Transketolase drives the development of aortic dissection by impairing mitochondrial bioenergetics
Chaoyun Wang and Li Zhang contributed equally to this study.
Abstract
Aim
Aortic dissection (AD) is a disease with rapid onset but with no effective therapeutic drugs yet. Previous studies have suggested that glucose metabolism plays a critical role in the progression of AD. Transketolase (TKT) is an essential bridge between glycolysis and the pentose phosphate pathway. However, its role in the development of AD has not yet been elucidated. In this study, we aimed to explore the role of TKT in AD.
Methods
We collected AD patients' aortic tissues and used high-throughput proteome sequencing to analyze the main factors influencing AD development. We generated an AD model using BAPN in combination with angiotensin II (Ang II) and pharmacological inhibitors to reduce TKT expression. The effects of TKT and its downstream mediators on AD were elucidated using human aortic vascular smooth muscle cells (HAVSMCs).
Results
We found that glucose metabolism plays an important role in the development of AD and that TKT is upregulated in patients with AD. Western blot and immunohistochemistry confirmed that TKT expression was upregulated in mice with AD. Reduced TKT expression attenuated AD incidence and mortality, maintained the structural integrity of the aorta, aligned elastic fibers, and reduced collagen deposition. Mechanistically, TKT was positively associated with impaired mitochondrial bioenergetics by upregulating AKT/MDM2 expression, ultimately contributing to NDUFS1 downregulation.
Conclusion
Our results provide new insights into the role of TKT in mitochondrial bioenergetics and AD progression. These findings provide new intervention options for the treatment of AD.
1 INTRODUCTION
Aortic dissection (AD) is an acute cardiovascular condition characterized by the separation of layers of the aortic wall which is usually caused by an intimal tear.1 Advances in medical technology, diagnostic techniques, and treatment options for AD have significantly improved; however, the morbidity and mortality rates associated with AD remain high.2 Therefore, elucidating the underlying mechanisms of AD progression is crucial.
Glucose metabolism disorders are characterized by abnormal glucose metabolism,3 and abnormal blood glucose levels can be a predictor of cardiovascular disease risk.4 Fasting glucose levels negatively correlate with aortic diameter and dilation of the aortic lumen is the cause of dissection rupture.5 Other studies have shown that patients with diabetes are less prone to AD than patients without diabetes and exhibit a thicker mid-vascular layer.6 Pharmacological inhibition or activation of transporter proteins or key enzymes in the glucose metabolic pathway, such as SGLT2 and LDHA, can reduce the progression of aortic diseases.7, 8 Consequently, targeting glucose metabolism is an alternative strategy for relieving symptoms and improving vascular function.
TKT is a thiamine-dependent enzyme that plays a role in glucose metabolism by linking the pentose phosphate pathway (PPP) to the glycolytic pathway.9 One study noted that the specific knockdown of TKT in adipocytes inhibited glycolysis and increased gene expression in the mitochondrial electron transport chain.10 Vascular smooth muscle cells (VSMCs) are major components of the aorta and are activated by glycolysis.11 Glycolysis provides L-lactic acid as a substrate to increase mitochondrial reserve capacity, which prevents “ATP crises” and prolongs cell survival.12 Moreover, the aortic VSMCs phenotype in patients with AD is converted from contractile to synthetic, which increases glycolytic flux and reduces glucose oxidation.13 However, there are few studies on TKT in cardiovascular disease, and even fewer studies have focused on its pathogenesis. A recent proteomic study showed that TKT is significantly elevated in the hearts of post-myocardial infarction (MI) mice.14 However, the role of TKT in AD has not yet been elucidated.
In this study, we aimed to explore the role of TKT in AD. Reduction in TKT expression alleviates BAPN and Ang II-induced AD. We further found that TKT may impair mitochondrial bioenergetics-mediated AD occurrence by downregulating NDUFS1 via the AKT/MDM2 signaling pathway. These findings are valuable in the clinical application of TKT in AD.
2 RESULTS
2.1 Enhanced glycolysis and upregulation of TKT in aortic dissection
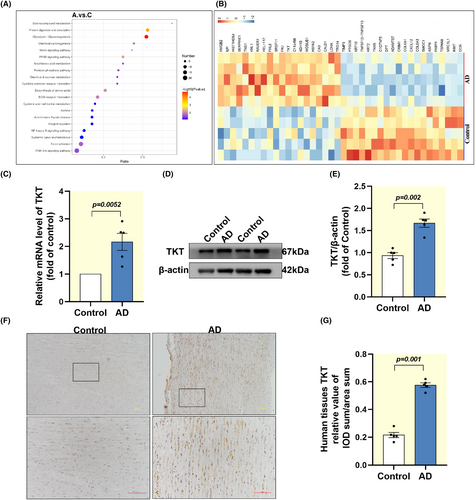
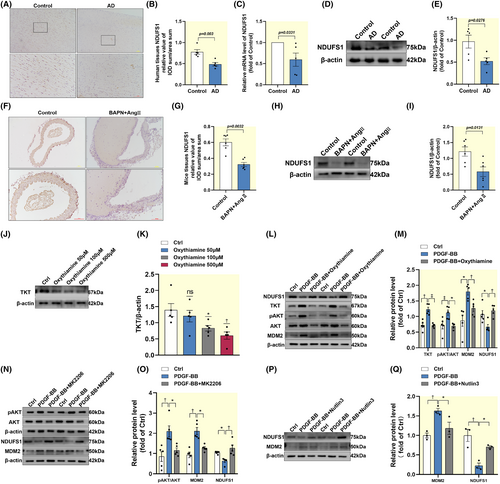
There were 370 proteins significantly changed in AD, among which 131 proteins were increased and 239 proteins were decreased (Figure S1A–C). The KEGG results suggested that proteins associated with the glycolysis/gluconeogenesis pathway were most significantly changed in AD (Figure 1A). To identify candidate proteins that may be associated with both AD and glycolysis, we plotted heat maps to show differentially expressed proteins. The results showed that TKT was upregulated in AD patients (Figure 1B). We found that TKT mRNA was upregulated in AD tissue, in contrast to normal thoracic aortic tissue (Figure 1C). Relative differences in TKT mRNA were replicated at the protein level, confirming that TKT protein present in AD tissue is the product of local gene expression (Figure 1D,E). IHC results similarly indicated that TKT expression was upregulated in AD tissues (Figure 1F,G). Taken together, these findings suggest that TKT is upregulated in AD and may be regarded as the pathogenesis of AD.
2.2 Enhanced mitochondrial fission in AD patients
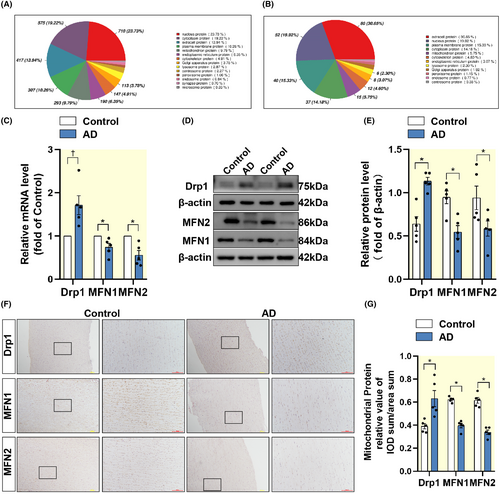
We found that apart from the nucleus, mitochondria are the organelle with the highest percentage of proteins, both total and differentially expressed proteins (Figure 2A,B). RT-qPCR analysis of human aortic tissue showed that Drp1 mRNA was upregulated in AD tissue, compared with normal thoracic aortic tissue, while MFN1/2 expression was downregulated (Figure 2C). In addition, immunoblotting results (Figure 2D,E) and immunohistochemical (Figure 2F,G) analysis were consistent with mRNA expression. Similarly, we found enhanced mitochondrial fission in AD mice (Figure S2A–D).
2.3 | Oxythiamine treatment attenuates BAPN combined with AngII-induced aortic dissection in mice
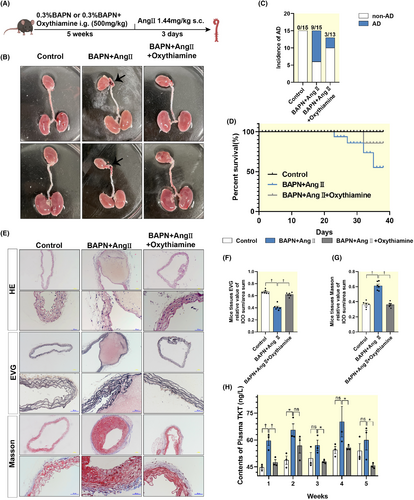
To further investigate the role of TKT in AD, we constructed a mice model of AD using BAPN combined with AngII, while administering the TKT inhibitor OT for treatment (Figure 3A). We found that OT was effective in preventing aortic dilatation and intramural hematoma formation and reduced the incidence of AD from 60% in the AD group to 23% in the treatment group (Figure 3B,C). Survival curves showed an increase in mortality in the AD group compared with the treatment group (Figure 3D). Morphological staining of the aorta showed that the aorta in the treated group was structurally intact, with neatly arranged elastic fibers and less collagen deposition than the AD group (Figure 3E–G). Interestingly, we examined the expression of TKT in mice plasma and found that its expression was also upregulated in AD and downregulated by the administration of OT, with the most significant reduction at week five (Figure 3H). Therefore, these findings suggest that reducing the abundance of TKT may provide an effective strategy to protect the aorta from BAPN combined with AngII-induced AD.
2.4 Enhanced glycolysis, lactate accumulation, and upregulation of TKT in AD mice
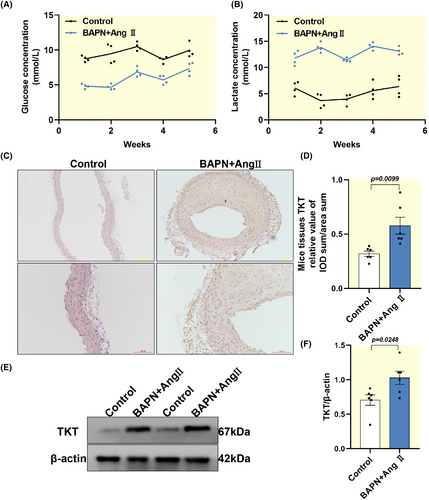
Given the role of glucose metabolism in AD, we measured the plasma concentrations of glucose and lactate in mice (Figure 4A,B). We found enhanced glucose utilization and high lactate concentrations in AD mice compared with controls, confirming that there is an enhanced glycolytic capacity during the progression of AD. To further explore, we examined the expression of TKT in the BAPN combined with AngII experimental mice model. We found that TKT was upregulated in the SMC of AD mice (Figure 4C–F). Support the notion of the TKT involvement in the setting of AD.
2.5 | Pharmacological inhibition of TKT attenuates PDGF-BB-induced impairment of mitochondrial bioenergetics in HAVSMCs
To determine whether TKT regulates energy metabolism in HAVSMCs in vitro, we constructed a cellular AD model by administering 20 ng/mL PDGF-BB to imitate the AD pathological environment and manipulated TKT expression by administering a TKT inhibitor on this basis. We found that partial inhibition of TKT attenuated PDGF-BB induced an increase in glucose consumption, lactate accumulation, and a decrease in ATP production (Figure 5A–C). JC-1 staining showed that the mitochondrial membrane potential was partially restored after partial attenuation of TKT expression (Figure 5D,E). Inhibition of TKT restored mitochondrial respiratory capacity, including basal respiration, ATP production, maximal respiration, and spare respiratory capacity (Figure 5F,G). We also found that reducing the abundance of TKT expression restored PDGF-BB-induced abnormalities in mitochondrial morphology, including the disappearance of cristae, the appearance of vacuoles, and lysis (Figure 5H,I). At the same time, Mito-tracker staining showed abnormal mitochondrial density (Figure 5J). To validate these results, we evaluated the protein expression of Drp1 and MFN1/2. We found that the upregulation of Drp1 expression and the downregulation of MFN1/2 expression after PDGF-BB induction were partially restored after TKT inhibitor treatment (Figure 5K,L). In summary, these data suggest that TKT may be involved in AD pathogenesis by impairing mitochondrial bioenergetics. Furthermore, we found that the impaired mitochondrial energetics caused by TKT are closely related to apoptosis, which is a major pathological feature of AD (Figure S3A–E).
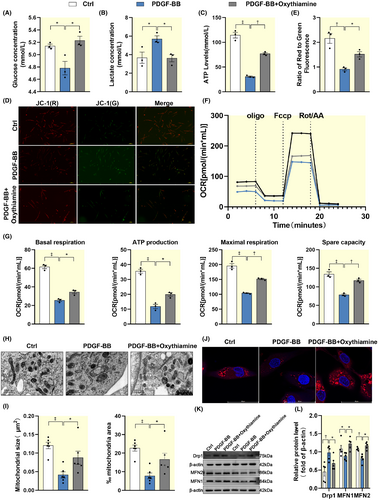
2.6 TKT may mediate mitochondrial bioenergetic disorders through AKT/MDM2/NDUFS1 in HAVSMCs
We investigated the signaling pathways by which TKT mediates AD genesis. We found the mRNA and protein levels of NDUFS1 were expressed downregulated in AD (Figure 6A–E). This result was also replicated in AD mice (Figure 6F–I). We selected the optimal inhibitory concentration of TKT for HAVSMCs and found that partial inhibition of TKT alleviated the PDGF-BB-induced increase in AKT phosphorylation, upregulation of MDM2 expression, and downregulation of NDUFS1 expression (Figure 6J–M). Utilizing MK2206 to inhibit AKT phosphorylation, we found that MDM2 was partially inhibited in PDGF-BB-treated HAVSMCs, while NDUFS1 was partially restored (Figure 6N,O). Our results confirmed that MDM2 has a negative regulatory effect on NDUFS1 by employing Nutlin3 to inhibit MDM2 (Figure 6P,Q).
3 DISCUSSION
Aortic dissection is a life-threatening global problem with a high mortality rate. To date, there are no effective preventive drug therapies, and surgery is the only definitive treatment. Although there is a consensus that the best strategy for AD management emphasizes early intervention, limited progress has been made regarding therapies for this disease; therefore, elucidating the pathogenesis of AD is imperative. Here, we report the significance of glucose metabolism in AD and the upregulated expression of the glucose metabolism enzyme TKT in the aorta of patients and mice with AD. The use of BAPN coupled with Ang II is a well-established method for constructing animal models of AD. ΒAPN is a lysyl oxidase factor that inhibits elastin and collagen cross-linking.15 AngII can activate the renin-angiotensin-aldosterone system and has the effect of promoting vasoconstriction, proliferation of vascular smooth muscle cells, and inducing an inflammatory response, which can mediate the occurrence of aortic dissection.16 We found that reducing TKT expression reduced the incidence and mortality induced by BAPN coupled with Ang II and alleviated AD pathology (such as elastin fiber breakage and collagen deposition). We further explored the mechanism in HAVSMCs and found that TKT may impair mitochondrial bioenergetics-mediated AD occurrence by downregulating NDUFS1 via the AKT/MDM2 signaling pathway. Thus, this study broadens our understanding of AD progression and suggests interventions for restoring mitochondrial function.
Mitochondria are the center of cellular energy metabolism, and those within VSMC are thought to play a role in maintaining vascular tone, facilitating cellular transport, and generating energy.17 Previous studies have reported the presence of abnormal morphology and reduced mitochondrial size in tissues affected with AD,18 confirming that enhanced fission may be present in patients and mice with AD, as proposed in our study. Mitochondria regulate its structure and function through division and fusion.19 Dynamic-related protein 1 (Drp1) mediates fission, while membrane GTPases mitofusin (MFN) 1 and 2 are responsible for mitochondrial fusion.20 In the present study, we found that DRP1 expression was upregulated and MFN1/2 expression was downregulated in the tissues of patients and mice with AD; and in HAVSMC, the Mito-tracker showed reduced mitochondrial density in the AD cell model. Oduro et al. showed that elevated levels of free fatty acids and sustained elevation of blood glucose can impair vascular mitochondrial function.21 We found that AD mice had abnormally elevated blood glucose concentrations and PDGF-BB-induced aberrant mitochondrial bioenergetics in HAVSMC as evidenced by the incomplete mitochondrial bilayer membranes, loss of cristae, reduced respiratory capacity, and increased apoptosis. Furthermore, studies have shown that the reduction in mitochondrial mtDNA quantity, enhancement of oxidative stress, imbalance of division and fusion, and occurrence of autophagy were all involved in the development of related cardiovascular diseases by affecting the metabolic function and phenotypic transformation of VSMCs.22 Therefore, further studies are needed to determine these relationships.
TKT is a key enzyme in PPP.23 OT is one of the most commonly used TKT inhibitors and is considered a potential drug for treating malignant tumors.24 Once combined with chemotherapeutic agents, OT improves the effectiveness of chemotherapeutic strategies by inhibiting tumor proliferation and enhancing ROS levels.25 TKT is a crucial factor in preventing hyperglycaemia-induced vascular cell dysfunction.26 However, the mechanism by which TKT affects AD progression remains unclear. Here, we revealed that TKT mRNA and protein levels were significantly upregulated in the aortas of patients and mice with AD. By administering the TKT inhibitor OT, we confirmed that suppression of TKT decreased the incidence and fatality of AD while preventing aortic dilation and middle-layer degradation and decreasing excessive glycolysis levels. To our knowledge, this is the first study to report TKT as a metabolic regulator of AD progression.
Given the key role of TKT in the AD process, we investigated its downstream signaling pathways. Protein kinase B (PKB/AKT) plays important roles in cell metabolism, growth, proliferation, and survival.27 The PI3K-AKT signaling pathway is expressed in AD and induces a phenotypic switch to HAVSMC.28, 29 Regulation of glycolysis by TKT is dependent on AKT phosphorylation in colorectal cancer.30 SM22α is a major marker of the contractile phenotype of VSMC, and its disruption impairs vascular function by promoting VSMC phenotype switching. Studies have indicated that increased SM22α expression inhibits activation of the AKT/MDM2 pathway.31 Murine double minute 2 (MDM2) is known to be upregulated in the aortic media of patients with thoracic aortic dissection (TAD), and studies suggest that this is due to a disturbed balance of the MDM2 and p53 feedback loops.32 This leads to the degradation of the aortic media. NADH Dehydrogenase (Ubiquinone) Fe-S Protein 1 is one of the core subunits of mitochondrial complex I that regulates mitochondrial oxidative phosphorylation.33 Further studies point to a negative regulation of NDUFS1 mediated by MDM2.34 Here, we found that NDUFS1 mRNA and protein levels were significantly downregulated in the aortas of patients and mice with AD. Enhanced platelet-derived growth factor-BB (PDGF-BB) promotes the development of TAD and induces vascular remodeling involved in the development of AD. Therefore, in the present study, PDGF-BB was used to stimulate HAVSMCs and establish AD cell models.
The present study revealed that TKT mediates mitochondrial bioenergetics via AKT/MDM2/NDUFS1 and is an important contributor to AD. This study had some limitations. First, the number of clinical sample cases is small, and second, it was not possible to determine whether TKT can also mediate other regulatory mitochondrial function-related proteins other than NDUFS1 to affect mitochondrial bioenergetics. Given the profound clinical importance of mitochondrial bioenergetics in AD, our findings provide new insights to further elucidate its pathogenesis.
4 MATERIALS AND METHODS
4.1 Human aorta samples
We collected discarded aortas from patients with AD after aortic replacement in the Fujian Medical University Union Hospital. Patients are seen immediately after the onset of clinical symptoms, and surgery is usually performed on the same day or the next day. AD was diagnosed by clinical history, physical examination, and imaging. Normal thoracic aortic tissue was obtained from heart transplant organ donors. None of these patients had cardiovascular disease and their vessels served as controls. This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved and conducted under the guidance of the Ethics Committee of Fujian Medical University Union Hospital (No. 2019-36). All subjects signed a written informed consent form. Dissected arteries were collected during the procedure and some tissues were immediately stored at −80°C for subsequent experiments, and the remaining tissues were placed in 4% paraformaldehyde for pathological staining. The general information of the patients was recorded and listed in Table 1.
| Group | ID | Gender | Age | BP (mmHg) | EF (%) | Diameter (mm) |
|---|---|---|---|---|---|---|
| Con | 1179626 | Female | 38 | 113/70 | 63.4 | 23.3 |
| 1180052 | Female | 56 | 99/64 | 63.2 | 27.4 | |
| 1179766 | Male | 61 | 110/83 | 44.4 | 31.4 | |
| 1179645 | Male | 44 | 107/60 | 59.9 | 27.8 | |
| 1179837 | Male | 67 | 139/76 | 71.0 | 31.7 | |
| AD | 1113857 | Female | 63 | 148/86 | 62.0 | 41.5 |
| 1101866 | Female | 53 | 135/78 | 60.8 | 40.1 | |
| 1101702 | Female | 49 | 182/79 | 63.7 | 42.5 | |
| 1098739 | Male | 48 | 145/89 | 63.9 | 45.5 | |
| 1115602 | Male | 47 | 130/60 | 60.9 | 37.7 |
4.2 Extracting total protein
The collected vascular tissues of AD patients were ground into powder by liquid nitrogen and transferred to the centrifugal tubes immediately. Mixed the powder and an appropriate amount of protein lysates was added. Sonicated the mixture on ice for 5 min to fully lyse. Centrifuged at 12 000g for 15 min at 4°C, took the supernatant, added 10 mM DTT at final concentration, and reacted for 1 h at 56°C. Then added enough IAM and reacted at room temperature for 1 h in the dark. Precooled acetone of four times volume at −20°C was added to precipitation at least 2 h at −20°C, and centrifuged at 12 000g for 15 min at 4°C to collect the precipitation. After that, 1 mL precooled acetone at −20°C was added to resuscitate and clean the precipitation. The precipitation was collected and dried by centrifugation at 4°C and 12 000 g for 15 min. An appropriate amount of proteolysis solution (6 M urea, 100 mM TEAB, pH = 8.5) was added to dissolve the precipitate.
4.3 TMT-labeled mass-spectrometry
Took 120 μg of each protein sample, added protein solubilizing solution to make up the volume to 100 μL, added 3 μL of 1 μg/μL trypsin and 500 μL of 50 mM TEAB buffer, mix and digest at 37°C overnight. Added equal volume of 1% formic acid, mixed and centrifuged at 12 000 g for 5 min at room temperature. Took the supernatant and slowly passed it through the C18 desalting column, and then used 1 mL of washing solution (0.1% formic acid, 4% acetonitrile) three consecutive times. Another 0.4 mL of eluent (0.1% formic acid, 75% acetonitrile) was added for consecutive elution two times. The eluent sample was lyophilized after being combined. Added 100 μL of 0.1 M TEAB buffer to re-dissolve and another 41 μL of acetonitrile-dissolved TMT labeling reagent to be a mixture. The mixture was reacted by inversion at room temperature for 2 h. After that, the final concentration of 8% ammonia water to terminate the reaction and mix it with the equal volume of the labeled sample. Lyophilized them after desalination.
4.4 Mouse model
In this study, the experimental protocol was approved by the Ethical Review Committee of Fujian Medical University Hospital (IACUC FJMU-Y-0763) and complied with animal welfare and ethical requirements. C57BL/6J mice were divided into three groups. Only male mice were studied in this experiment because of the relatively high incidence of AD and the smaller sex hormone changes.35 The control group was given a normal diet daily, the AD group was given 0.3% BAPN in drinking water, the treatment group was given an additional 500 mg/kg of oxythiamine (04000-5 g, Sigma) gavage daily in addition to 0.3% BAPN, and the remaining two groups were given drinking water by gavage. AngII (4474913, Tocris) was injected subcutaneously on day 35 at a total dose of 1.44 mg/kg in 5 injections, each 1 h apart. The AD model mice were modified from that reported in the literature by using a subcutaneous injection of AngII instead of a subcutaneous slow-release pump, which reduced pain in the mice.36
4.5 Cell culture and treatment
The HAVSMC line T/G HAVSMC (HTX2061, Otwo Biotech) was maintained and cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/G-streptomycin sulfate in a 5% CO2 atmosphere with a constant temperature of 37°C. HAVSMC (4th–8th generation) were inoculated in culture flasks. Cells were starved with medium containing 2% FBS for 24 h and then treated with different drugs: PDGF-BB 20 ng/mL (P5579-10 μg, Beyotime), PDGF-BB 20 ng/mL, and Oxythiamine 500 μmol/L (HY-107430-5 g, MedChemExpress).
4.6 Immunoblot
Proteins were extracted from human aortic tissue, mouse aortic tissue, and HAVSMCs, and then concentrations were determined by bicinoline quinone (BCA) protein assay (P0010S, Beyotime). Proteins were separated by SDS-PAGE and transferred to a 0.2 μm pore size polyvinylidene fluoride membrane (Immobilon-P PVDF membrane, Millipore). The membranes were placed in a quick blocking buffer for 15 min at room temperature and then incubated with primary antibodies at 4°C overnight. Primary antibodies used were as follows: TKT (11039-1-AP), NDUFS1 (12444-1-AP), Drp1 (12957-1-AP; all from Proteintech), AKT (AA326), pAKT (Ser473, AA329), MDM2 (AF7449), MFN1 (AF7461), MFN2 (AF74773; all from Beyotime), and β-actin (BM0627, BOSTER). Then washed with TBST and incubated with HRP-conjugated secondary antibody. Signals were detected by GE AI680 and band density was quantified using Image J.
4.7 RT-qPCR
Total RNA was extracted from human aortic tissue using Trizol, and cDNA was synthesized using a reverse transcription kit (TransGen Biotech, Beijing). Amplification was performed using SYBR green dye on the Biosystems QuanStudio® 5 (Applied Biosystems). The primer sequences were designed as follows:
TKT, TAGTGGTGTTACTGGTGATTGGA and TCCTGTCGTGTGATACAACCT; Drp1, GAATGACCAAGGTGCCTGTAG and GTGCCTCTGATGTTGCCATAT; MFN1, GTGGCAAACAAAGTTTCATGTG and CACTAAGGCGTTTACTTCATCG; β-actin, AGACGTGGACATCCGCAAAG and CTGGAAGGTGGACAGCGAGG; MFN2, AGATTACGGAGGAAGTGGAGAG and AACCTTGAGGACTACTGGAGAA; and NDUFS1, AGAAGATCTGGCTATGTTTCGG and TAGTTCCACTGCCTTCTCTGTA.
4.8 Hematoxylin–eosin (H&E), Masson, and EVG staining
Formalin-fixed, paraffin-embedded mice tissues were sectioned into 3 μm slices and stored at room temperature. Sections were dewaxed and dehydrated. For H&E staining, sections were stained with hematoxylin for 2 min and then with hydrochloric acid ethanol for 1 s to remove excess hematoxylin. The sections were then stained with eosin for 30 s, rinsed with water, and then dehydrated and sealed. Masson (G1340, Solarbio Life Science) and EVG staining (R203913, Genye Biotechnology) were performed following the manufacturer's instructions. Finally, those sections were observed with a Nikon fluorescence microscope (Nikon ECLIPSE Ni, Japan) and statistically analyzed with Image-Pro Plus 6.0 (IPP 6.0, USA).
4.9 Immunohistochemistry
Formalin-fixed, paraffin-embedded human and mice tissues were sectioned into 3 μm slices and stored at room temperature. Sections were dewaxed and dehydrated. The paraffin slices were treated in the oven (65°C, 30 min) and then dewaxed by dipping in xylene and ethanol with different concentration gradients. According to the instructions of the Immunohistochemical Kit (UItraSensitive SP mouse/rabbit, MXB), the paraffin sections were treated, during which the suitable first antibody was selected and incubated overnight at 4°C. Use the DAB staining kit (DAB-0031, MXB) to dye according to the instructions. Statistical analyses were carried out by a Nikon fluorescence microscope (Nikon ECLIPSE Ni, Japan) and Image-Pro Plus 6.0 (IPP 6.0, USA).
4.10 Cellular lactate production and glucose consumption measurement
Approximately, 5000 cells were inoculated in 96-well plates and then subjected to different administration treatments for 24 h. Afterward, supernatant cultures were collected and centrifuged, and the concentration of lactic acid in the medium was determined according to the instructions of the Lactic Acid Test Kit (A019-2-1, Established Institute of Biological Engineering). For glucose concentration determination, follow the glucose determination kit (A154-1-1, Established Institute of Biological Engineering).
4.11 JC-1 staining
Cells were inoculated in small 35 mm dishes and treated differently for 24 hours. Cells were washed with PBS and were performed according to the mitochondrial membrane potential assay kit (C2006, Beyotime). The cells were stained with JC-1 at 37°C dark environment for 20 min. After incubation, the supernatant was aspirated and washed twice with JC-1 staining buffer (1X). Then the cells were photographed using Nikon fluorescence microscope (Nikon ECLIPSE Ni, Japan).
4.12 Measurement of ATP content
The ATP assay kit was from Beyotime (S0026, Beyotime) and the assay was performed according to manufacturer's instructions. After centrifugation to remove cell debris, the supernatant was added to the ATP assay working solution. The luminescence was recorded at the multi-mode reader (BioTek).
4.13 TUNEL
Apoptotic cells were analyzed using the TUNEL Apoptosis Detection Kit (P-CA-302, Pricella). Briefly, cells were washed with PBS and fixed with 4% paraformaldehyde for 15 min, and then the cells were permeabilized with PBS containing 0.3% Triton-100. After washing again, the cells were incubated with the TUNEL reaction mixture at 37°C for 1 h. Finally, the nuclei were stained again using DAPI. Images were taken using a fluorescence microscope (Nikon ECLIPSE Ni, Japan).
4.14 Annexin V-FITC/PI
Cells after different treatments were digested down with EDTA-free trypsin, centrifuged, and 1–5 × 105 cells were collected, washed with cooled PBS, and resuspended with Binding Buffer. Annexin V-FITC and PI dye were added separately and incubated for 20 min at room temperature. Finally, detection was performed by flow cytometry (FACSCanto (TM) II, BD).
4.15 Transmission electron microscopy (TEM) analysis
After trypsin digestion, cell samples were centrifuged and then added to 2.5% glutaraldehyde and Milonig's phosphate buffer (pH = 7.3) and fixed at 4°C for 1 day. Cell samples were washed three times with PBS, followed by gradient dehydration, embedding, and finally ultrathin sectioning (50–100 nm) using an ultrathin sectioning machine (EM UC7, Germany). Specimens were double stained with 3% uranyl acetate and lead nitrate, observed, and photographed under a transmission electron microscope (Tecnai Gl2, USA).
4.16 Confocal imaging
Cells were inoculated in confocal dishes and treated with 20 ng/mL PDGF-BB or 500 μmol Oxythiamine for 24 h after reaching 50% confluency. After removal of the medium, medium containing 200 nM Mito-Tracker (C1049B-50 μg, Beyotime) was added to the dishes for 30 min at 37X°C in a 5% CO2 incubator. Cells were washed thrice with PBS and then treated with 4% paraformaldehyde for 15 min at room temperature to fix the cells. After three further washes with PBS, the dishes were treated with DAPI (C1005, Beyotime) for 15 min and then washed again with PBS. Then, the samples were photographed by laser confocal microscopy (Leica SP5, Germany).
4.17 Mitochondrial respiratory function assay
Respiration of human smooth muscle cell mitochondria was assessed with Oxygraph-2K (Oroboros O2k, Austria) at 37°C. Briefly, human smooth muscle cell cells were metabolically perturbed by the sequential injections of oligomycin, Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, rotenone, and antimycin A, and oxygen consumption rates (OCR) were recorded.
4.18 ELISA
Blood was taken from the inner eye canthus of mice for five consecutive weeks and plasma was obtained by centrifugation. Plasma levels of TKT (MEIMIAN) are measured using an enzyme-linked immunosorbent assay (ELISA). All plasma samples were tested without any dilution.
4.19 Statistical analysis
All graphs and calculations were performed using GraphPad Prism 8.0.1(San Diego, CA). Values are expressed as individual data points, percentages, or means ± SEM when appropriate. The normality of the data was assessed via the Shapiro–Wilk test. Differences between the two groups were compared using two-tailed unpaired Student's t tests when normal distribution was satisfied, otherwise, the Mann–Whitney nonparametric test was used. Differences between more than two groups were compared using one-way or two-way analysis of variance (ANOVA) followed by Dunnett's post-hoc test or Tukey's post-hoc test when the assumption satisfies the normal distribution. Otherwise, the nonparametric Kruskal–Wallis test was used for further analysis. Statistical significance was considered at *p < 0.05, †p < 0.01, ‡p < 0.001.
AUTHOR CONTRIBUTIONS
Chaoyun Wang, Li Zhang: Writing—Original Draft. Qinghua Zhang, Hui Zheng, and Xi Yang: Data analysis. Weixing Cai, Qiuying Zou, Jingjing Lin: Methodology. Lin Zhang, Lin Zhong, Xinyao Li, Yuqing Liao, Qin Liu: Investigation. Yumei Li and Liangwan Chen: Writing—Review and Editing.
ACKNOWLEDGMENTS
The authors thank the Union Hospital of Fujian Medical University and the Experimental Animal Center of Fujian Medical University for their support of the experiments. The authors thank the patients and medical staff for their support of this study.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (82241210, 82241209, U2005202, 82370470) and the Natural Science Foundation of Fujian Province, China (2022J01285).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




