Lipopolysaccharide exacerbates depressive-like behaviors in obese rats through complement C1q-mediated synaptic elimination by microglia
Titikorn Chunchai and Thirathada Chinchapo have contributed equally to this study.
Abstract
Aim
Prolonged high-fat diet (HFD) consumption has been shown to impair cognition and depression. The combined effects of HFD and lipopolysaccharide (LPS) administration on those outcomes have never been thoroughly investigated. This study investigated the effects of LPS, HFD consumption, and a combination of both conditions on microglial dysfunction, microglial morphological alterations, synaptic loss, cognitive dysfunction, and depressive-like behaviors.
Methods
Sixty-four male Wistar rats were fed either a normal diet (ND) or HFD for 12 weeks, followed by single dose-subcutaneous injection of either vehicle or LPS. Then, cognitive function and depressive-like behaviors were assessed. Then, rats were euthanized, and the whole brain, hippocampus, and spleen were collected for further investigation, including western blot analysis, qRT-PCR, immunofluorescence staining, and brain metabolome determination.
Results
HFD-fed rats developed obese characteristics. Both HFD-fed rats with vehicle and ND-fed rats with LPS increased cholesterol and serum LPS levels, which were exacerbated in HFD-fed rats with LPS. HFD consumption, but not LPS injection, caused oxidative stress, blood–brain barrier disruption, and decreased neurogenesis. Both HFD and LPS administration triggered an increase in inflammatory genes on microglia and astrocytes, increased c1q colocalization with microglia, and increased dendritic spine loss, which were exacerbated in the combined conditions. Both HFD and LPS altered neurotransmitters and disrupted brain metabolism. Interestingly, HFD consumption, but not LPS, induced cognitive decline, whereas both conditions individually induced depressive-like behaviors, which were exacerbated in the combined conditions.
Conclusions
Our findings suggest that LPS aggravates metabolic disturbances, neuroinflammation, microglial synaptic engulfment, and depressive-like behaviors in obese rats.
1 INTRODUCTION
Obesity has become a major global public health concern, primarily caused by the chronic consumption of a high-fat diet (HFD).1, 2 HFD consumption promotes inflammation, increases oxidative stress, impairs blood–brain barrier (BBB) integrity, and decreases neurogenesis.3-6 Chronic HFD consumption also increased gut dysbiosis, which led to an increase in gut permeability and Gram-negative LPS-containing bacteria.7 This, in turn, resulted in elevated levels of LPS in the serum, leading to chronic systemic inflammation.7 Furthermore, HFD-induced obesity has been associated with changes in brain structures and functions, leading to cognitive impairments and neuropsychiatric disorders such as anxiety and depression.8, 9
Lipopolysaccharide (LPS), a gram-negative bacterial cell wall component, activates the TLR4-MD2 complex, leading to the production of pro-inflammatory cytokines.10 Previous studies demonstrated that LPS administration caused brain pathologies,11-13 led to alterations in brain structures and functions,14 and contributed to cognitive deficits and neuropsychiatric conditions including anxiety and depression.15 Microglia are the resident immune cells in the brain that play crucial roles in normal brain functions.16 In recent years, “primed microglia” have emerged as a result of microglial alterations in response to stimuli exposure. These microglia also exhibit a hyperresponse to subsequent stimuli. Previous studies demonstrated that a single dose of intraperitoneal LPS injection developed innate immune memory (IIM) and long-lasting primed microglia, which enhanced and amplified microglial responses to a second inflammatory insult.17, 18 Our previous studies reported that chronic HFD consumption altered the morphology and function of microglia.4, 5, 19 However, the effect of HFD on microglial priming has not been elucidated. Microglia also participate in synaptic pruning via the complement component C1q during brain development.20 However, the synaptic pruning by microglia in LPS-stimulated HFD-primed conditions has never been investigated.
Despite the recognized effects of HFD and LPS on metabolic and immune functions, their combined impact on systemic and brain inflammation and associated mechanisms remain insufficiently understood. This study aims to investigate the comprehensive effects of long-term HFD consumption and single LPS administration on metabolic parameters, inflammation, immune activation, neurogenesis, cognition, and depressive-like behaviors in rats. We hypothesized that (1) both LPS and long-term HFD consumption induce microglial dysfunction, microglial morphological alterations, synaptic loss, cognitive dysfunction, and depressive-like behaviors, and (2) LPS injection following long-term HFD consumption-induced microglial priming aggravates microglial dysfunction, microglial morphological alteration, synaptic loss, cognitive dysfunction, and depressive-like behaviors.
2 RESULTS
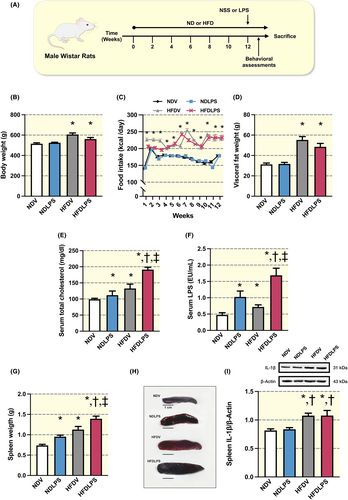
2.1 Long-term HFD consumption induced metabolic disturbance with splenomegaly and single LPS administration exacerbated these adverse effects in HFD-fed rats
After 12 weeks of HFD consumption, but not a single administration of LPS, showed a significant increase in body weight, food intake, and fat weight, when compared to ND-fed rats (Figure 1B–D). Both long-term HFD consumption and LPS administration led to increased serum total cholesterol, serum LPS levels, and spleen weight, and a more pronounced increase was observed in the combined group, suggesting the synergistic effect of HFD and LPS on metabolic parameters, endotoxemia, and splenomegaly (Figure 1E–H). Further analysis revealed that all HFD-fed rats, but not ND-fed rats receiving LPS, equally increased IL-1β levels in the spleen when compared with ND-fed rats receiving the vehicle, suggesting that LPS does not influence the spleen's IL-1β protein (Figure 1I).
2.2 Only long-term HFD consumption increased peripheral/hippocampal oxidative stress and decreased hippocampal tight junction protein expression, but both HFD consumption and LPS administration altered pro-inflammatory genes in microglial/astrocytic-enriched fractions
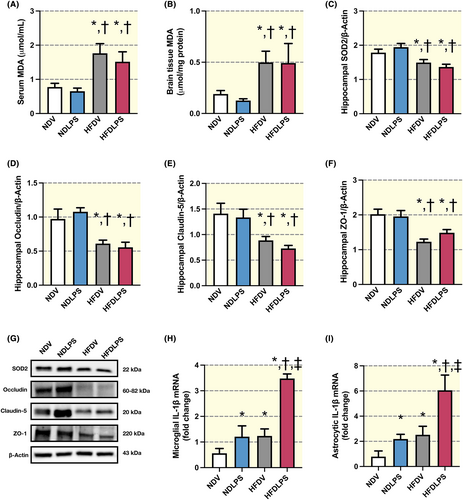
Chronic consumption of HFD, but not LPS administration, led to a significant increase in serum and whole brain MDA levels and a decrease in hippocampal SOD2 levels when compared to ND-fed rats (Figure 2A–C,G). These results indicated that HFD consumption, but not single LPS administration, led to an imbalance of oxidative/anti-oxidative status in the peripheral, brain, and hippocampus. Consistently, chronic HFD consumption, but not LPS administration, led to a significant impairment of tight junction protein expression in the hippocampus, as evidenced by decreased levels of occludin, claudin-5, and ZO-1 when compared to ND-fed rats (Figure 2D–F,G). In addition, both HFD and LPS administration led to a significant increase in IL-1β mRNA levels in microglial- and astrocytic-enriched fractions from the whole brain lysate, and a greater effect was observed in the combined group (Figure 2H,I). These findings suggested that microglia were efficiently primed by both LPS administration and HFD consumption, which led to glial cells in the brain increasing IL-1β mRNA in response to LPS administration and HFD consumption.
2.3 Long-term HFD consumption altered the morphologies of microglia and astrocytes, and a single LPS administration intensified these effects in HFD-fed rats
We determine the morphological alterations of microglia and astrocytes in CA1 region of the hippocampus by using Iba1 and GFAP markers for immunofluorescent staining, respectively. The results revealed that, among all groups, only HFD-fed rats receiving LPS had an increased microglial number (Figure 3A,B). In addition, both HFD consumption and single administration of LPS increased soma area when compared to ND-fed rats receiving the vehicle, and a greater effect was observed in the combined group (Figure 3C). Shortened filament length was observed in rats receiving HFD, LPS, or the combined treatment when compared with ND-fed rats receiving vehicle (Figure 3D). Furthermore, rats receiving either HFD or LPS led to a reduction in microglial branch complexity, including the number of terminal points and branches, as well as area under the curve of Sholl intersections, when compared to ND-fed rats receiving vehicle, and a greater effect was observed in combined treatment (Figure 3E–H).
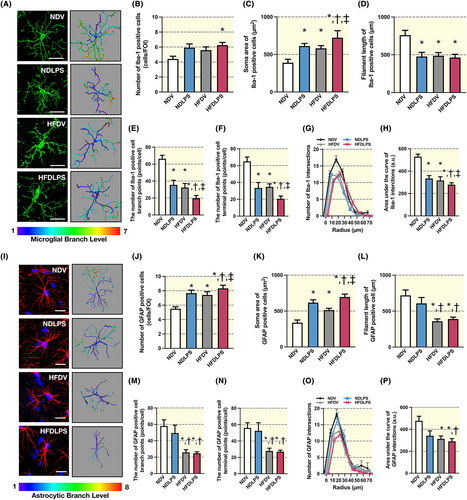
In addition, both HFD consumption and LPS administration equally increased the astrocyte number and soma area when compared to ND-fed rats receiving the vehicle, and a greater effect was observed in the combined treatment (Figure 3I–K). Moreover, all HFD-fed rats with or without LPS, but not ND-fed rats receiving LPS, showed a reduction in astrocyte complexity, including filament length, the number of branches, the number of terminal points, and the area under the curve of Sholl intersections, when compared to ND-fed rats receiving vehicle (Figure 3L–P). Collectively, these findings suggest that HFD consumption or LPS exposure triggers the microglia/astrocyte response in the hippocampus, which is associated with changes in morphology and complexity, and the combined HFD and LPS administrations aggravate these alterations.
2.4 Both a single LPS administration and chronic HFD consumption caused microglial synaptic engulfment in the hippocampus, and the combined treatments worsened these effects
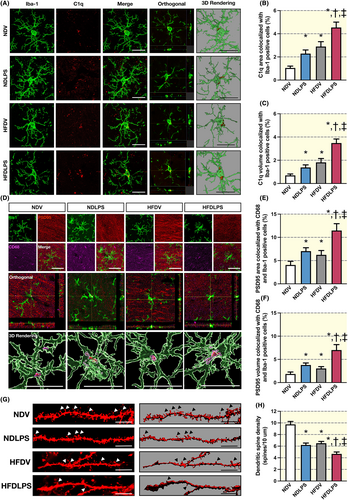
To determine microglial synaptic engulfment, the colocalization of C1q with Iba1 and PSD95 with CD86 as well as Iba1 was performed. Then, their respective areas and volumes were measured in the CA1 region of the hippocampus (Figure 4A–F). The results revealed that both LPS administration and HFD consumption increased the area and volume of C1q colocalization and PSD95 colocalization in microglia when compared to ND-fed rats receiving vehicles, and the combined treatments showed the greatest colocalization among all groups (Figure 4A–F). Furthermore, a decrease in dendritic spine density was observed following exposure to either LPS or HFD alone, when compared to ND-fed rats receiving vehicle, and the combined treatments showed the lowest dendritic spine density among all groups (Figure 4G,H). These findings suggest that HFD and LPS cause an increase in C1q, which tags synapses and leads to microglial synaptic engulfment, resulting in dendritic spine loss in the CA1 region of the hippocampus, which worsens with combined treatment.
2.5 Long-term HFD consumption, but not a single dose of LPS administration, impaired neurogenesis
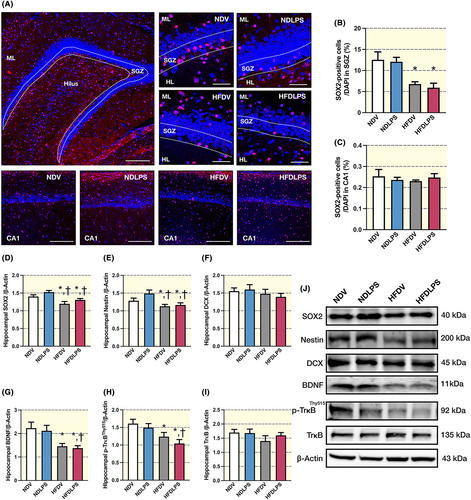
The results demonstrated that all HFD-fed rats, but not ND-fed rats receiving LPS, decreased the number of SOX2-positive cells in the subgranular zone (SGZ) in the dentate gyrus of the hippocampus when compared to ND-fed rats receiving vehicle, whereas this parameter did not alter in the CA1 region of the hippocampus (Figure 5A–C). A reduction was also observed in the expression of neurogenesis-related proteins in the hippocampus, including SOX2, Nestin, BDNF, and p-TrkBThy515, in all HFD-fed rats but not ND-fed rats receiving LPS, when compared to ND-fed rats receiving vehicle (Figure 5D,E,G,H,J). However, the expression of DCX and TrkB in the hippocampus did not show any significant change among all groups (Figures 5F,I–J). These findings suggest that only chronic HFD consumption, but not LPS administration, impairs neurogenesis by reducing the number of SOX-positive cells in SGZ in the dentate gyrus of the hippocampus and the expression of neurogenesis-related proteins in the hippocampus.
2.6 Long-term HFD consumption and a single dose of LPS administration altered depressive behaviors-related neurotransmitters and shifted the glucose metabolic pathway in the brain
To assess depressive behavior-related neurotransmitters, we measured brain tryptophan, serotonin, and kynurenine levels from the whole brain lysate. Either LPS or HFD led to an increase in tryptophan levels when compared to ND-fed rats receiving the vehicle, and the combined treatment showed the greatest brain tryptophan level among all groups (Figure 6A). The administration of LPS led to an increase in kynurenine levels in both ND-fed rats and HFD-fed rats, when compared to ND-fed rats receiving vehicle (Figure 6B). Furthermore, HFD-fed rats receiving vehicles decreased serotonin levels when compared to ND-fed rats receiving vehicles (Figure 6C). Taken together, these findings suggest that both HFD and LPS alter depressive behavior-related neurotransmitters in the brain.
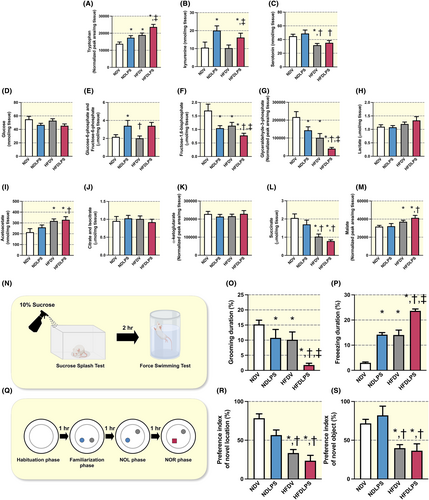
Next, to investigate the brain glucose metabolic pathway in the brain, substrates of glycolysis and the Krebs cycle were measured from the whole brain lysate. There was no difference in glucose and lactate levels among all groups (Figure 6D,H). Findings revealed that both LPS administration and chronic HFD consumption altered glycolysis substrates (Figure 6E–G). Moreover, HFD-fed rats also altered several Krebs cycle substrates, including acetoacetate, succinate, and malate levels, whereas citrate isocitrate and α-ketoglutarate did not alter among all groups (Figure 6I–M). Overall, the results suggest that HFD consumption and LPS administration affected both glycolysis and the Krebs cycle in the brain, leading to alterations in their respective substrates.
2.7 Both a single LPS administration and chronic HFD consumption induced depressive-like behaviors, but only chronic HFD consumption impaired cognitive function
The results showed that grooming duration during the sucrose splash test (SST) was decreased, whereas freezing duration during the forced swimming test (FST) was increased in rats receiving HFD or LPS administration, when compared to ND-fed rats receiving vehicles, and the combined treatment showed the highest alteration of these parameters among all groups (Figure 6N–P). These findings suggest that both LPS and HFD cause depressive-like behaviors, which are aggravated by the combination of HFD consumption and LPS administration. In addition, our findings showed that HFD-fed rats receiving either vehicle or LPS, but not ND-fed rats receiving LPS, decreased the preference index of the object in a novel location during the novel object location test (NOL) and the novel object during the object recognition test (NOR; Figure 6R,S). These results indicate that chronic HFD consumption, but not LPS administration, induces cognitive impairment.
3 MATERIALS AND METHODS
3.1 Animals and diets
The Laboratory Animal Center, Chiang Mai University approved animal studies in compliance with NIH guidelines (permit number: 2564/RT-00019). Sixty-four male Wistar rats (180–200 g) were obtained from Nomura Siam Company (Bangkok, Thailand) and were pair-housed in a temperature-controlled environment with a 12:12 h light–dark cycle. The age of the rats was 5–6 weeks old at the beginning of the protocol and 17–18 weeks old at the end of the protocol. After 1 week of acclimatization, rats were randomly assigned to receive either a normal diet (ND; 19.77% energy from fat, n = 32) or a HFD (59.28% energy from fat, n = 32) for 12 weeks. Food intake was recorded daily, and body weight was recorded weekly. After week 12, all rats in each dietary group were subsequently assigned into two subgroups (n = 16/subgroup) to receive either a single dose of vehicle (1 mL of 0.9% saline solution), intraperitoneal (i.p.) injection as ND-fed rats treated with vehicle (NDV) and HFD-fed rats treated with vehicle (HFDV) or LPS (0.5 mg/kg, i.p.) as ND-fed rats treated with LPS (NDLPS) and HFD-fed rats treated with LPS (HFDLPS). For 16 hours after the injections, rats from each subgroup were separated into two cohorts for different behavioral tests, to avoid the confounding factor toward the experiments. The first cohort (n = 8/subgroups) measured locomotor activity and cognitive function, whereas the other cohort measured depressive-like behaviors. All rats were anesthetized by isoflurane (2% isoflurane in oxygen, 1.5 L/min) for blood collection, and euthanasia by overdose of isoflurane (4%–5% isoflurane in oxygen, 1.5 L/min, until respiratory arrest occurs for 60 s), respectively.62 The brain and spleen were quickly collected for further investigation (Figure 1A).
3.2 LPS preparation and injection
The stock of LPS (Sigma-Aldrich, Missouri, USA) purified from Salmonella enterica was opened under sterile conditions. Then, the LPS stock was diluted with sterile PBS to a working concentration of 1 mg/mL. The amount of LPS injected was calculated based on the weight of the animal at a dose of 0.5 mg/kg of body weight, which was previously demonstrated to cause brain inflammation in rodents.17 Then, the rat was held in a dorsal recumbent position, and the LPS was injected into the low abdomen on the left or right side of the midline.
3.3 Metabolic parameters determination
A colorimetric assay (Biotech in Bangkok, Thailand) was used for measuring the cholesterol levels. The Pierce® LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific, USA) was used for measuring the serum LPS concentrations as described previously.4 In addition, to evaluate peripheral and brain oxidative stress, we measured the levels of malondialdehyde (MDA) in the serum and hippocampus using the high-performance liquid chromatography method as previously described.63
3.4 Immunoblotting of hippocampal anti-oxidation, the blood–brain barrier, inflammation, and neurogenesis proteins
The homogenate hippocampi were used to measure the expression of proteins related to anti-oxidation, the blood–brain barrier, inflammation, and neurogenesis as described previously.64 Briefly, the hippocampus tissue was homogenized, then protein levels in all samples were measured, and electrophoresis was performed. The nitrocellulose membrane was used for transferring the protein to membrane, and subsequently blocked and probed with primary antibodies overnight. All membranes were incubated with a secondary antibody conjugated with horseradish peroxidase (1:2000; #7074; Cell Signaling Technology, MA, USA). The protein bands were visualized on ChemiDocO™ touch imaging system (Bio-Rad, CA, USA) using ECL Western blot detection reagents (Bio-Rad Laboratories, Inc.).
3.5 Microglial- and astrocytic-enriched fraction and quantitative real-time PCR analysis
Microglia and astrocyte isolation were performed according to a previous study.19 The fractions were subsequently extracted RNA as previously described.65 Each step of glial isolation and qRT-PCR was detailed in Methods S1.
3.6 Immunofluorescent labeling for microglia, astrocyte, neurogenesis, and dendritic spine
The staining were performed according to a regular immunofluorescence staining technique as described in the previous study.4 Each step of the immunofluorescence staining protocol was detailed in Methods S1.
3.7 Brain metabolome extraction and determination
The brain metabolomics, including neurotransmitters, glycolysis metabolomes, free fatty acids, ketones, Krebs cycle metabolomes, and ATP levels, were measured by Liquid chromatography coupled with mass spectrometry (LC/MS)-based targeted metabolomics method as described previously.66 Each step of brain metabolome extraction and determination was detailed in Methods S1.
3.8 Determination of cognitive function
The cognitive function was measured by the modified NOL and NOR as described previously.67, 68 Briefly, animals were introduced to the apparatus, initially exploring two identical objects. Afterward, one of the objects was moved to a new location. Subsequently, animals were allowed to explore the apparatus with one familiar and one novel object for 10 min. Exploration durations were measured, and the % preference index was calculated.
3.9 Determination of depressive-like behaviors
The depressive-like behaviors in rats were assessed using the SST and FST, respectively. Rats were sprayed with a 10% sucrose solution on the dorsal surface, and grooming behavior to remove the solution was measured.69 After resting in the home cage, rats were further placed in a transparent cylinder tank containing water for 5 min. The duration of immobility was measured.70
3.10 Statistical analysis
The results of each experiment were expressed as the mean ± SEM. The data for all multiple comparisons were analyzed using a two-way ANOVA followed by Tukey's post-hoc test. A p-value below 0.05 was considered statistically significant.
4 DISCUSSION
Regarding the metabolic parameters in the present study, we found that long-term HFD consumption and LPS administration independently induced dyslipidemia and endotoxemia, with a more significant increase observed when HFD and LPS were combined. Consumption of HFD has been shown to alter the gut microbiota, leading to an increase in gram-negative LPS-containing bacteria, resulting in higher LPS levels in the gut lumen and serum.4, 19, 21 The rise in serum LPS levels in HFD-fed rats may trigger an immune response.22 Furthermore, the simultaneous presence of HFD and LPS administration appears to have a synergistic impact on dyslipidemia and endotoxemia, as both factors can independently lead to gut barrier disruption23, 24 and liver dysfunction.25, 26 These findings suggest that HFD consumption can cause metabolic disruptions and inflammation, which can be further exacerbated by LPS administration. In addition, exogenous LPS administration contributes to splenomegaly by stimulating the immune system, resulting in the production of white blood cells and an increase in spleen size,27 whereas chronic HFD consumption increases serum LPS levels and free fatty acids,26 which subsequently trigger peripheral immune cells and fat cell responses, resulting in the production of inflammatory cytokines such as IL-1β.28 Overall, these findings suggest that HFD and LPS may jointly amplify inflammatory responses, and LPS can exacerbate inflammation in the context of HFD-induced obesity. In contrast, LPS injection did not cause either peripheral/brain oxidative stress or a decrease in tight junction protein expression in hippocampus, like chronic HFD consumption, possibly due to the dosage used or frequency of injection, which was different from previous studies.12
Microglial response is recognized as changes in many layers of complexity, including gene expression, morphology, and phagocytic function, whereas primed microglia are capable of amplifying their response following a secondary immune challenge.16, 29-32 Microglial responses are associated with the alteration of MHCII, IL1β, and CX3CR1 genes in neurological disorders.32 Our investigation extends these observations by revealing that the mRNA levels of IL-1β are heightened in both microglia and astrocytes following exposure to either HFD or LPS, with a more pronounced effect evident in the LPS-stimulated HFD-primed condition, suggesting the microglia were primed following chronic HFD exposure.
In addition to microglial/astrocytic profiles, morphological alterations in glial cells fortify the concept of microglial response.4 Microglia transition from a ramified-like shape to an amoeboid-like shape in response to several detrimentral stimuli.33 Likewise, reactive astrogliosis exhibits increased volume and branch thickness.34 Expanding on these observations, our study demonstrates that both HFD and LPS trigger morphological alteration in both microglia and astrocytes. Notably, this effect is heightened in the LPS-stimulated HFD-primed condition, underscoring the primed state of microglia induced by chronic HFD consumption. Although the immunofluorescent staining using Iba1 and GFAP markers clearly illustrated microglial and astrocyte morphologies, there are some limitations of these markers. For example, Iba1 is a cytoplasmic protein expressed in monocyte lineage cells.35 Therefore, Iba1 markers are also expressed by other myeloid cells, such as infiltrating macrophages, perivascular macrophages, neutrophils, or T cells,36 which may affect the total number of Iba1 positive cells. In addition, it is also worth noting that not all astrocytes in vivo express GFAP.37-39
A recent study emphasized the role of microglial-mediated synaptic pruning through C1q in normal brain circuit development.40 However, complement pathway dysregulation can lead to excessive synapse elimination.20, 41 C1q binding triggers C3 cleavage into C3b, tagging synapses for elimination.40, 42 Our study builds on this, revealing that chronic HFD or acute LPS exposure increases C1q and PSD95 colocalization with microglia in the CA1 region of the hippocampus, indicating heightened synaptic pruning and subsequent dendritic synaptic loss. These changes contribute to cognitive impairment and depressive-like behavior.43, 44 Our findings suggest that microglial-mediated synaptic pruning may underlie the reduced dendritic spine density observed after chronic HFD or acute LPS exposure. Moreover, the exacerbated effect in obese rats with LPS exposure implies microglial priming resulting from chronic HFD exposure. Notably, male rats were used in the present study due to potential alterations in sex hormones, including estrogen, during menstrual cycles in female rats, which may impact glial function.45-47 Further studies are needed to evaluate the sex differences in glial function in response to LPS, HFD, and the combined effects.
Recent studies have associated microglia with the regulation of neurogenesis in the dentate gyrus.48, 49 Our study extends this research, revealing that chronic HFD consumption reduces the number of SOX2-positive cells in the SGZ of the hippocampus, which are crucial transcription factors for stem cell development.50 However, acute LPS exposure alone has no significant impact on neurogenesis in the SGZ of the hippocampus. These findings emphasize the substantial effect of chronic HFD consumption, as opposed to LPS alone, on neurogenesis in the SGZ of the hippocampus. Importantly, chronic LPS exposure or high doses may have effects on neurogenesis and brain function.13 Further research is necessary to explore the consequences of chronic LPS exposure and its interaction with obesity on neurogenesis.
Tryptophan is crucial in mood regulation,51 and can be metabolized through the serotonin and kynurenine pathways.52 Activation of the kynurenine pathway produces kynurenine and neurotoxic metabolites.52 Both HFD consumption and LPS administration also elevated brain tryptophan levels, and their combined administration further amplified this increase, possibly as an adaptive response to counteract heightened tryptophan metabolism along the kynurenine pathway. Significantly, we observed a marked decrease in brain serotonin levels in HFD-fed rats, regardless of LPS administration. This suggests that prolonged HFD consumption disrupts the brain serotonin pathway, potentially contributing to depressive-like behaviors.53
In addition, we found a decrease in brain glycolytic activity, suggesting disturbances in the normal brain glucose metabolic pathway caused by HFD consumption and LPS administration.54, 55 Moreover, elevated levels of acetoacetate and malate in HFD-fed rats indicate a shift toward fatty acid metabolism as an energy source,56 which can lead to oxidative stress and inflammation in the brain.57 Overall, the combined effects of long-term HFD consumption and a single LPS administration significantly impact metabolic pathways in the brain, altering glycolysis and Krebs cycle substrates. These alterations may signify a shift toward fatty acid oxidation in the brain's metabolic pathways, potentially resulting in detrimental effects on cognitive function58, 59 and depressive-like behavior.60, 61
5 CONCLUSION
The present study offers significant insight into the intricate relationship between chronic HFD consumption, LPS administration, and the multiple factors that influence depressive-like behaviors and cognitive function in rats. The findings of this study strengthen the statement that chronic HFD consumption or acute exposure to LPS effectively primed microglia and triggered microglial responses, leading to alterations of the microglial profile, morphology, and synaptic pruning function, resulting in brain inflammation, synaptic loss, the reduction of neurogenesis, cognitive decline, and rising depressive-like behaviors. The findings that microglia are primed by chronic HFD consumption, thus exacerbating the deleterious effects upon subsequent immune challenges, underscore the crucial role of microglia in cognitive function and depressive-like behaviors. These findings have significant implications for understanding the pathophysiology of depressive-like behaviors and cognitive decline, suggesting new avenues for the development of novel therapeutic strategies for neurodegenerative and psychiatric diseases.
AUTHOR CONTRIBUTIONS
Siriporn C. Chattipakorn: Conceptualization; funding acquisition; writing – original draft; writing – review and editing; supervision; validation; formal analysis. Titikorn Chunchai: Conceptualization; investigation; funding acquisition; writing – original draft; writing – review and editing; methodology; validation; visualization; formal analysis. Thirathada Chinchapo: Investigation; methodology; validation; visualization; writing – review and editing; writing – original draft; formal analysis. Jirapas Sripetchwandee: Investigation; methodology; writing – review and editing; validation; formal analysis. Chanisa Thonusin: Investigation; methodology; validation; writing – review and editing; formal analysis. Nipon Chattipakorn: Conceptualization; funding acquisition; writing – review and editing; supervision; formal analysis.
ACKNOWLEDGMENTS
This work was supported by the Research Grant for Young Researchers from the National Research Council of Thailand (NRCT) and Chiang Mai University (N42A660463 to TC); the Distinguished Research Professor (DPG) Grant from the National Research Council of Thailand and Chiang Mai University (N42A660301 to SCC); the Chiang Mai University Center of Excellence Award (NC); the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC). TCH gratefully acknowledges the master's degree program in Physiology in Faculty of Medicine, Chiang Mai University, under the CMU Presidential Scholarship. The authors would like to thank Dr. Hiranya Pintana, Dr. Benjamin Ongnok, Dr. Kewarin Jinawong, Patcharapong Pantiya, Busarin Arunsak, Sornram Janjek, Sasiwan Kerdphoo, Wichwara Nawara, Assistant Prof. Dr. Nattayaporn Apaijai, and Associate Prof. Dr. Wasana Pratchayasakul for the laboratory technical assistance.
FUNDING INFORMATION
This work was supported by the Research Grant for Young Researchers from the National Research Council of Thailand (NRCT) and Chiang Mai University (N42A660463 to TC); the National Research Council of Thailand (Distinguished Research Professor Grant: N42A660301 to SCC); the Chiang Mai University (CMU Excellent Center Award: NC); National Research Council of Thailand (NC) and the National Science and Technology Developement Agency Thailand (NC). TCH gratefully acknowledges the master's degree program in Physiology in Faculty of Medicine, Chiang Mai University, under the CMU Presidential Scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




