pressure) and heart rate, driven by catecholamine release and peripheral vasoconstriction, aiming to preserve myocardial perfusion despite reduced coronary blood flow [28]. Furthermore, CSNS modulated inflammatory cytokines, reduced collagen deposition, and improved hemodynamic parameters, reinforcing its electroceutical potential in mitigating systemic inflammation and protecting against CRS1 progression. These findings provide valuable insights into the interplay between cardiac and renal dysfunction and highlight innovative electroceutical approaches for early intervention.
The AMI model adopted was appropriate for the purpose of the current study, especially in the early period—3 days—after AMI. Histological analysis of the kidney and heart tissues revealed structural impairment in the infarcted group. In the heart, there was a considerable area of infarction (32.7%) in relation to the total circumference of the left ventricle. In the kidney, there was an increase in cell apoptosis. In the context of AMI [29], it is common to observe the existence of tissue necrosis, surrounded by an adjacent region of viable cells; but, with reduced levels of oxygenation, known as the risk area [22]. The correlation between the tissue necrosis and the risk area increases progressively throughout ischemia, a phenomenon known as infarct expansion [22]. Infarct expansion occurs in the first few hours and peaks 72 h after AMI [30]. In the present study, an ischemic area greater than 30% was observed 3 days after AMI, and the CSNS reduced it, indicating that this electroceutical approach slowed the progression of ischemic damage, thus limiting further myocardial injury. Importantly, although CSNS induced a reduction in arterial pressure, this effect did not appear to exacerbate hemodynamic instability or compromise myocardial function. On the contrary, it was associated with a reduced infarct size and attenuation of inflammation. This suggests that the pressure-lowering effect of CSNS may reflect a modulatory influence on sympathetic overactivation rather than a detrimental hypotensive outcome. Therefore, the observed reduction in MAP should not be interpreted as harmful in this context, but rather as a consequence of autonomic and anti-inflammatory balance induced by CSNS [15, 16, 31]. This finding supports the notion that electroceutical interventions play a crucial role in controlling the progression of ischemic heart disease (IHD). A thorough understanding of their effects and the identification of suitable parameters for clinical implementation are essential [32]. In alignment with these findings, previous studies indicate that early intervention in myocardial infarction can help stabilize the injury and preserve myocardial function, suggesting that, whereas electroceutical interventions may not completely reverse the infarction, they play an important role in limiting the progression of damage and protecting cardiac function [33]. Although electroceutical interventions may not fully reverse AMI-related infarction, they play a key role in limiting ischemic damage and protecting cardiac function. In agreement with this, CSNS has been shown to induce coronary vasodilation and increase blood flow in ischemic myocardial regions, mitigating injury and improving cardiac outcomes [33].
Of note, PSR staining has proven particularly effective for evaluating the early stages of myocardial infarction, when the fibrosis is not yet fully established. This technique allows for a more accurate assessment of the infarcted area, on the basis of tissue impairment resulting from sustained ischemia. In contrast to classical methods like TTC staining, which is reliable but less sensitive in detecting smaller areas of necrosis [34], PSR highlights areas of sustained ischemia by marking excess extracellular matrix and collagen deposition. These factors are critical for characterizing post-ischemic tissue remodeling and monitoring the progression to heart failure [23]. Thus, PSR not only circumvents the limitations of traditional methods but also serves as a vital tool in studies correlating early structural alterations with functional changes in the myocardium. Its high sensitivity and specificity make it an indispensable choice for analyzing experimental infarction models.
Upon analyzing the results related to cardiac function parameters, a slight tendency toward functional impairment was observed in −dP/Dtmin and HR in the AMI group. These alterations may be associated with the maintenance of secondary cardiac function parameters during the hyperacute phase, with the HR increment likely resulting from enhanced adrenergic stimulation in the initial days post-AMI [35]. This finding suggests an early alteration in the cardiac functional balance in rats following AMI. Compared with previous studies, it is well to remember that Pfeffer [36] defined the relationship between infarct size and ventricular performance in rats, 21 days after left coronary occlusion. Rats with small myocardial infarctions (4%- 30%) showed no significant impairment in baseline cardiac parameters, peak pumping rates, or pressure generation compared to non-infarcted rats. In contrast, rats with moderate infarctions (31%–46%) exhibited normal baseline cardiac function, but reduced peak flow rates and pressure development [19, 36]. Therefore, cardiac functional impairment was only noticeable with infarct sizes greater than 46% [15]. Taking into account that the current study observed an infarct area of 32.7%, this finding is in line with the literature, indicating that there is no significant cardiac functional impairment despite the structural injury.
Hemodynamic parameters (MAP, SAP, DAP, and HR) observed within the 24-h period showed distinct variations among the four groups analyzed. These fluctuations are likely related to a necessary post-surgical adaptation period, considering that ketamine/xylazine was used as an anesthetic. According to recent studies [33], the administration of this anesthetic affects mean arterial pressure, sympathetic activity, and heart rate regulation. These findings suggest that the hemodynamic effects of ketamine and xylazine may extend beyond the anesthetic period, influencing cardiovascular parameters for up to 24 h post-exposure. This adjustment is crucial to minimize the influence of surgical stress on hemodynamic impairment. In the current study, MAP, DAP, SAP, and HR showed an increase in the AMI group, starting at 48 h and sustained at 60 h after the AMI. These changes were promising, proving that, although the increase was slight, it may indicate the beginning of hemodynamic impairment in the development of the pathophysiology of CRS1. Moreover, a previous study [37] has indicated that in post-AMI rats it is common to observe an increase in DAP, corroborating the results from the current study. Although some hemodynamic changes were identified, they were not sufficient to indicate significant hemodynamic impairment, which is in line with the study from Kato [34], who found that moderate infarctions may not affect hemodynamic parameters but cause structural changes which, in the long term, can lead to tissue injury and impairment of cardiac function.
Although more pronounced alterations in renal functional parameters are required to definitively confirm renal impairment, the observed changes suggest early functional alterations that, over time, may progress to kidney dysfunction. Significant variations in GFR and plasma creatinine levels may indicate renal impairment in rats, given the inverse relationship between plasma creatinine and GFR [38]. Both are well-established markers of renal dysfunction.
Plasma creatinine remains the standard laboratory test for assessing kidney function and estimating GFR. However, its sensitivity is limited in the early stages of kidney impairment, as subtle changes may not be immediately detectable. Although this study showed changes in plasma creatinine that compromised GFR, its values remained close to those of animals without functional alterations. For this reason, plasma creatinine alone is not specific for detecting early renal dysfunction, particularly in conditions such as cardiorenal syndrome [39]. Therefore, in order to improve the diagnostic accuracy, a comprehensive evaluation combining plasma creatinine with more sensitive biomarkers, such as NGAL, kidney injury molecule-1 (KIM-1), and quantification of apoptotic cells in renal tissue is essential. These biomarkers can detect kidney damage before significant alterations in plasma creatinine levels, underlining the need for a multifactorial approach in identifying acute kidney injury (AKI) [40].
The integration of functional and damage-specific biomarkers plays a crucial role in improving the accuracy of early detection and risk stratification in AKI. This comprehensive approach minimizes the risk of false negatives, particularly in subtle kidney dysfunction, providing a more complete understanding of renal injury [41]. In this context, renal dysfunction in CRS1 is classically associated with hemodynamic impairment, when reduced cardiac output leads to decreased renal perfusion, ultimately lowering the GFR and increasing plasma creatinine levels [42].
However, although hemodynamic alterations are central to CRS1, initial observations indicate that the timing of AKI development is a key factor in determining disease progression. Early identification of renal dysfunction is essential to prevent further complications, especially in acute decompensated heart failure (ADHF), where reduced renal blood flow contributes to worsening CRS1 [43]. Given this, therapeutic strategies aimed at restoring hemodynamics may help normalize both renal and cardiac parameters.
Beyond hemodynamics, growing evidence suggests that CRS1 pathophysiology is also influenced by neurohumoral activation and inflammation. The overactivation of the sympathetic nervous system, the RAAS, chronic inflammation, and oxidative stress imbalance contribute significantly to disease progression [44]. These factors, commonly observed in ADHF patients, have been linked to dysregulated monocyte apoptosis and increased inflammatory activity, exacerbating renal injury [43].
In particular, inflammatory mechanisms directly impact the renal tubular epithelium, a structure highly susceptible to ischemic damage [45]. The resulting apoptosis and necrosis of tubular cells lead to significant loss of epithelial integrity and function, reinforcing further the complex link between intra-renal inflammation and renal dysfunction [45]. The findings from the current study support this notion, as increased apoptotic cell death (TUNEL staining) was observed in the infarcted group, an expected consequence of ischemic injury in multiple organs [46]. The presence of apoptotic cells in renal tissue serves as an early marker of structural impairment, highlighting the importance of quantifying apoptosis to assess whether AMI-induced renal dysfunction aligns with CRS1 pathophysiology.
The consequences of ischemic injury extend beyond the heart; since systemic inflammation plays a pivotal role in CRS1 development. Elevated levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, are recognized contributors to CRS1 progression [47]. TNF-α, in particular, is a key apoptotic stimulator [48], and its increased presence in the infarcted group in the present study corroborates previous studies on inflammation-driven renal injury [6]. Notably, CRS1 patients exhibit significantly higher cytokine concentrations than healthy individuals [49], whereas experimental models confirm a rapid elevation of IL-6 and TNF-α post-AMI [6, 8]. Similarly, the current study identified increased TNF-α levels in both cardiac and renal tissues, alongside a trend toward higher IL-6 levels in cardiac tissue 3 days after AMI.
With respect to the potential mechanisms from the CSNS playing a favorable role in early cardiac and renal dysfunction in CRS1 and the possible role of respiratory changes as well, are highlighted in Figure 7, which displays briefly the central (Medulla) and afferent branches of the autonomic nervous system (sympathetic and parasympathetic) involved in baroreflex activation (inhibiting sympathetic fibers and stimulating the parasympathetic fibers) and chemoreflex activation (stimulating parasympathetic and sympathetic fibers and respiratory movements) [50, 51].
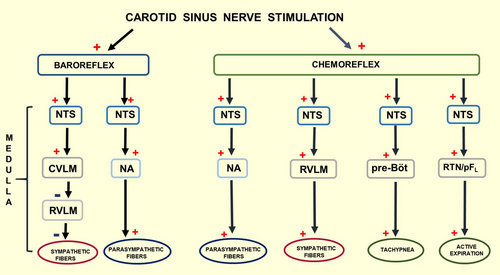
A previous study from our laboratory [52] demonstrated that CSNS, in conscious rats, activates both the carotid baroreflex and the carotid chemoreflex, driving an increase in ventilation and a decrease in arterial pressure. Notably, respiratory changes were not evaluated in the current study. However, this approach certainly warrants further investigation regarding the CSNS's potential favorable role in early cardiac and renal dysfunction in CRS1.
Besides, Tracey's Group [53, 54] provided a remarkable contribution to the literature proposing the existence of the inflammatory reflex associated with the autonomic nervous system, focusing on the parasympathetic efferences for controlling the inflammatory responses. Later on, in contrast to this original concept of the inflammatory reflex, Davide Martelli's group [55], proposed that the efferent arm of the inflammatory reflex involves the splanchnic sympathetic nerves, not the vagus as previously proposed by Tracey's group [53, 54]. Notably, despite these dissimilar mechanisms highlighted above, that is, parasympathetic versus sympathetic afferences, the complexity of the mechanisms involved in the neural regulation of inflammatory responses is centered on the autonomic nervous system (sympathetic and parasympathetic branches).
Thus, these concepts focused on the autonomic nervous system (sympathetic and parasympathetic branches) provided an excellent opportunity for our laboratory to investigate the role played by the parasympathetic and sympathetic branches involved in the CSNS in some experimental protocols, for instance, innate immune response to lipopolysaccharide [31], experimental colitis [56], and experimental periodontitis [15, 16].
CSNS plays a crucial role in modulating the innate immune response, as it simultaneously activates the baroreflex and chemoreflex [31]. Although the baroreflex inhibits sympathetic activity, the chemoreflex concurrently stimulates both autonomic branches [50]. This autonomic interplay fosters a strong anti-inflammatory response, significantly reducing pro-inflammatory cytokines such as IL-6 and IL-1β [16]. In the context of systemic inflammation, CSNS has demonstrated efficacy in mitigating inflammatory responses, as evidenced by reduced IL-6 and IL-1β levels in the heart [15]. Prior studies from our laboratory have shown that CSNS effectively attenuates systemic and local inflammation in experimental periodontitis in rats, reinforcing its broader immunomodulatory potential. Furthermore, in the AMI-induced CRS1 model, CSNS exhibited protective effects by reducing collagen deposition in cardiomyocytes, lowering inflammatory cytokines, and improving hemodynamic parameters. The observed reductions in diastolic, systolic, and mean arterial pressure at different time points further highlight the electroceutical potential of CSNS in systemic inflammatory conditions.
5 Conclusion
The findings from this investigation provide crucial insights into the early stages of CRS1 following AMI, demonstrating the utility of the CRS1 model in assessing both cardiac and renal dysfunction. Moreover, electrical CSNS was shown to reduce the infarct size, improve hemodynamic parameters, and mitigate inflammatory responses and renal apoptosis. These results underscore the potential of CSNS as an electroceutical strategy for the early stages of CRS1. Unlike studies focused on established CRS1, this research highlights the significance of identifying and intervening in the initial phases of the syndrome, paving the way for preventive therapies to counteract its progression.
Author Contributions
Rollssman de Oliveira Cavalheiro: conceptualization, writing – original draft, investigation, validation, visualization, methodology, writing – review and editing, project administration, formal analysis, software, data curation. Fernanda Brognara: formal analysis, validation, methodology, writing – review and editing. Carlos Alberto Aguiar da Silva: methodology. Jaci Airton Castania: methodology. Carlos Augusto Fernandes Molina: formal analysis, visualization. David Murphy: writing – review and editing, visualization, formal analysis. Minna Moreira Dias Romano: writing – review and editing, conceptualization, formal analysis. Helio Cesar Salgado: funding acquisition, investigation, visualization, writing – review and editing, supervision, resources, formal analysis, validation, conceptualization.
Acknowledgments
The authors thank Dr. Helio Cesar Salgado for his invaluable guidance and support throughout this study. Appreciation is also extended to the technical staff for their skilled assistance in the experimental procedures. The emotional support provided by companion animals during demanding periods is gratefully acknowledged. The authors also acknowledge the contributions of fellow graduate students from the laboratory and the department for their collaboration and academic engagement. This research was funded by FAPESP (São Paulo Research Foundation, Process # 2020/06043-7, and F.B. by Process #2021/03764-8.), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Process 423999/2021-4), and the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)—Brasil—PROEX for R.O.C—Financial Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




