Ruxolitinib: A new hope for ventilator-induced diaphragm dysfunction
[Correction added on May 3, 2024 after first online publication. The author name has been corrected from ‘Anna Widegren’ to ‘Anna Widgren’ in this version.]
Abstract
Aim
Mechanical ventilation (MV) results in diminished diaphragm size and strength, termed ventilator-induced diaphragm dysfunction (VIDD). VID increases dependence, prolongs weaning, and increases discharge mortality rates. The Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway is implicated in VIDD, upregulated following MV. JAK/STAT inhibition alleviates chronic muscle wasting conditions. This study aimed to explore the therapeutic potential of Ruxolitinib, an FDA approved JAK1/2 inhibitor (JI) for the treatment of VIDD.
Methods
Rats were subjected to 5 days controlled MV (CMV) with and without daily Ruxolitinib gavage. Muscle fiber size and function were assessed. RNAseq, mitochondrial morphology, respirometry, and mass spectrometry were determined.
Results
CMV significantly reduced diaphragm size and specific force by 45% (p < 0.01), associated with a two-fold P-STAT3 upregulation (p < 0.001). CMV disrupted mitochondrial content and reduced the oxygen consumption rate (p < 0.01). Expression of the motor protein myosin was unaffected, however CMV alters myosin function via post-translational modifications (PTMs). Daily administration of JI increased animal survival (40% vs. 87%; p < 0.05), restricted P-STAT3 (p < 0.001), and preserved diaphragm size and specific force. JI was associated with preserved mitochondrial content and respiratory function (p < 0.01), and the reversal or augmentation of myosin deamidation PTMs of the rod and head region.
Conclusion
JI preserved diaphragm function, leading to increased survival in an experimental model of VIDD. Functional enhancement was associated with maintenance of mitochondrial content and respiration and the reversal of ventilator-induced PTMs of myosin. These results demonstrate the potential of repurposing Ruxolitinib for treatment of VIDD.
1 INTRODUCTION
Mechanical ventilation (MV) is a life-saving intervention for intensive care unit (ICU) patients incapable of adequately ventilating. However, controlled mechanical ventilation (CMV) promotes loss of diaphragm mass and strength, termed ventilator-induced diaphragm dysfunction (VIDD).1 VIDD increases ventilator dependence and prolongs weaning in ~35% of patients, while increasing age worsens prognosis.2 Weaning complications significantly impact 1-year discharge mortality rate (50%), while less than 10% of survivors report good health outcomes.3, 4 MV represents a major medical and societal issue, with no available effective treatment.
Enhanced oxidative stress, mitochondrial dysfunction, and pronounced inflammation are central to the etiology of VIDD, triggering catabolic processes and muscle degradation.5 The Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway was recently implicated in VIDD, upregulated in the diaphragm following MV and limb muscles during critical care defined by the STAT3 activation.6-9 JAK proteins are activated by cytokines, hormones, and growth factors, leading to phosphorylation of STAT proteins, promoting nuclear translocation and transcriptional regulation of many processes imperative to skeletal muscle function.10 STAT3 regulation is required for effective muscle growth and regeneration, while chronic activation is implicated in muscle wasting and dysfunction conditions including cancer cachexia.11, 12 Recently, suppression of STAT3 by JAK1/3 inhibition partially prevented ventilator-induced reduction in diaphragm contractile force and muscle atrophy in an acute 18 h model of MV.8, 9 However, the effect of STAT3 inhibition following longer durations of MV, which recapitulate the conditions experienced in critical care, is currently unknown.
STAT3 is imported into mitochondria, localized to the inner mitochondrial membrane (IMM), implicated in regulation of complex I and II of the electron transport chain (ETC) and production of reactive oxygen species (ROS).13 Mitochondrial ETC is reduced in the diaphragm following MV. Intact fibers exhibit reduced respiration following acute 18 h MV, while Complex IV activity progressively declines as MV duration increases to 5 days.8, 14 Loss of mitochondrial respiration is associated with disrupted intermyofibral and subsarcolemmal mitochondrial morphology, characterized by swollen mitochondria with less organized cristae and electron-dense matrix.14 Morphological alterations result after just 6 hours of MV, as intermyofibrillar mitochondria were found to be shorter, more rounded, and less morphologically complex.5 Thus, mitochondrial structure and respiration appear important in the early development of VIDD. Promotion of an oxidative environment in VIDD cannot be discounted, as regulation of STAT3 improves indirect measures of oxidative stress and respiration in acutely ventilated diaphragm, which was associated with augmented diaphragm force.8, 9 Given its IMM localization and regulation of ROS, STAT3 may be involved in mitochondrial structure and function in VIDD.
VIDD worsens in a time-dependent manner.15 While targeting STAT3 activity has benefit for the treatment of MV acutely, treatment efficacy needs to be ascertained in models representative of the prolonged nature of VIDD. Here, using a unique experimental rat model representative of the clinical pathophysiology, we explore the therapeutic potential of Ruxolitinib, a JAK1/2 inhibitor (JI) for treating VIDD. Ruxolitinib is FDA and EMA approved for treatment of myelofibrosis and splenomegaly-related disease following COMFORT-I/II trials.16 Ruxolitinib holds benefit for muscle-specific conditions, including critical illness myopathy.7 Thus, we hypothesize that Ruxolitinib will reduce MV-induced diaphragm loss of CSA and force. Here, JI preserved diaphragm muscle fiber CSA and specific force, associated with regulation of STAT3, preservation of mitochondrial content and respiration.
2 RESULTS
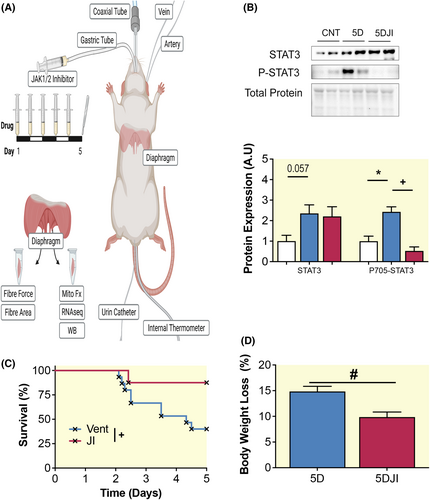
2.1 JI promotes survival during critical care
Following 5D CMV, the JAK/STAT pathway was elevated as signified by a two-fold upregulation of STAT3 and its phosphorylation at tyr705 (p = 0.057 and p < 0.001, respectively; Figure 1B; Supplementary Figure S1). JI reduced P-STAT3 to control levels (p < 0.001). Given the increased incidences of morbidity and mortality with increasing time in critical care, we examined the efficacy of JI for survival. Mortality in the initial 48 h of experiments is primarily related to technical problems of instrumentation or surgical complications in the experimental model used here. Thus, survival was assessed after the initial 48 h of CMV. While only 40% of ventilated rats examined survived for the 5-day experimental period, 87% of 5DJI rats reached 5 days in critical care, demonstrating increased survival following JI (log-rank, p = 0.048; Figure 1C). Consistent with this, 5DJI attenuated the body weight loss observed following CMV. 5D reduced body weight by 15%, while 5DJI reduced the loss of body weight to just 10% (p = 0.012; Figure 1D). Inhibition of JAK/STAT pathway promotes survival in response to 5 days CMV.
2.2 JI preserves ventilator-induced diaphragm muscle dysfunction
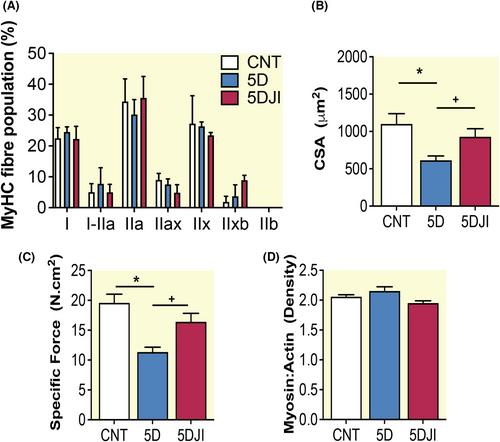
To ascertain the effect of JI on diaphragm performance, we isolated single fibers to examine diaphragm cross-sectional area (CSA) and force normalized to CSA (specific force). Here, 145 fibers passed the inclusion criteria (average of 13 fibers per animal; Supplementary Methods). To assess any fiber type specific effects, we determined myosin heavy chain (MyHC) fiber type distribution. No difference was observed in examined MyHC isoform between groups, thus fibers were combined and assessed irrespective of fiber type (2A). 5D reduced diaphragm fiber CSA and specific force by 40%–45% (p < 0.001; Figure 2B,C). Consistent with previous studies by Corpeno et al., neither 5D nor 5DJI influenced diaphragm myosin: actin ratio (Figure 2D).17 JI significantly improved diaphragm fiber CSA and specific force such that it was not different from control (p < 0.001; Figure 2). JI augments diaphragm function during long-term CMV.
2.3 Five days VIDD has little effect on mitochondrial morphology
To elucidate causative mechanisms in VIDD, we performed RNAseq of the diaphragm. 5D reduced gene ontology (GO) pathways including “Mitochondrion,” “Mitochondrial Inner Membrane,” “Cellular Respiration,” and “Oxidoreductase Complex” (Figure 3A). Downregulation of these processes suggests mitochondria are central to the loss of diaphragm function, supporting the involvement of mitochondrial impairments as a causative factor in VIDD.5 Enrichment of mitochondrial membrane GO led us to explore the effects of 5 days CMV on diaphragm intermyofibrillar mitochondrial morphology in a subset of samples. Mitochondrial number tended to be decreased following 5D and increased with JI treatment, however statistical significance was not achieved (Figure 3H). Mitochondrial size, defined by area, minimal feret and circumference was similar between control and 5D, tended to increase following JI, yet failed to reach significance (Figure 3I–K).
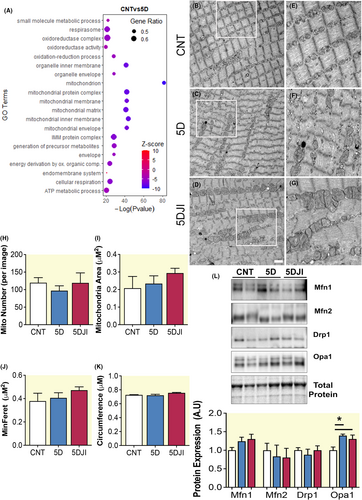
Given the enrichment of mitochondrial membrane GO term, we examined key regulators of mitochondrial dynamics. Here, neither outer mitochondrial fission nor the fusion proteins, DRP1, MFN1, and MFN2, were altered by 5D or 5DJI (Figure 3L). However, expression of IMM fusion protein, OPA1, was enhanced following CMV, irrespective of JI (p = 0.033; Figure 3L). OPA1 may be important in regulating IMM fusion or cristae structure following CMV; however, exploration of cristae structure with high resolution TEM may provide further insight.
2.4 JI augments mitochondrial respiration
GO terms pertaining to mitochondrial respiratory function were enriched in the diaphragm following 5D (Figure 3A). To identify key genes involved in this enrichment, DEGs were identified in complex I-IV. 5D reduced multiple genes in all respiratory complexes (Figure 4A). To ascertain the functional implication of 5 days CMV on mitochondrial respiration, mitochondrial-rich diaphragm preparations were subjected to respirometry. Here, 5D reduced the OCR of complex I, II, and IV (p = 0.004, 0.0005, and 0.007, respectively; Figure 4B). Administration of JI improved complexes I, II, III, and IV OCR, such that they become equal to or greater than control (p = 0.04). Reduced respiration was associated with reduced complex I protein expression (~40%; p = 0.05; Figure 4C), while complexes II–V were unaltered. Improvement of respiration following JI was associated with increased complex I protein expression (p = 0.05; Figure 4C). To assess if decreased complex I was due a reduction in mitochondrial content in the diaphragm following CMV, mtDNA was assessed relative to genomic DNA. 5D reduced mtDNA by ~50%, which was preserved following 5DJI (p = 0.001; Figure 4D). Mitochondrial transcription factor A (Tfam), a key activator of mitochondrial transcription and mtDNA replication,18 was similarly reduced following 5D and maintained at control levels with 5DJI (p = 0.003; Figure 4E). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1α) expression was determined to ascertain if such alterations were dependent on promotion of mitochondrial biogenesis. 5D-enhanced Pgc1α transcript expression (p = 0.0166), yet 5DJI failed to affect Pgc1α expression (Figure 4F).
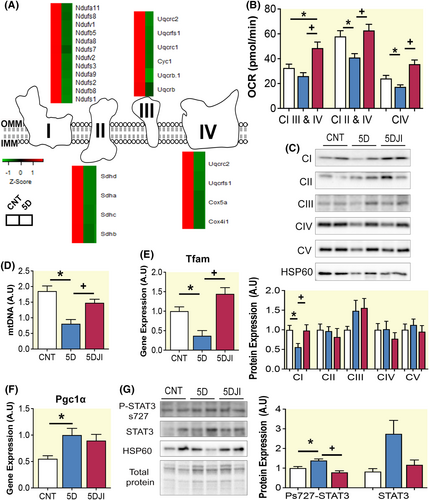
STAT3 is best characterized by its role as a nuclear transcription factor, yet STAT3 is also present in mitochondria bound with GRIM19 and implicated in regulating complex I respiratory chain.13 While phosphorylation of STAT3 at tyrosine 705 promotes translocation to the nucleus, phosphorylation on serine 727 is associated with a mitochondrial localization. 5D increased s727 P-STAT3 expression in mitochondrial-rich protein lysates, which was reduced following 5DJI (p = 0.050; Figure 4G). Total STAT3 tended to be increased following 5D in mitochondrial-rich lysate but failed to reach statistical significance (p = 0.08; Figure 4G). Therefore, 5DJI may prevent the VIDD-associated mitochondrial dysfunction by regulating P-STAT3 at s727, preserving mitochondrial abundance and ETC functions.
2.5 Oxidative stress is buffered during long-term CMV
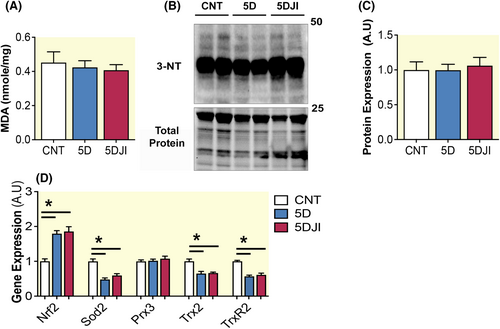
Activation of mitochondrial STAT3 at t727 increases complex I of the ETC and ROS production. Complex I is a particularly active producer of ROS. Given the regulation of ROS by STAT3 and the involvement of increased oxidative stress in the etiology of VIDD, we examined indirect levels of ROS in diaphragm via lipid peroxidation. Here lipid peroxidation levels were also assessed by detection of malondialdehyde, confirming neither 5D nor 5DJI had any effect on diaphragm lipid peroxidation (Figure 5A). As acute ventilation has previously been shown to promote nitrosylation of the diaphragm, protein lysates were separated and subjected to 3NT. Consistent with oxidation, nitrosylation was unaffected by 5D and 5DJI (Figure 5B,C). To determine if the lack of oxidative stress observed following MV was buffered by antioxidant activity, key redox sensor, and master antioxidant regulator, nuclear erythroid 2-related factor 2 (Nrf2), was assessed at the transcript level. Nrf2 was increased following 5D and unaltered by 5DJI (p = 0.0278; Figure 5D). To see if this antioxidant response was also observed in key mitochondrial localized antioxidant enzymes, superoxide dismutase (Sod2), peroxiredoxin (Prx3), thioredoxin 2 (Trx2), and thioredoxin reductase 2 (TrxR2) were measured. Surprisingly, Sod2, Trx2, and TrxR2, were reduced in 5D (p < 0.0001, p = 0.009 and p = 0.002, respectively). Consistent with the general antioxidant response, 5DJI had no effect on mitochondrial antioxidants. These results suggest improvements in muscle function with JI are associated with improved mitochondrial respiration independent of oxidative stress regulation.
2.6 The effect of JI on myosin PTMs
The motor protein myosin is responsible for cross bridge cycling and thus contractile function. Our group has previously reported that myosin from limb and respiratory muscles undergoes PTMs, which negatively affect myosin function.14, 19 Here, we examined PTMs of type I, II, and IIx myosin isoforms of the diaphragm by mass spectrometry. Many myosin PTMs were observed following 5D (Table 1). We focused on modifications induced by 5 days of CMV with or without JI. The selection criteria were defined as: (I) the absence or presence in ≥50% of all MV rats and (II) PTMs should be either absent or present in ≤25% of control or treated animals. Selected modifications were verified by manual inspection of the mass spectra.
| Position | Modification | Frequency | |||
|---|---|---|---|---|---|
| CNT | 5D | 5DJI | |||
| MyHC2—A0A0G2K0F5 | 44 | P-Pro → pyro-Glu | 3 | 5 | 2 |
| 215 | N-Term (Acetyl) | 2 | 2 | 5 | |
| 217 | Q-Deamidation | 2 | 2 | 5 | |
| 1170 | H-Oxidation | 1 | 4 | 1 | |
| 1212 | Q-Deamidation | 4 | 1 | 5 | |
| 1311 | K-Acetyl | 1 | 1 | 5 | |
| 1536 | N-Term (Acetyl) | 4 | 0 | 0 | |
| 1615 | N-Deamidation | 3 | 1 | 2 | |
| 1682 | R-Oxidation | 4 | 2 | 2 | |
| 2006 | N-Deamidation | 3 | 5 | 2 | |
| MyHC2x—Q0GC39 | 29 | N-Deamidated | 3 | 1 | 0 |
| 79 | P-Oxidation | 2 | 4 | 3 | |
| 96 | H-Oxidation | 1 | 4 | 2 | |
| MyHC1—P02564 | 17 | R-Oxidation | 1 | 3 | 1 |
| 777 | R-Oxidation | 0 | 3 | 1 | |
| 1193 | Q-Deamidation | 1 | 3 | 4 | |
| 1250 | N-Term (Acetyl) | 3 | 5 | 2 | |
| 1255 | Q-Deamidation | 3 | 5 | 2 | |
| 1318 | Q-Deamidation | 1 | 5 | 2 | |
| 1667 | Q-Deamidation | 3 | 5 | 0 | |
Following 5D, we confirmed two deamidation PTMs which were recovered in 5DJI (Table 2). Deamidation increases the negative charge of asparagine and glutamine residues which progressively disrupt structural integrity, biological activity and promotes protein degradation.20 Thus, deamidation is implicated in the pathophysiology of various conditions including aging skeletal muscle.21, 22 In type I myosin, 5D increased deamidation at position 1656 in the rod region compared to control. 5DJI had no such deamidation at position 1656. Interestingly, the biological consequence of protein deamidation may not be exclusively negative. Deamidation can modify biomolecule bioactivity, longevity, and efficacy of therapeutics. Deamidation at position 1212 in the rod region of type II myosin was lost during 5D yet retained in 5DJI. JI also increased the presence of deamidation within the motor domain at position 217, which may augment function.
| Position | Modification | Location | Frequency | |||
|---|---|---|---|---|---|---|
| CNT | 5D | 5DJI | ||||
| MyHC2—A0A0G2K0F5 | 44 | P-Pro → pyro-Glu | Head | 3 | 4 | 2 |
| 96 | H-Oxidation | Head | 2 | 2 | 0 | |
| 217 | Q-Deamidation | Head | 2 | 2 | 5 | |
| 1212 | Q-Deamidation | Rod | 4 | 1 | 4 | |
| 2006 | N-Deamidation | Rod | 3 | 4 | 2 | |
| MyHC1—P02564 | 1318 | Q-Deamidation | Rod | 0 | 1 | 0 |
| 1656 | Q-Deamidation | Rod | 2 | 5 | 0 | |
3 DISCUSSION
MV is a life-saving therapy for critically ill patients, but promotes the development of VIDD. As the JAK/STAT pathway was recently implicated in the regulation of VIDD, this study aimed to examine the therapeutic potential of repurposing Ruxolitinib, a JAK1/2 inhibitor, for reducing the effects of long term CMV. 5D diaphragm fiber CSA and specific force were significantly reduced, associated with elevated P-STAT3. Daily Ruxolitinib treatment reduced P-STAT3, prevented the loss of diaphragm fiber CSA and specific force, and increased animal survival. Preservation of function was associated with maintained mitochondrial content, and respiratory function. Ruxolitinib treatment may combat mitochondrial dysfunction associated with VIDD and preserve diaphragm fiber size and specific force.
Mitochondrial dysfunction is strongly implicated in the etiology of VIDD. Acute MV results in significant reduction in diaphragm mitochondrial function, indicated by reduced cytochrome c oxidase and succinate dehydrogenase activity,5 impaired respiration in intact diaphragm fibers,8 and spectrophotometric analysis.14 These enzymatic and respiratory dysfunctions are associated with reduced markers of mitochondrial content, including transcriptional regulator Tfam,5 but also independent of mitochondrial reductions.14 Here, following 5D, mitochondrial respiratory capacity was decreased in complex I–IV of mitochondrial-rich diaphragm muscle preparations, associated with reduced mtDNA content. Upregulation of the mitochondrial biogenesis marker Pgc1α, was observed following 5D, indicating possible compensatory promotion of mitochondrial generation. However, this did not result in recovery of mitochondrial content. JI prevented the loss of mitochondrial content preserved complex I–IV respiration during CMV. Thus, regulation of mitochondrial content by Ruxolitinib may protect from defective mitochondrial respiration following MV.
Loss of mitochondrial integrity promotes cellular stress, reduces respiration, and drives muscle degeneration in various myopathies.23 Mitochondrial dynamics regulate mitochondrial morphology, number, and connections, and in turn respiration.24 Mitochondrial dynamics can mitigate oxidative stress by fusing or separating damaged areas for mitophagy. However, when in excess, oxidative stress promotes fragmentation and apoptosis. Picard et al. demonstrated acute MV exposure can promote smaller, rounder, and less complex IMF mitochondria.25 Associated with increases in the fission protein, DRP1, acute MV appears to promote more fragmented mitochondria.25 Increased durations of MV tend to reduce both pro fusion and fission proteins, with significant reduction of DRP1 protein following 10 days of CMV. Here, mitochondria were more vesicular with less organized cristae and less electron-dense matrix.14 As ventilator duration is a critical factor for disease progression, weaning, and prognosis, it is interesting that following 5 days of CMV, this study observed a different expression pattern of mitochondrial dynamics proteins and limited alteration of mitochondrial morphology. Specifically, CMV-enhanced OPA1 fusion protein, while having no effect on MFN1, MFN2, or DRP1. This increase in OPA1 may be triggered through upregulation of Pgc1α, as Pgc1α overexpression promotes a pro-fusion phenotype in muscle, including OPA1.26 Thus, while JI maintains mitochondrial content and respiratory capacity, these improvements are independent of mitochondrial dynamics or alteration of gross morphology, suggesting dynamics alone do not facilitate the improvements in mitochondrial respirometry function during VIDD.
Mitochondria are a major producer of ROS in skeletal muscle. Excessive ROS promotes apoptotic, proteasome, autophagy, and other protein degradation signaling pathways, resulting in enhanced degradation of diaphragmatic proteins. MV trigger proteolytic responses not limited to ubiquitination, SUMOylation, calpains, and caspase systems.14, 27 Surprisingly, no increases in indirect measures of oxidative stress, lipid peroxidation, or protein nitrosylation were observed, following 5 days CMV. Acute rodent models of MV and diaphragm of MV brain dead organ donors have previously demonstrated a significant oxidative stress response following MV, indicated by increased lipid peroxidation, nitrosylation, and protein carbonylation.5, 8 Promotion of these systems has been forwarded as a major trigger to MV-associated mitochondrial dysfunction. However more recently, van den Berg et al., observed no oxidative activation in diaphragms of MV patients, rather a small reduction in nitrosylation and lipid peroxidation.28 Consistent with our findings, this lack of oxidative activation was associated with enhanced expression of key redox sensor Nrf2, suggesting an adequate antioxidant response.28 It must however be noted that diaphragm fiber size and strength were significantly reduced irrespective of the oxidative environment following 5 days of CMV.
The contractile apparatus can be impaired or modulated by various PTMs. Previously, our group has shown that long-term CMV causes several PTMs, including oxidation, deamidation, and carbonylation of the motor protein myosin, associated with reduced fiber size and specific force.14 Here, we observed 20 PTMs of MyHC I and II isoforms comprising deamidation, acetylation, and oxidation modifications. Manual confirmation highlights two key rod modifications and one modification adjacent to the catalytic site. PTMs of the rod region are prevalent in VIDD and associated with the slowing of myosin speed with increasing age or oxidative stress environments.14, 21 While the rod region is not as well characterized as the motor domain, perturbations in the myosin rod can disturb myofilament and sarcomeric formation culminating in impaired force production.29 Protection of ventilator-induced PTMs by BGP15 is associated with improved diaphragm and soleus muscle force and presents a strong candidate drug for the treatment of ventilator-induced myopathies.14, 19 Here, JI reduced ventilator-induced deamidation of glutamines in the rod region, while enhancing the prevalence of glutamine deamidation close to the catalytic site of MyHC II isoform. While these alterations were associated with modulation of fiber force, the nature of these modifications requires further clarification in the context of muscle function and VIDD, specifically. Nonetheless, Ruxolitinib treatment may in part modulate myosin PTMs and limit ventilator-induced loss of diaphragm function.
An important consideration and limitation of this study is the prominence of skeletal muscle fiber type and its regulation of metabolic and functional capacity.30 Fiber type characteristics including mitochondrial structure and function can alter drastically between slow and fast phenotype muscle to meet diverse functional needs of skeletal muscle. Slow fiber type muscles possess higher mitochondrial content which correlates with increased oxidative activity, while mitochondrial morphology also differs and can closely match differences in energetic demands.31-33 Interestingly, CMV promotes muscle atrophy and reduced specific force in both fast and slow fibers in patients and rodent models.34 No difference was observed in the proportions of skeletal muscle MyHC over the duration of this study and thus analysis was performed independent of fiber type. However, fiber type specific alteration of mitochondria cannot be discounted, and future work should examine the implication of fiber type in the context of VIDD etiology and the benefits of Ruxolitinib.
4 METHODS
4.1 Animals ICU model
All procedures were approved by Karolinska Institute ethical committee (N263/14) and conducted in accordance with their ethical standards for animal research. Adult female Sprague–Dawley rats were divided into control (CNT; n = 5) or experimental groups exposed to deep sedation with isoflurane, pharmacologically paralyzed post-synaptically with alpha cobra toxin, and CMV, for 5 days (5D; n = 6) or 5D with 60 mg/kg JAK 1/2 inhibitor (5DJI; n = 7), administered daily via gastric tube as shown in Figure 1A and previously described.7 Following exposure to critical care, deeply sedated rats were weighed and euthanized by heart removal. A detailed description of our experimental rodent model can be found in supplement. For survival analysis, data from rats included in previously published work by Cacciani et al., 2020 were included.19 Here, rats were exposed to ICU condition for durations up to 5 days, but no less than 2 days. Female rats were used to allow urine bladder catheterization via the urethra.
4.2 Western blotting
Diaphragm muscle was lysed in sample buffer (glycerol, 10% SDS, 0.5 M Tris buffer at pH 6.8, Bromophenol blue solution, dithiothreitol, and leupeptin (5 mg/mL)) or mitochondrial-rich protein lysates (described below) were separated on a TGX Stain-Free™ criterion gel (Bio-Rad). The gel was activated and proteins visualized using a Chemidoc™ XRS system (Bio-Rad) and transferred to PVDF membranes. Membranes were blocked with 5% BSA, prior to overnight incubation in primary antibody (Supplementary Table S1). After HRP-linked secondary antibodies incubation the membrane was imaged using ECL chemiluminescence (Bio-Rad). Protein expression was normalized to the optical density of the total protein on the stain-free protein gel, while mitochondrial-rich fractions were normalized to the mitochondrial content marker, HSP60.
4.3 Contractile measurements
The midcostal diaphragm was dissected, and part of it was placed in relaxing solution for muscle bundle preparation. Remaining muscle was snap frozen and stored at −140°C for further analyses. Bundles were membrane-permeabilized and cryoprotected before freezing. Cryoprotectant was removed before measuring contractility, where a single muscle fiber segment was isolated and attached between connectors in the setup. Fiber CSA, maximum force, and specific force (normalized to CSA) were calculated as the difference between maximal isometric force and resting tension. A detailed description of the single fiber preparation and contractile measurements is found in supplement. Following contractile testing, fibers were placed in sample buffer and proteins separated on 6% SDS-PAGE, gel silver stained and scanned to identify myosin heavy chain isoform specificity as previously described.35
4.4 Myosin: Actin ratio
Myosin and actin contents were separated on 12% SDS-PAGE, stained and densitometry quantified as previously described.36 Myosin and actin protein content are represented as myosin:actin ratio.
4.5 RNA sequencing
RNA was extracted from the diaphragm as previously described (36 and supplementary methods), and strand-specific sequencing libraries were prepared using poly-A selection and Illumina TruSeq strand-specific kits (Illumina). Libraries were sequenced (Illumina NovaSeq S4 platform), mapped to the rattus norvigicus Ensemble reference using the NF-Core RNAseq pipeline,37 and analyzed using EdgeR and Goana pipeline on R.38 Genes with a false discovery rate (FDR) of p < 0.05 were reported as significantly differentially expressed genes (DEGs).
4.6 Transmission electron microscopy
Frozen diaphragms were fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer, pH 7.4, postfixed in 2% osmium tetroxide in 0.1M phosphate buffer, pH 7.4, and stepwise dehydrated in ethanol and acetone prior to embedding in LX-112 (Ladd). 60–80 nm sections were cut and contrasted with uranyl acetate and lead citrate prior to examination in a HT7700 (Hitachi High-technologies) transmission electron microscope at 100 kV and equipped with a 2kx2k Veleta CCD camera (Olympus Soft Imaging System).
To analyze intermyofibrillar mitochondria, five to ten randomly selected longitudinally orientated ×10 000 magnification electron micrographs underwent point counting stereology (81-point grid) using ImageJ software (Version 1.52h, National Institutes of Health). An average of 107 ± 12 mitochondria was analyzed per animal. Each mitochondrion was manually traced and morphology (area, circumference, and minimal feret) assessed to determine mitochondrial morphology.
4.7 Mitochondrial Respirometry
Mitochondrial complex respiration was measured with Seahorse XF96 (Agilent), as previously described with minor modifications.39 Briefly, 10–20 mg of frozen muscle were thawed in ice cold PBS, minced, and mixed with 500 μL of mitochondria assay solution without BSA (MAS; 220 mM d-Mannitol, 70 mM sucrose, 10 mM of KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, pH to 7.4 at 37°C) with addition of collagenase type II (0.25 mg/mL final). The tube was incubated at 37°C for 30 min with seldom agitation. The digested tissue was mechanically homogenized by 10 strokes with a Dounce homogenizer. A further 500 μL of MAS of fatty acid-free BSA 0.2% (w/v) in ultrapure H2O, pH to 7.2 at 37°C was added to each sample and the samples centrifuged 1000× g 10 min at 4°C. 6-8ug of protein supernatant was loaded in a XF microplate in 20ul MAS and centrifuged 2000× g 5 min at 4°C (no brake). For each well, 130 μL of pre-warmed (37°C) MAS with ADP (final concentration 4 mM) was added and the plate placed at 37°C in a non-CO2 incubator for the time of cartridge calibration. The oxygen consumption rate (OCR) was measured at basal level, after addition of MAS with NADH (to measure activity of CI + III + IV) (1 mM) or succinate (5 mM) + rotenone (2 μM) (to measure activity of CII + III + IV), rotenone+antimycin to inhibit complexes I and III, ascorbate (1 mM) + TMPD (0.5 mM) to measure complex VI activity, and finally sodium azide (50 mM) to inhibit complex IV. Respiration was measured using cycles of 1 min mix and 5 min measure. The experiment was run using five rats (biological replicates for each condition) and three wells (technical replicates). Data are presented by mean + CI of the wells pooled together after outlier calculation (Grubb's test) to avoid loss of data information. Statistical significance was calculated from the average of each data per mouse using one-way ANOVA and Tukey post hoc test.
4.8 Real-time quantitative PCR (qPCR)
RNA was extracted from diaphragm, reverse transcribed and qPCR performed as previously described (7 and Supplementary Methods). Ct values were normalized to the geometric average of three housekeeping genes (beta-actin, alpha-tubulin, and GAPDH) and expressed in arbitrary units (A.U.). DNA from diaphragm fractions was extracted using a DNAeasy kit, as per the manufacturer's instructions (Quiagen) and mtDNA versus gDNA content was measured by qPCR using Dloop2 and 18s primers, respectively. mtDNA content was normalized to gDNA.
4.9 MDA assay
Diaphragm lysis was prepared, and lipid peroxidase content measured in the supernatant using malondialdehyde (MDA) Assay Kit (MAK085; Sigma Aldrich) according to the manufacturer's instructions. MDA content was normalized to tissue wet weight, expressed as nmols/mg.
4.10 Mass spectrometry for PTMs
Total myosin heavy chain (MyHC) were separated on a 12% SDS-PAGE and Coomassie-stained bands corresponding to MyHC were excised, subjected to in-gel protein digestion and peptides analyzed by liquid chromatography - high resolution tandem mass spectrometry, as described previously19 and in supplement.
4.11 Statistics
Statistical analysis was performed using GraphPad Prism software (LaJolla, USA). One-way ANOVA with Tukey's post hoc test or t-test was used to compare treatment groups where appropriate. p < 0.05 was considered statistically significant. Data presented as means ± standard error of the mean (SEM) unless otherwise stated.
5 CONCLUSION
Our findings demonstrate the benefit of Ruxolitinib for the prevention of VIDD. Ruxolitinib increased animal 5-day survival, restored P-STAT3 expression, and prevented loss of diaphragm fiber size and specific force associated with VIDD. Preservation of function was associated with maintained mitochondrial content, and respiratory capacity, potentially through regulation of mitochondrial P-STAT3. Ruxolitinib has been repurposed, demonstrating potential for conditions with exacerbated inflammation including polycythemia,19 atopic dermatitis,35 steroid-refractory acute graft-versus-host disease,36 hemophagocytic lymphohistiocytosis,37 and COVID-19.38 This study highlights the potential of repurposing Ruxolitinib for the treatment of VIDD. Combined with previous work in critical illness myopathy,7 we forward Ruxolitinib as a potential therapy in the fight against critical illness-associated myopathies.
AUTHOR CONTRIBUTIONS
Alex B. Addinsall: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; validation; visualization; writing – review and editing; formal analysis; data curation. Nicola Cacciani: Conceptualization; methodology; writing – review and editing; formal analysis. Noah Moruzzi: Methodology; validation; formal analysis; writing – review and editing. Hazem Akkad: Formal analysis; writing – review and editing. Alice Maestri: Methodology; validation; formal analysis; writing – review and editing. Per-Olof Berggren: Methodology; validation; writing – review and editing; formal analysis. Anna Widgren: Methodology; validation; writing – review and editing; formal analysis. Jonas Bergquist: Methodology; validation; writing – review and editing; formal analysis. Tamara Tchkonia: Methodology; validation; writing – review and editing. James L. Kirkland: Methodology; validation; writing – review and editing. Lars Larsson: Conceptualization; funding acquisition; investigation; validation; writing – review and editing; visualization; project administration; resources; supervision; writing – original draft.
ACKNOWLEDGMENTS
This study was supported by Swedish Research Council (8651, 7154, 1001), Swedish Heart and Lung Foundation, Erling-Persson Foundation, Stockholm City Council (Alf; 20150423, 20170133), Centrum för Idrottsforskning (CIF; 2020-0014; 118-2021), and Karolinska Institute to LL. CIF Postdoctoral Fellowship (D2020-0018) and Loo and Hans Osterman Foundation to ABA. JLK and TT are supported by the Noaber Foundation, Connor Fund, and Robert J. and Theresa W. Ryan. The authors acknowledge support from the National Genomics Infrastructure in Stockholm.
CONFLICT OF INTEREST STATEMENT
Per-Olof Berggren is cofounder and CEO of Biocrine AB.All other authors declare no financial or non-financial conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




