Multiple ions control astroglial excitability, or “Nein, Kalzium, ist NICHT alles”
Otto Loewi exclaimed “Ja, Kalzium, das ist alles” in 1959. This paradigm was written in stone for glial cells, for which Ca2+ signalling is considered paramount for excitability. Fancy cytosolic Ca2+probes, such as Quin-1 and Fura-2 introduced by Roger Tsien in early 1980 led to a remarkable boost in studies on neuroglia. For the first time, it became possible to monitor in real time cellular responses to various forms of chemical and mechanical stimulation (electrophysiology did not reveal much because glial cells are electrically non-excitable and cannot generate action potentials). Extensive investigations in the 1990s discovered a wide array of receptors and channels expressed in glial cells, and in astrocytes in particular. Activation of glial receptors or opening of channels almost invariably triggered cytosolic Ca2+ responses; glial cells were also found to generate spontaneous Ca2+ events. The discovery of propagating Ca2+ waves,1 traveling over long distances in confluent astrocytic cultures, endowed astrocytes with the means for long-range signalling, and Ca2+ waves were considered to be functionally analogous to action potentials in neurones. The concept of astroglial Ca2+ excitability was born,2 and the majority of physiological studies of astroglia since included Ca2+ imaging, which was performed in vitro, in situ (in brain slices) and in vivo. Astrocyte Ca2+ signalling was extensively used in modelling of astrocytic networks and astroglia-neuronal interactions, with various forms of encoding and decoding being considered.3
This coincided with the emergence of the concept of the tripartite synapse, which postulated that glutamate release from astrocytes contributes to synaptic transmission.4 Neuronal-astrocytic signalling was posited to be mediated by astrocytic metabotropic glutamate receptors, which triggered inositol-trisphosphate (InsP3)-induced Ca2+ release from the endoplasmic reticulum; elevated cytoplasmic Ca2+ in turn instigated exocytosis of astrocytic glutamate-containing vesicles. This concept quickly became popular and widely accepted and continues to dominate modern views on bidirectional communication between astroglia and neurones. However, the experimental proof that this concept is indeed the universal basis of neurone–glia interaction has never been conclusively demonstrated. First, it was found that a suppression of astrocytic Ca2+ signalling by cell-specific deletion of InsP3 receptor type 2 (predominant in astrocytes) did not translate into any major phenotype and did not affect synaptic transmission. Second, astrocytic terminal processes (known as leaflets5) appeared to be devoid of endoplasmic reticulum; nor did they often contain small secretory vesicles. Another observation challenging the tripartite synapse concept is that the expression of metabotropic glutamate receptors type 1 and 5 (proposed to be the main pathways initiating astroglial Ca2+ signalling) is strongly downregulated in the course of astrocyte maturation. Meanwhile, a plethora of additional pathways generating Ca2+ signalling has been identified, among them influx from the extracellular space through reversal of Na+/Ca2+ exchangers.6, 7 Notably, NCX reversal is initiated by an intracellular Na+ increase—and Ca2+ only appears secondary to this initial signal. What is then the role of Ca2+ signals in astrocytic physiology? Naturally, cytosolic Ca2+ signals are fundamental for excitation-metabolic (by stimulating mitochondrial ATP production) and excitation-protein synthesis (by stimulating translation in the nucleus) coupling. Moreover, extensive studies performed in the last decades revealed substantial molecular and functional heterogeneity of astrocytes, and the question how Ca2+ signals exactly translate into astrocyte-specific physiological responses that feed back onto nervous tissue therefore appears highly specific for a given preparation and network.
The fundamental function of astrocytes, their raison d'etre, is to maintain the homeostasis of the CNS.8 Astrocytes control the ionic composition of the extracellular milieu (this function is known as ionostasis), being in particular engaged in K+ buffering that sustains neuronal excitability. Astrocytes regulate the excitatory/inhibitory balance in the CNS, through the glutamate(GABA)-glutamine shuttle that removes potentially toxic glutamate, regulates glutamate and GABA dynamics in the synaptic cleft thereby shaping postsynaptic currents, and feeds neurones with glutamine, the obligatory precursor of glutamate and (by proxy of glutamate) of GABA. Astrocytes regulate CNS pH homeostasis, control reactive oxygen species by supplying neurones with glutathione and cysteine needed for glutathione synthesis, take up catecholamines and degrade them with astrocyte-resident monoamine oxidase B; astrocytes detoxify ammonium, secrete synaptogenetic factors, and perform many more homeostatic functions. Astrocytic homeostatic responses must be tightly coordinated with neuronal activity, and this coordination is the function of sodium ions. The absolute majority of homeostatic transporters expressed in astrocytes are Na+-dependent.6 In addition, these Na+-dependent transporters generate Na+ influx; similarly, Na+ influx is generated by the opening of ionotropic receptors (glutamate and purinoceptors) and various cationic channels operating in the astrocytic plasmalemma. Neuronal activity thus triggers Na+ entry into astrocytes, which generates substantial and long-lasting changes in cytosolic Na+ concentration ([Na+]i).
These [Na+]i transients (or Na+ signals) accompanying neuronal activity have an amplitude of ~10–20 mM and last for many seconds.9 Increases in [Na+]i modulate all Na+-dependent transporters and Na+-dependent intracellular enzymes (Figure 1).9 The glutamate-glutamine shuttle has a particular association with astrocytic Na+ signalling. First, astrocytic EAAT1/2 (excitatory amino acid transporters 1/2) that takes up the bulk of glutamate in the CNS generate massive Na+ influx: every single glutamate molecule translocated into the astrocyte is accompanied by three Na+. Glutamatergic transmission therefore generates large Na+ signals.9 Increased [Na+]i in turn stimulates glutamine synthetase and astrocytic SNAT3/5 glutamine transporters exporting glutamine into the extracellular space to be subsequently captured by neurones. Increases in [Na+]i accompanying glutamatergic synaptic transmission also affect other transporters. In particular, an increase in [Na+]i may reverse astrocytic GABA transporters. An increase in [Na+]i also reverses the Na+-Ca2+ exchanger (NCX), resulting in Ca2+ influx and an increase in astrocytic [Ca2+]i, which activates, for example, the bestrophin-1 anion channel, which acts as a GABA conduit. Finally, an increase in astrocytic [Na+]i may reverse the glycine transporter GlyT1, which supplies glycine to modulate NMDA receptors. Thereby, glutamatergic transmission acting through Na+ signalling triggers a chain of events, which modulate neuronal activity. In this paradigm activity-dependent astrocytic [Na+]i fluctuations act as a "first responder": a rapid signalling system that coordinates neuronal firing and synaptic transmission with the astrocytic homeostatic response.7
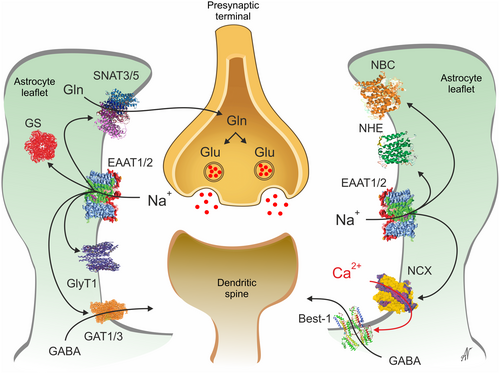
Besides Na+, astrocytes use Cl− as a signalling anion. In the mature CNS, Cl− is the anion that mediates inhibition by GABAA and glycine receptors, which are pentameric ligand-gated anion channels. Neurones maintain a low cytoplasmic Cl− concentration ([Cl−]i) and hence opening of GABAA or glycine receptors generates Cl− influx that mediates an outward, hyperpolarising current. In contrast to neurones, mature astroglial cells have a much higher [Cl−]i ranging from 30 to 50 mM.10 This results in relatively positive Cl− reversal potential (ECl ~ −30 mV), and hence the opening of anion channels in astrocytes generates a Cl− efflux that mediates a depolarising inward current. Astrocytic leaflets covering neuronal inhibitory synapses possess GABAA receptors which are opened by neuronal GABA in the course of inhibitory transmission. Opening of astrocytic GABAA receptors generates a Cl− efflux stabilising the Cl− concentration in the synaptic cleft, thus sustaining inhibitory transmission10; of note, Cl− also may exit astrocytes through Best-1, Pannexin 1 or volume regulated anion channels, VRACs. Manipulation of astrocytic [Cl−]i affects inhibitory transmission in vivo in freely behaving animals.10 Astrocytic [Cl−]i is highly dynamic and dependent on the brain state: during sleep [Cl−]i is stable and relatively high; arousal and activity decrease resting astrocytic [Cl−]i and stimulate activity-dependent [Cl−]i oscillations. These changes in [Cl−]i are also used to regulate several physiological processes, including proliferation, differentiation, activity of certain enzymes and apoptosis. Last but not least, it is long established that even small shifts in pH significantly impact neuronal excitability. The brain therefore controls its acid–base status within very narrow limits. Astrocytic NBCe1, which couples astrocytic homeostasis of Na+ and pH, contributes to buffering extracellular pH by either mediating inward or outward transport of HCO3−. Inward NBCe1 is stimulated by neuronal activity, and the resulting alkalinisation a key element in the neuro-metabolic coupling.9
Thus, astrocytic excitability is based on cytosolic signals generated not only by Ca2+ but also by Na+ and Cl− and H+ (and possibly even by K+, which remains to be investigated). Moreover, Na+ signalling is fundamental for real time coordination between neuronal activity and astroglial homeostatic support, which is critical for normal CNS function. Astrocytes, through their complex excitability and multitude of homeostatic pathways appear to be particularly important for the excitatory-inhibitory balance in the nervous tissue. Astrocytes regulate excitatory transmission through controlling glutamate and inhibitory transmission by controlling Cl− movement. Based on these clear findings and well-established principles, the multiunit nature of astroglial excitability needs to be fully appreciated, because Kalzium, ist eben nicht alles.
ACKNOWLEDGMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
We declare no conflict of interest.




