Loss of ceramide synthase 5 inhibits the development of experimentally induced aortic valve stenosis
Abstract
Aim
Inflammation and calcification are hallmarks in the development of aortic valve stenosis (AVS). Ceramides mediate inflammation and calcification in the vascular tissue. The highly abundant d18:1,16:0 ceramide (C16) has been linked to increased cardiovascular mortality and obesity. In this study, we investigate the role of ceramide synthase 5 (CerS5), a critical enzyme for C16 ceramide synthesis, in the development of AVS, particularly in conjunction with a high-fat/high-cholesterol diet (Western diet, WD).
Methods
We used wild-type (WT) and CerS5−/− mice on WD or normal chow in a wire injury model. We measured the peak velocity to determine AVS development and performed histological analysis of the aortic valve area, immune cell infiltration (CD68 staining), and calcification (von Kossa). In vitro experiments involved measuring the calcification of human aortic valvular interstitial cells (VICs) and evaluating cytokine release from THP-1 cells, a human leukemia monocytic-like cell line, following CerS5 knockdown.
Results
CerS5−/− mice showed a reduced peak velocity compared to WT only in the experiment with WD. Likewise, we observed reduced immune cell infiltration and calcification in the aortic valve of CerS5−/− mice, but only on WD. In vitro, calcification was reduced after knockdown of CerS5 in VICs, while THP-1 cells exhibited a decreased inflammatory response following CerS5 knockdown.
Conclusion
We conclude that CerS5 is an important mediator for the development of AVS in mice on WD and regulates critical pathophysiological hallmarks of AVS formation. CerS5 is therefore an interesting target for pharmacological therapy and merits further investigation.
1 INTRODUCTION
Aortic valve stenosis (AVS) is the most common and most deadly valvular heart disease in humans, with a prevalence of 2.8% in patients above 75 years of age. AVS is the cause of considerable mortality, with a two-year mortality rate of severe AVS of around 50% for symptomatic patients.1, 2 Until now, no pharmacological treatment of AVS has been available, leaving the replacement of the aortic valve (AV) the only treatment option. AVS development is an inflammatory process that involves immune cell infiltration and calcification of valvular interstitial cells.3, 4 Well established risk factors for the development of AVS are, among others, obesity and insulin resistance.5, 6
Ceramides are biologically active sphingolipids that are produced in a complex network of enzymes. The main regulators of ceramide synthesis are sphingomyelinases and ceramide synthases (CerS). Herein, the different isoenzymes of CerS produce ceramides with specific acyl lengths in the de-novo-synthesis pathway.7-10 CerS5 and CerS6 predominantly produce C16 ceramide, which has been associated with adverse cardiovascular outcomes in observational studies.11 Ceramides have been linked to diet-induced obesity and insulin resistance.12-15 Loss of CerS5 function has been shown to reduce diet-induced obesity in mice.14 In the context of atherosclerotic plaque formation, the global inhibition of ceramide production has been shown to be effective in the regulation of inflammation.16-19 In addition, it has been suggested that ceramides play a critical role in calcification, a hallmark of AVS development.20
However, to date, it remains unclear if the ceramide metabolism influences the development of AVS and how dietary factors interfere with AVS development in ceramide-deficient organisms. This is particularly relevant as ceramides mediate inflammation and diet-related effects, which are thought to promote AVS development independently.5, 21, 22 In this article, we describe the effect of a loss of CerS5 function in combination with high-fat/high-cholesterol diet (Western diet, WD) on AVS development. We show that loss of CerS5 function reduces the inflammation of the AV and the development of AVS in mice with WD, but not with normal chow. We also find CerS5 to drive calcification of valvular interstitial cells (VIC).
2 METHODS
2.1 Animals and wire-injury
CerS5 constitutional knockout (CerS5−/−) mice were generated by Gosejacob et al. on a C57BL/6-J background.14 C57BL/6-J wild type (WT) mice were purchased from Janvier Labs. For the experiments, 14–16 –week-old mice were kept at 22° in a room with 12-h-light/dark cycle and fed with a high-fat/high-cholesterol diet (Western diet, WD) containing 21% fat and 0.25% cholesterol (Sniff Cat# E15721-347) or with normal chow ad libitum. The WD diet was started 3 days before the wire injury. For the induction of AVS development, we used the wire injury model, originally published by Honda et al.23 A wire injury of the AV was performed according to the protocol of Niepmann et al.24 Under echocardiographic control, a coronary angiography wire with a 15° angled tip was inserted into the left ventricle, pushed back and forth 20 times over the AV, and then rotated 200 times on the AV level. All animal experiments were performed according to the German animal protection law and were approved by the National Office for Nature, Environment and Consumer Protection in Recklinghausen, North Rhine-Westphalia.
2.2 Echocardiography
Echocardiography was performed as previously reported.24 Mice were anesthetized with 1.5% isoflurane. For echocardiographic analysis, a Fujifilm Visualsonics Vevo 2100 Ultra High Frequency Imaging Platform was used. The left ventricular ejection fraction was measured in parasternal long-axis views with the Vevo LV-Trace function. AV peak velocity was measured in suprasternal view using a pulse-wave doppler. The development of AV regurgitation was monitored and only mild regurgitation was detected.
2.3 Histology and Immunofluorescence
Forty-two days after the wire injury, the mice were sacrificed for histological analysis. The mice were anesthetized with ketamine and xylazine, followed by the collection of blood samples. The heart was flushed with a 0.9% sodium chloride solution, embedded in tissue freezing medium, and sectioned at 8 μm. H&E staining was performed to measure the AV area, as previously reported.24 For von Kossa staining to detect calcification, the slides were incubated for 30 min in 2% silver nitrate (Sigma, Cat# S6506-25G), followed by washing with tap water, 5 min incubation with sodium thiosulfate (Sigma, Cat# 217263-250G) and counterstaining with nuclear fast red-aluminum solution 0.1% (Millipore Sigma, Cat# 1001210500) for 3 min. Anti-CD68 staining to detect macrophage infiltration was performed by fixation of the slides for 20 min in −20° acetone, washing in PBS, blocking the slides with 2% BSA in PBS for 30 min, followed incubation with the primary antibody (rat anti-mouse, antibodies online, Cat# ABIN 181836) 1:100 diluted in 2% BSA in PBS at 4° overnight. After washing, the slides were incubated with the secondary antibody (Cy3, AffiniPure donkey anti-rat, Jackson ImmunoResearch, Cat#712-165-153) 1:500 diluted in 2% BSA in PBS for 1 h at room temperature. Images were taken with an Axio observer microscope (Zeiss, Germany) at 10X magnification. Von Kossa positive area and Anti-CD68 positive area were automatically quantified by Zen3.2 pro software. Using von Kossa staining, AV pigmentation can be mistaken, as calcium. To assure that this does not distort our results, we measured the AV pigmentation and found a low pigmentation that is similar between the groups.
2.4 Cell culture and siRNA transfection
All cells were purchased by Lonza and kept under 37°, 100% humidity and 5% CO2. For VICs (human valvular interstitial cells), DMEM, low glucose, GlutaMAX Supplement, and pyruvate (Thermo Fisher Scientific, Cat# 21885108) containing 10% Fetal Bovine Serum (FBS) with 1% Penicillin/Streptomycin (P/S) were used. For knockdown of CerS5 and CerS6, CerS5 siRNA (Invitrogen, Cat# AM16708, Assay ID 131807), CerS6 siRNA (Invitrogen, Cat# AM16708, Assay ID 149484), and silencer negative control No.1 siRNA (Invitrogen, Cat# AM4611) were used at a final concentration of 10 nM. The knockdown was performed with antibiotic-free medium, OPTI-MEM reduced serum medium (Gibco, 31 985-062), and Lipofectamine RNAiMAX Transfection reagent (Invitrogen, Cat# 13778150) at a final concentration of 3 μL/mL. The efficacy of the knockdown was confirmed using quantitative real-time PCR (rt PCR).
2.5 RNA-isolation and rt PCR
For rt PCR, RNA was isolated with Trizol (Invitrogen, Cat# 15596026) and chloroform. Cells were washed and then lysed with Trizol. Isolation was performed with chloroform and precipitation with isopropanol 1:1, followed by washing with 75% ethanol and resuspending it in water. Concentrations were assessed with a Nanodrop 2000 (Thermo Fisher Scientific). Reverse transcription of 0.5 μg RNA was performed using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Cat# 43-688-13) with RNase Inhibitor (Thermo Fisher Scientific, Cat# N8080119) following the manufacturer's protocol. For rt PCR, cDNA was diluted to 1 ng/μL with water. And then 6.75 μL of cDNA with 8.25 μL of Taqman Gene Expression Master Mix (Applied Biosystems, Cat# 4440040) and the Taqman probes for RUNX-2, CerS5, CerS6, and GAPDH (all from Thermo Fisher Scientific: RUNX-2 Cat# 4351370, Hs01047973_m1, CerS5 Cat# 4448892, Hs00332291_m1, CerS6 Cat# 4448892, Hs00826756_m1, GAPDH Cat# 4351368, Hs02758991_g1) were used to perform rt PCR. A QuantStudio 5 (Thermo Fisher Scientific) instrument was used. GAPDH served as internal control and relative expression was calculated as 2^(-ddCT).
2.6 THP-1 ELISA
THP-1 cells are a widely used human monocyte-like cell line, isolated from a patient with acute monocytic leukemia.25 To mimic high-fat/high-cholesterol diet, THP-1 cells were stimulated with a lipid mixture (LM) (Sigma, L0288, diluted 1:200) containing 10 μg/mL each of linoleic, linolenic, myristic, oleic, palmitic, and stearic fatty acids, 2 μg/mL arachidonic fatty acids, and 0.22 mg/mL cholesterol.26, 27 Cells were stimulated with a lipid mixture or control for 12 h, followed by stimulation with 50 ng/mL LPS (LPS from E. coli, 0111:B4, Sigma, L2630) for 24 h.28, 29 TNF-α and IL-1β concentrations were measured in THP-1 cell supernatants using the DuoSet ELISA kit for IL-1β (Human IL-1b/IL-1F2, R&D Systems, #DY201-05) and TNF-α (Human TNFa, R&D Systems, # DY210-05) with the TMB Substrate reagent set (BioLegend, #421101) following the manufacturer's protocol. Absorption was measured in a TECAN, Infinite M Plex, at a wavelength of 450 nm and with a reference wavelength of 570 nm. 4-PL analysis was performed in GraphPad Prism10. Cell viability was measured with an MTT cell growth kit (Merck, #CT02).
2.7 In vitro calcification
Human valvular interstitial cells (VICs) were isolated from Lonza upon individual request. At least three independent donors were used per experiment. In vitro calcification experiments with VICs were incubated with pro-calcifying medium (PCM) for 7 or 21 days, refreshed three times per week. PCM contains DMEM with 5% FBS, 2 mmol/L NaH2PO4 (pH 7.4), and 50 μg/mL L-ascorbic acid.30 For calcium staining in vitro, alizarin red (Sigma Cat# A5533-25G) was dissolved in distilled water to generate a 2% solution with an adjusted PH of 4.2. The cells were fixed with 4% formaldehyde solution (EMD Millipore Cat# 1.00496.5000) for 30 min at 4°, followed by washing the cells with 200 μL distilled water, and incubation with 100 μL alizarin red solution for 30 min at room temperature. After this, the cells were washed again two times with distilled water and alizarin red was detected in an Axio observer microscope (Zeiss, Germany). Quantification was carried out by dissolving Alizarin staining in a 100 mmol/L Hexadecylpyridinium chloride monohydrate (Sigma Cat# C9002) aqueous solution. The solution was transferred to a 48-well plate, and absorption was measured in a TECAN, Infinite M Plex at a wavelength of 540 nm. The absorption was normalized using the control wells with PCM treatment only.
2.8 Statistical analysis
For all statistical analyses, Graph Pad Prism10 was used. All data are represented as mean ± standard error of mean (SEM). For statistical analysis of two groups, an unpaired t-test was performed; for more than two groups, a one-way ANOVA followed by Tukey's multiple comparisons test was performed. For time-course experiments, a two-way ANOVA followed by Tukey's multiple comparisons test was performed. All p values are two-sided.
3 RESULTS
3.1 CerS5−/− reduces echocardiographic development of AVS in mice on WD
To evaluate the effect of loss of CerS5 function on the development of AVS in mice on WD, an echocardiographic analysis was performed before, 14, 28, and 42 days after wire injury (Figure 1). Two weeks after wire injury, peak velocity was significantly increased in WT and CerS5−/− mice compared to baseline. CerS5−/− mice showed a significantly lower peak velocity at 28 and 42 days after injury in comparison to WT mice. In CerS5−/− mice, the effect of the wire injury was completely abolished after 42 days, as the peak velocity returned to baseline. (Figure 2A). Left ventricular function, evaluated by ejection fraction, did not change in both groups after wire injury and showed no difference between CerS5−/− and WT (Figure 2B). The corrected left ventricular mass increased only in WT mice after wire injury, but without difference between WT and CerS5−/− mice 42 days after wire injury (Figure 2B). The WD resulted in a body weight increase of around 20% without a significant difference between CerS5−/− and WT mice (Figure S1).
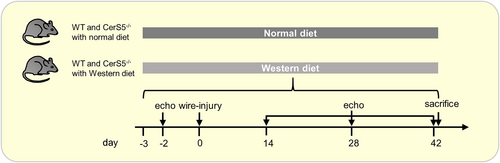
3.2 CerS5−/− counteracts inflammation and calcification of the AV in mice with WD
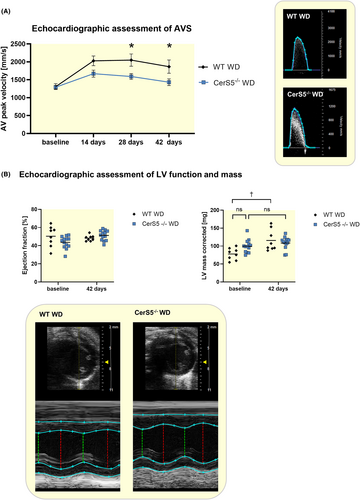
Histological staining against CD68 showed that CerS5−/− exhibits significantly lower levels of macrophage infiltration in the AV after wire injury (Figure 3A). Von Kossa staining revealed a lower amount of calcification of the AV of CerS5−/− mice (Figure 3B). AV thickness and fibrosis, stained with Sirius red, did not show any difference between WT and CerS5−/− (Figure 3C + D).
3.3 CerS5−/− has no effect on AVS development in mice with normal chow
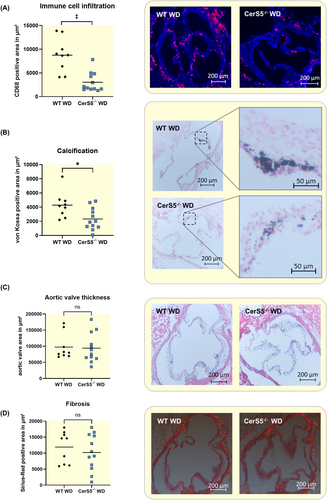
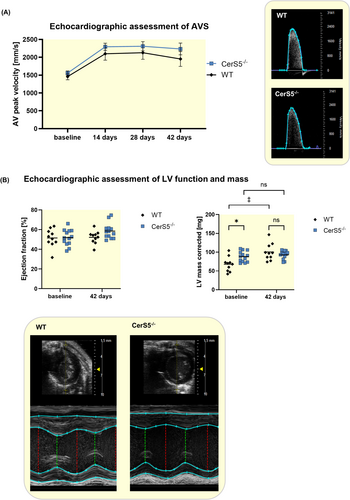
In order to evaluate whether the effect of reduced AVS development in CerS5−/− mice is dependent on WD, the same experiments were also performed with normal chow. In this experiment, the peak velocity exhibited no significant difference between CerS5−/− and WT (Figure 4A). Again, neither wire injury nor CerS5−/− significantly affected the left ventricular function (Figure 4B). At baseline, the corrected left ventricular mass was higher in CerS5−/− mice compared to WT. Forty-two days after wire injury, the corrected left ventricular mass increased in WT but not in CerS5−/− mice, without differences between WT and CerS5−/− (Figure 4B). Histological assessment of the AV using anti-CD68 staining revealed no difference in macrophage infiltration between both groups (Figure 5A). Moreover, von Kossa staining showed comparable amounts of AV calcification in WT and CerS5−/− mice (Figure 5B). AV area and fibrosis were not significantly different between CerS5−/− and WT animals (Figure 5C + D).
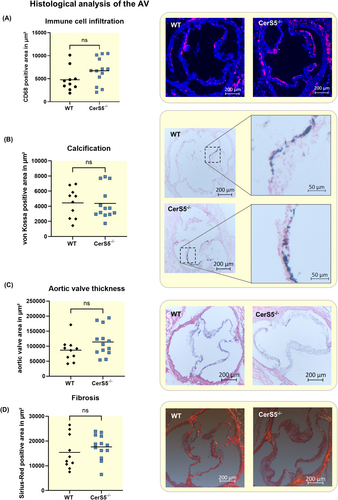
3.4 Knockdown of CerS5 reduces calcification in vitro
To analyze calcification in VICs, the cells were treated with pro-calcifying medium for 7 or 21 days. After stimulation of VICS with pro-calcifying medium for 7 days, CerS5 was slightly upregulated and CerS6 was strongly upregulated (Figure 6A). Stimulation for 7 days with pro-calcifying medium led to an upregulation of RUNX-2, a promotor of AV calcification, while this effect was not present after 21 days of stimulation (Figure 6B). In order to analyze the effect of CerS5 and CerS6 on the calcification of VICs, the cells were treated with RNAi for 24 h and consecutively with PCM for 7 or 21 days. The downregulation was shown to be stable for at least 1 week (Figure S3). Alizarin red staining of the VICs revealed that CerS6 KD and CerS5 KD alone, as well as the combination of both, reduced the calcification of VICs after treatment with pro-calcifying medium after 7 and 21 days of stimulation (Figure 6C).
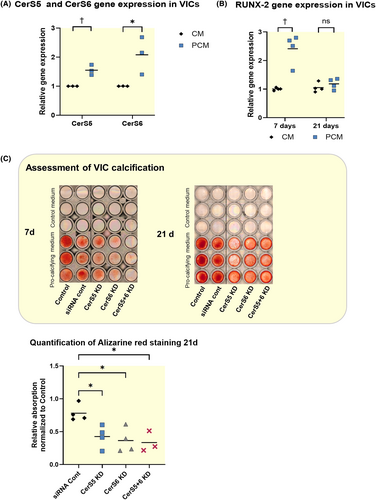
3.5 Lipids and knockdown of CerS5 reduce TNF-α and IL-1β secretion in THP-1
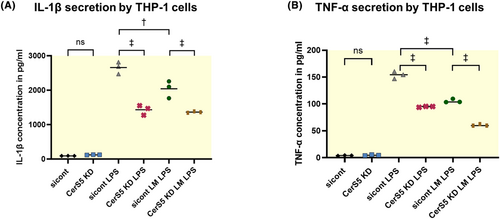
In order to further investigate potential mechanisms how CerS5 is involved in immune cell activation as observed in murine aortic valves, we performed in vitro experiments with THP-1 cells. TNF-α and IL-1β are important cytokines that regulate inflammation. To stimulate IL-1β and TNF-α secretion, THP-1 cells were stimulated with 50 ng/mL LPS.28, 29 A high-fat/high-cholesterol diet was mimicked in vitro by the use of a lipid mixture (LM), as previously reported.26, 27 THP-1 cells were pre-incubated with LM for 12 h before LPS treatment for 24 h (LPS + LM) (Figure 7A + B). In all groups, CerS5 KD strongly reduces IL-1β and TNF-α secretion when cells are stimulated with LPS. Interestingly, the lipid mixture attenuated IL-1β and TNF-α secretion. The MTT assay for viability shows no reduced viability of cells treated with LM (Figure S4B).
3.6 CerS5 knockout does not lead to an CerS2 or CerS6 upregulation
Additionally, we investigated if loss of CerS5 would lead to a compensatory upregulation of either CerS6 or CerS2. Neither in the aortic tissue of CerS5−/− mice on WD or normal chow nor in vitro, a significant upregulation of CerS2 or CerS6 was detected (Figure S2).
4 DISCUSSION
In the present manuscript, we show that the loss of CerS5 reduces the development of AVS in animals on WD. Ceramides have been shown to be involved in cardiovascular disease and obesity, but their impact on AVS has not been investigated.13, 15
The impact of ceramides on obesity and lipid metabolism is well studied.14, 31, 32 It has been shown that CerS5 is essential to maintain systemic C16 ceramide levels in mice.14 CerS5−/− mice are partially protected from an increase in ceramide serum levels due to high-fat diet.14
Interestingly, in our experiments, we did not detect a difference in the body weight between CerS5−/− and WT animals on WD. This could be explained by the shorter observation time of only 6 weeks, where the mice showed only moderate weight gain. Our study revealed anti-inflammatory effects of CerS5−/− in mice on WD, which were not present in mice with normal chow. This effect appears to be independent of body weight.
Inflammation is described as a hallmark of early AVS development.3, 4 In other non-cardiovascular contexts, C16 functions as a modulator of inflammation.8 Previous work indicates that in CerS5-deficient animals high-fat diet might induce an anti-inflammatory phenotype similar to our observations in the aortic valve.14 Likewise, we found a strongly reduced valvular immune cell infiltration in CerS5−/− mice on WD compared to WT mice on WD. In murine macrophages, the impact of lipid excess on ceramide-regulated inflammation has already been studied: Schilling et al. described how lipopolysaccharide, only together with the saturated fatty acid palmitate, leads to a C16 ceramide production of the macrophages, which then leads to an increased inflammatory response of these macrophages.18 This mechanism could explain why only CerS5−/− mice on WD, in comparison to mice on normal chow, showed less valvular inflammation than WT. Here, for the first time, we show that loss of a ceramide synthase in combination with WD can reduce the inflammatory response to an injury to the AV in vivo. As described for macrophages, we also found this effect to be dependent on the high presence of fat.
Our experiments with THP-1 cells were performed with a lipid mixture containing cholesterol, saturated, and unsaturated fatty acids in order to simulate the situation in our animals on WD as closely as possible. We detect reduced secretion of TNF-α and IL-1β from monocytes after treatment with fatty acids and LPS. There are previous reports that lipids activate pro-inflammatory ceramide producing pathways.18, 33, 34 In line with our in vivo experiments, CerS5 KD strongly reduced the inflammatory response of monocytes, independent of lipid excess. While it has been reported that inhibition of neutral sphingomyelinase 2, another ceramide-producing enzyme, reduces the inflammatory response, these effects have not yet been described for CerS5.16 The anti-inflammatory effect of loss of CerS5 function was not abrogated but at least partially enhanced by additional lipid supply. The stronger anti-inflammatory effect of a loss of CerS5 function on monocytes in the presence of excess lipids may explain why only the combination of WD and CerS5 knockout leads to a significant reduction in aortic valve inflammation and stenosis formation in our in vivo model.
Calcification of the AV is a fundamental mechanism in the development of AVS.4, 35 In general, ceramides influence calcification, but the specific role of CerS5 has not been investigated yet.20, 31, 36 Our work shows that inhibition of CerS5 leads to reduced calcification of VICs in vitro. Moreover, we showed a reduced calcification of the AV in CerS5−/− mice with WD.
As previous studies have shown a certain amount of cross-compensation of different CerS isoenzymes, we hypothesized that loss of CerS5 function could lead to an increased expression of CerS6, which also produces C16 ceramide, or CerS2, which would produce ceramides with longer acyl chains that exert anti-inflammatory properties.10, 37 This could have explained the anti-inflammatory effect of the loss of CerS5 function under WD.37 In our models, compensation for the loss of CerS5 by upregulation of CerS6 or CerS2 was not observed and does not seem to be a relevant mechanism to explain this effect.
In our experiments, we found a reduction in AVS development only in mice on WD. It appears that loss of CerS5 protects from an enhanced inflammation caused by WD. However, our experiments show that the AV inflammation in CerS5−/− mice on WD is even lower than in WT mice with normal chow. Therefore, it can be speculated that loss of CerS5 function in combination with WD has anti-inflammatory effects with yet-unknown mechanisms. Our THP-1 experiments suggest anti-inflammatory effects of downregulation of CerS5 in combination with lipid excess, which could explain why inflammation in CerS5−/− mice on WD is even lower than in WT mice with normal chow.
THP-1 cells are a well-established model cell line for human monocytes, and as their overall inflammatory responses are similar to those of circulating monocytic cells, they are representative of human inflammatory cells. However, there are some limitations, as the LPS responses of THP-1 cells are substantially lower compared to other peripherally circulating monocytic cells.16, 25, 38
The human AVS pathophysiology and AV anatomy differ from those of mice. Mice do not have the trilayer morphology of human, porcine, or rabbit aortic valves.4, 39 In addition, C57BL/6-J wild-type mice do not develop aortic valve calcification with age.39 Yet, the murine wire injury model is well established and reflects the hallmarks of human AVS development, like inflammation, calcification, and accelerated aortic valve flow.24 In contrast to other AVS models, the wire injury model does not require dietary intervention or extreme hypercholesterolemia.40 To date, it is unknown how the findings of the murine wire injury model translate to humans.
In our model, we showed both a lower infiltration of macrophages in the AV of mice on WD as well as a direct reduction of calcification related to the loss of CerS5. This was mirrored by an abrogation of AVS development in CerS5−/− mice on WD.
In summary, our work highlights the importance of ceramides in the development of AVS in mice on a high-fat/high-cholesterol diet by influencing inflammatory processes. Although the underlying mechanism of these effects is only incompletely understood, CerS5 could serve as an interesting target to counteract AVS development.
AUTHOR CONTRIBUTIONS
Laurine Reese: Investigation; methodology; writing – original draft; formal analysis; visualization; data curation; conceptualization; validation. Sven Thomas Niepmann: Writing – review and editing; conceptualization; methodology; investigation; visualization. Philip Düsing: Writing – review and editing; investigation; methodology; data curation. Lea Hänschke: Methodology; writing – review and editing; resources. Thomas Beiert: Writing – review and editing; data curation; formal analysis. Sebastian Zimmer: Writing – review and editing; investigation; validation; conceptualization; resources. Georg Nickenig: Writing – review and editing; funding acquisition; supervision; project administration; resources. Reinhard Bauer: Writing – review and editing; methodology. Felix Jansen: Writing – review and editing; funding acquisition; conceptualization; project administration; supervision; resources. Andreas Zietzer: Project administration; formal analysis; supervision; data curation; writing – review and editing; conceptualization; investigation; funding acquisition; methodology; visualization; validation; resources.
ACKNOWLEDGEMENTS
Open Access funding enabled and organized by Projekt DEAL. We thank Paula Levermann, Anna Flender, Pia Fuchs, Henning Panatzek, Gloria Martinac for excellent technichal support. We further thank Sandra Adler and Marta Stei for coordinating and supervising the animal experiments.
FUNDING INFORMATION
This work was supported by the medical faculty of the University of Bonn (BONFOR grant no. 2020-2A-05 to A.Z.), the German Cardiac Society (DGK16/2018 to A.Z.), the Ernst & Berta Grimmke Foundation (13/19 to A.Z.), the Deutsche Forschungsgemeinschaft (Project-ID 397484323—TRR 259 to L.R., F.J., S.Z., G.N., Project-ID 514976099 to A.Z.), and the Corona-Foundation (to F.J.).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available on request.




