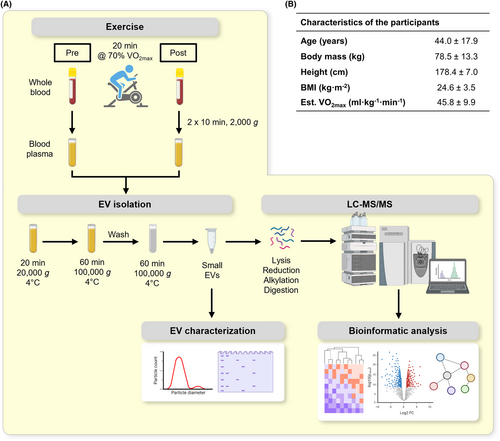
Acute exercise-induced release of innate immune proteins via small extracellular vesicles changes with aerobic fitness and age
Abstract
Aim
Physical exercise triggers the secretion of small extracellular vesicles (sEVs) into the circulation in humans, enabling signalling crosstalk between tissues. Exercise-derived EVs and their cargo have been proposed to mediate adaptations to exercise; however, our understanding of how exercise-derived EV protein cargo is modulated by factors such as aerobic fitness and age of an individual is currently unknown. Here, we examined the circulating sEV proteome following aerobic exercise in healthy males of different ages and aerobic fitness to understand exercise-induced EV response during the aging process.
Methods
Twenty-eight healthy men completed a bout of 20-min cycling exercise at 70% estimated VO2peak. Small EVs were isolated from blood samples collected before and immediately after exercise, and then quantified using particle analysis and Western blotting. Small EV proteome was examined using quantitative proteomic analysis.
Results
We identified a significant increase in 13 proteins in small plasma EVs following moderate-to-vigorous intensity exercise. We observed distinct changes in sEV proteome after exercise in young, mature, unfit, and fit individuals, highlighting the impact of aerobic fitness and age on sEV protein secretion. Functional enrichment and pathway analysis identified that the majority of the significantly altered sEV proteins are associated with the innate immune system, including proteins known to be damage-associated molecular patterns (DAMPs).
Conclusion
Together, our findings suggest that exercise-evoked acute stress can positively challenge the innate immune system through the release of signalling molecules such as DAMPs in sEVs, proposing a novel EV-based mechanism for moderate-to-vigorous intensity exercise in immune surveillance pathways.
1 INTRODUCTION
Accumulating evidence shows that physical exercise contributes to overall health benefits, including enhanced cardiovascular fitness, elevated overall metabolism, improved immune competence, and the prevention of chronic diseases such as cardiovascular disease and cancer.1-3 Despite the impact of exercise on human health that is unequivocal, the exact mechanisms for how exercise promotes overall health remain poorly understood at the signalling level. During exercise, it is known that increased metabolic demands provoke stress and homeostatic challenge to the body in the acute phase.4 To maintain a whole-body homeostasis, a myriad of integrative responses takes place at the systemic level which consequently promote adaptations linked to health improvements when repeatedly challenged.5 Although numerous signalling pathways have been identified as part of the integrative response of exercise, our understanding of how these responses are linked to health benefits remains limited. In recent years, tissue crosstalk during exercise has emerged as an alternative mechanism by which exercise exerts its systemic effects.6, 7
Extracellular vesicles (EVs) encapsulating bioactive cargo such as proteins, lipids, and nucleic acids in the circulation are capable of transferring their cargo to elicit functional effects in recipient cells.8-10 The secretion of EVs has therefore been proposed as one of the important mechanisms of tissue crosstalk during exercise.11-14 We and others have reported the release of sEVs into the circulation in healthy males after acute aerobic exercise15-17; however, much of the attention has been put on the surface and size characterization of exercise EVs. To fully explore the functional effect of EVs on health, understanding the cargo of exercise sEVs is pivotal. Therefore, omics analysis of exercise sEVs could be highly valuable in the identification of factors responsible for health benefits of exercise.
Much of the current interest in exercise sEV cargo has been focused on microRNAs.18-21 Investigations into exercise sEV proteome are still insufficient, with only a limited number of reported studies that have been mostly conducted with high-intensity or exhaustive aerobic exercise, particularly in young individuals.22, 23 In addition, other factors that may affect the protein composition of circulating EVs after exercise such as age and aerobic fitness have not been examined.
Here, we used a label-free quantitative proteomic method to examine the changes in plasma sEV proteome in healthy adults after moderate-to-vigorous intensity exercise. Importantly, we have investigated both young and mature people of different aerobic fitness, to identify exercise sEV protein secretion in populations similar to that found in the general community. We demonstrate an increase in several sEV proteins after exercise, with the responses differing in individuals with different ages and aerobic fitness. The majority of the altered proteins are associated with immune modulation and antimicrobial activity, implicating a role for sEVs in exercise-induced immune surveillance.
2 RESULTS
2.1 The release of sEVs increases during exercise
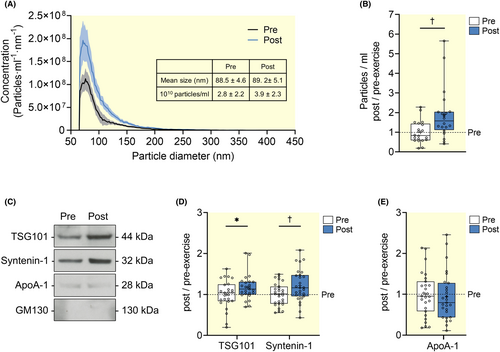
To examine the release of sEVs during moderate-to-vigorous intensity exercise, 28 healthy men performed a 20-min cycling exercise at 70% of the individual's estimated VO2peak. Small EVs were isolated from blood plasma samples withdrawn before and after exercise using differential ultracentrifugation (Figure 1A,B). We first conducted a nanoparticle count analysis using a Spectradyne nCS1 instrument. The EVs isolated at pre- and post-exercise displayed the size distribution attributed to sEVs with mean size (±SD) of 88.5 ± 4.6 nm and 89.2 ± 5.1 nm, respectively (Figure 2A). Paired t-test revealed a significant increase of 1.9-fold in the mean EV particle counts (±SD) from 2.8 × 1010 (±2.2 × 1010) particles/ml at pre-exercise to 3.9 × 1010 (±2.3 × 1010) particles/ml at post-exercise (p = 0.007, Figure 2B). It should be noted that some of the co-isolated non-EV particles are likely to contribute to the total particle count. To further characterize the isolated sEVs, we evaluated the presence of the commonly used sEV and non-EV markers using Western blot (Figure 2C). We observed significant increases in the amount of TSG101 and syntenin-1 released after exercise (p = 0.010 and p = 0.009, respectively, Figure 2C,D). Importantly, the amount of co-isolated apolipoprotein A1 (ApoA-1) remained unchanged from pre- to post-exercise (p = 0.623, Figure 2C,E), suggesting that the observed changes were mainly due to exercise-induced release of sEVs. Moreover, GM130 was not detected in both pre- and post-exercise EVs, suggesting the absence of contaminant proteins from intracellular compartment (Figure 2C). Overall, these results confirmed the release of a subset of sEVs into the circulation during moderate-to-vigorous intensity exercise.
2.2 Moderate-to-vigorous intensity exercise differentially alters sEV proteins
We next performed a label-free quantitative proteomic analysis of sEVs to identify the exercise-derived sEV proteome. We detected 1257 proteins in both pre- and post-exercise sEVs, of which 1162 and 1063 proteins overlapped with the human proteins annotated in the manually curated Vesiclepedia and ExoCarta databases, respectively (Figure 3A,B).24, 25 Of 1063 proteins overlapped with ExoCarta database, 87 proteins from the current study were common to the top 100 proteins that are often identified in exosomes (Figure 3B). Importantly, these proteins include sEV markers that are associated with multivesicular body formation (PDCD6IP (ALIX), TSG101, and SDCBP (syntenin-1)), chaperones (heat shock proteins), membrane transport and fusion (annexins, FLOT-1, FLOT-2, and RAB proteins), as well as vesicle adhesion molecules (tetraspanins (CD9, CD81, and CD82) and integrins) (Figure 3C). We also identified 18 of 22 proteins that were universally enriched in exosomes of different cell lines.26
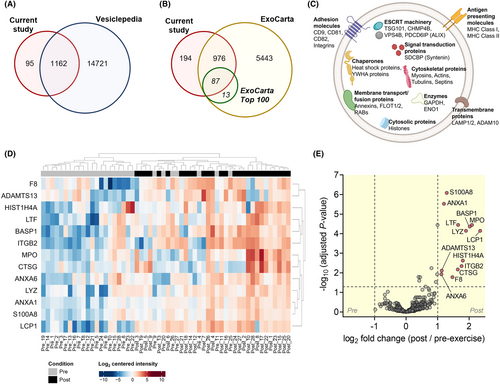
To investigate sEV protein composition in depth, we performed a quantitative comparison of the sEV proteome between pre- and post-exercise. A total of 13 proteins were identified to be differentially altered as a result of exercise in the mass spectrometry screen (Figure 3D). We observed significant increases in proteins including, a disintegrin and metalloproteinase with thrombospondin-type motifs 13 (ADAMTS13), annexin A1 (ANXA1), annexin A6 (ANXA6), brain acid-soluble protein 1 (BASP1), cathepsin G (CTSG), coagulation factor VIII (F8), histone H4 (HIST1H4A), integrin beta-2 (ITGB2), plastin-2 (LCP1), lactotransferrin (LTF), lysozyme C (LYZ), myeloperoxidase (MPO), and S100 calcium-binding A8 (S100A8) (Figure 3E; Table 1).
| Gene | Protein | UniProt accession | Molecular weight (kDa) | Pre vs. post adjusted p-value | Pre vs. post log2 fold change | Vesiclepedia | ExoCarta |
|---|---|---|---|---|---|---|---|
| ADAMTS13 | A disintegrin and metalloproteinase with thrombospondin motifs 13 | Q76LX8 | 153.6 | 0.00773 | 1.13 | + | + |
| ANXA1 | Annexin A1 | P04083 | 38.714 | 0.00000297 | 1.20 | + | + |
| ANXA6 | Annexin A6 | P08133 | 75.872 | 0.0119 | 1.12 | + | + |
| BASP1 | Brain acid-soluble protein 1 | P80723 | 22.693 | 0.0000431 | 2.02 | + | + |
| CTSG | Cathepsin G | P08311 | 28.837 | 0.00676 | 1.64 | + | |
| F8 | Coagulation factor VIII | P00451 | 267.01 | 0.0167 | 1.47 | + | + |
| HIST1H4A | Histone H4 | P62805 | 11.367 | 0.00238 | 1.8 | + | + |
| ITGB2 | Integrin beta-2 | P05107 | 78.354 | 0.00452 | 1.76 | + | + |
| LCP1 | Plastin-2 | P13796 | 70.288 | 0.0000713 | 2.35 | + | + |
| LTF | Lactotransferrin | P02788 | 77.969 | 0.0000359 | 1.65 | + | + |
| LYZ | Lysozyme C | P61626 | 16.537 | 0.0000713 | 1.90 | + | + |
| MPO | Myeloperoxidase | P05164 | 83.869 | 0.0000359 | 2.09 | + | + |
| S100A8 | S100 calcium-binding protein A8 | P05109 | 10.834 | 0.000000825 | 1.29 | + | + |
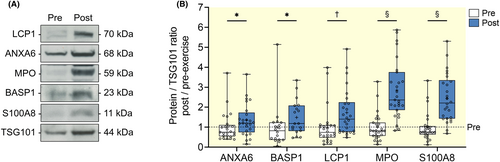
We further validated the abundance of several exercise-altered sEV proteins using Western blot (Figure 4A). The results from the Western blot analysis of exercise sEVs showed that ANXA6, a calcium-dependent membrane-binding protein, was increased significantly at post-exercise (p = 0.012, Figure 4B). A member of the family of neuronal growth-associated proteins BASP1 was also increased significantly after exercise (p = 0.046, Figure 4B). Significant increase in the abundance of LCP1, a leucocyte-specific protein, was observed at post-exercise (p = 0.004, Figure 4B). Other immune proteins MPO and S100A8 were also increased to higher levels post-exercise by 2.9-fold and 2.5-fold, respectively (all p < 0.001, Figure 4B). Taken together, these findings provide evidence that an acute moderate-to-vigorous intensity exercise transiently stimulates the release of systemic factors into circulation via sEVs.
2.3 Exercise releases sEV proteins that are involved in immune processes
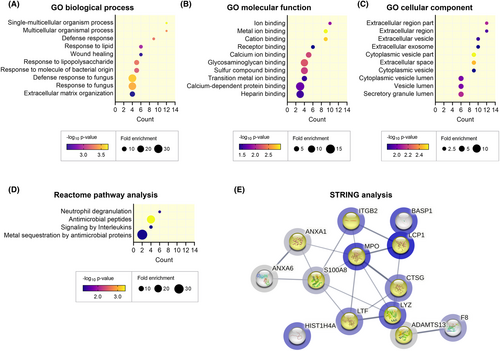
To gain insight into the potential biological functions of exercise sEVs, we carried out functional enrichment analysis on the 13 proteins that significantly increased post-exercise. In total, we observed significant enrichment in 67 GOBP, 11 GOMF, and 20 GOCC terms (p < 0.05, Tables S1–S3). Of the enriched terms, we observed significant enrichment in biological processes including response to stimulus (e.g., response to stress) and immune system process (Figure 5A). GOMF analysis showed significant enrichment in binding activities such as protein binding and ion binding (Figure 5B). We also observed that most of the significantly altered proteins were enriched in GOCC terms associated with extracellular compartments such as extracellular vesicles and exosomes (Figure 5C). This further supported the finding that exercise leads to the release of a host of factors packaged in sEVs involved in several biological functions. In addition, reactome pathway analysis revealed significant enrichment in pathways largely related to immune system (Figure 5D; Table S4). We further carried out analysis using the STRING database to understand the potential interactions among these proteins (Figure 5E). MPO appears to have close interactions with other proteins in the network including LCP1, S100A8, LTF, and CTSG. ADAMTS13 is closely interacting with F8, while BASP1 and HIST1H4A have indirect interactions with other proteins in this network. The proteins involved in immune processes cover most of the network (protein count = 9/13, highlighted in yellow in Figure 5E). Altogether, this indicates that exercise increases the release of sEV proteins that can modulate immune processes in the body.
2.4 Exercise regulates sEV proteins distinctly based on age and aerobic fitness
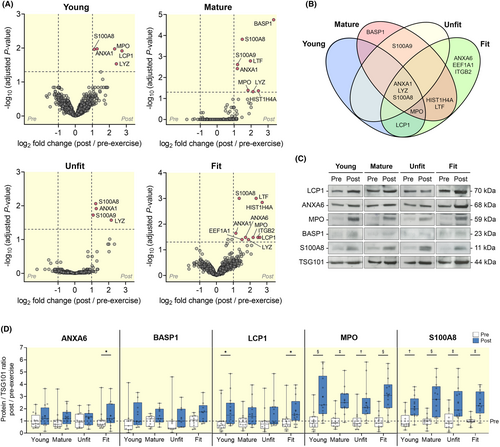
Next, we hypothesized that the release of sEV proteins could be affected by an individual's age and aerobic fitness. We therefore classified the participants into four subsets based on age (young, 18–35 years; and mature, 50–70 years) and aerobic fitness27 (unfit, estimated VO2peak < 45 mL kg−1 min−1; and fit, estimated VO2peak > 45 mL kg−1 min−1) (Table 2). There was a significant difference in age between young and mature subsets (p < 0.0001). There were also significant differences in body mass (p < 0.05), BMI (p < 0.05), and estimated VO2peak (p < 0.001) between unfit and fit subsets. Heart rate response and RPE during the 20-min cycling exercise for individual subset are presented in Table 2 and Figure S1. The release of sEVs from pre- to post-exercise across four participant subsets was examined (Figure S2). We observed a significant main effect for the abundance of TSG101 and syntenin-1 from pre- to post-exercise (p < 0.001, Figure S1A,B); however, post hoc pairwise comparisons did not detect differences in any of the subsets. This could be due to the smaller sample size in each subset. There was also no significant main effect for the abundance of ApoA-1 from pre- to post-exercise in all four subsets (Figure S1C). Quantitative comparison of sEV proteome between pre- and post-exercise showed distinct responses in the four subsets of participants (Figure 6A). In the young subset, we identified five significantly increased proteins at post-exercise (ANXA1, LCP1, LYZ, MPO, and S100A8), while eight proteins were significantly increased in the mature subset (ANXA1, BASP1, HIST1H4A, LTF, LYZ, MPO, S100A8, and S100 calcium-binding A9 (S100A9)). In the unfit subset, only four proteins were significantly increased (ANXA1, LYZ, S100A8, and S100A9). By contrast, we observed 10 proteins that were significantly increased in the fit subset (ANXA1, ANXA6, eukaryotic translation elongation factor 1 alpha 1 (EEF1A1), HIST1H4A, ITGB2, LCP1, LTF, LYZ, MPO, and S100A8). Since some of the exercise-altered proteins overlap, we visualized the data using a Venn diagram (Figure 6B). Among those proteins, ANXA1, LYZ, and S100A8 were significantly increased in all four subsets. BASP1, which has been shown to play important role in neurite outgrowth and nerve sprouting,28, 29 appears to be only significantly altered in the mature participants. LCP1 was observed to be increased in both young and fit subsets, while MPO was increased in all subsets except for unfit participants. Notably, the majority of the proteins were differentially altered in the fit participants, of which ANXA6, EEF1A1, and ITGB2 were increased specifically in the fit individuals. These findings from mass spectrometry analysis were further validated using results from Western blot analysis (Figure 6C,D). We observed that ANXA6 was significantly increased after exercise only in the fit subset (p = 0.024), while BASP1 did not show significant increases in all four subsets. In line with the mass spectrometry results in the Venn diagram, the abundance of LCP1 was significantly higher at post-exercise in both young and fit participants (p = 0.039 and p = 0.040, respectively). For MPO and S100A8, we observed that all four subsets of participants had significant increases in abundance after exercise (ranging from p < 0.001 to p = 0.003). While the exact functional relevance of each protein remains unclear, these results suggest distinct circulating sEV proteins in response to exercise in young, mature, unfit, and fit participants.
| Young (n = 14) | Mature (n = 14) | Unfit (n = 14) | Fit (n = 14) | |
|---|---|---|---|---|
| Age (years) | 27.1 ± 4.0 | 60.9 ± 6.1**** | 44.4 ± 18.7 | 43.7 ± 17.8 |
| Body mass (kg) | 78.6 ± 13.6 | 78.3 ± 13.6 | 83.4 ± 15.2 | 73.6 ± 9.2# |
| Height (cm) | 179.8 ± 5.6 | 177.1 ± 8.2 | 178.2 ± 7.3 | 178.6 ± 7.0 |
| BMI (kg m−2) | 24.4 ± 4.2 | 24.8 ± 2.6 | 26.2 ± 3.9 | 23.0 ± 2.0# |
| Est. VO2peak (mL kg−1 min−1) | 45.8 ± 11.8 | 45.8 ± 8.1 | 37.7 ± 5.5 | 53.9 ± 5.7#### |
| Average heart rate during exercise (bpm) | 147 ± 6 | 139 ± 9 | 139 ± 8 | 147 ± 6 |
| Average RPE during exercise (1–10) | 7 ± 1 | 6 ± 1 | 6 ± 1 | 7 ± 0 |
- Note: Young, 18–35 years; Mature, 50–70 years; Unfit, estimated VO2peak < 45 mL kg−1 min−1; Fit, estimated VO2peak > 45 mL kg−1 min−1.
- **** p < 0.0001 (Young vs. Mature);
- # p < 0.05;
- #### p < 0.0001 (Unfit vs. Fit).
3 DISCUSSION
Exercise elicits acute stresses and homeostatic challenge to the body with a myriad of integrative responses taking place at the cellular and systemic level.5 These responses include the activation of numerous signalling cascades and substantial crosstalk mechanisms between body tissues.4 While skeletal muscle adaptations and the release of humoral factors during exercise are widely studied, the discovery of EVs in cellular crosstalk during exercise is relatively new, and our knowledge of the influence of age and training status on exercise-derived sEV protein cargo is limited, particularly in the general population. Using quantitative proteomics of plasma sEVs collected from healthy men, we were able to detect changes in the sEV proteome after an acute bout of moderate-to-vigorous intensity exercise. We identified 13 proteins that were significantly increased after exercise in sEVs using mass spectrometry and correlated this increased release further using biochemical characterization. The majority of the increased sEV proteins were involved in the activation of immune system, and functional enrichment analysis further supported an increased response to immune system processes.
A strength of our study is the ability to interrogate changes in exercise sEV profiles from different ages and aerobic fitness, highlighting the changes that can occur in sEV protein secretion as we age or alter aerobic fitness levels. While ageing is associated with reduced immunity and elevated systemic inflammation (i.e., inflammaging), we show that exercise promotes the release of sEVs containing immune proteins after exercise, including ANXA1, LYZ, MPO, S100A8, and S100A9 in mature individuals. Of which, ANXA1, LYZ, MPO, and S100A8 were also found to be increased in young individuals. In particular, ANXA1, S100A8, and S100A9 are known danger/damage-associated molecular patterns (DAMPs).30 Although DAMPs are identified as pro-inflammatory mediators and often associated with diseases, recent evidence suggests that DAMPs-induced inflammatory responses following acute stress are important to prime and enhance innate immune function.31-34 Here, we observed that exercise results in the increase in sEV-associated DAMPs, suggesting that exercise-evoked acute stress can positively challenge the innate immune system by the release of DAMPs in sEVs.
Comparing the release of exercise sEVs across aerobic fitness levels, it is notable that a higher number of sEV proteins were altered in fit individuals as compared to unfit individuals; these were predominantly immune proteins. While it is unclear why a greater immune response was observed in fit individuals, it could be indicative that these individuals may have a higher immune competency as a response to regular physical exercise. In contrast to the fit group, which had 10 proteins significantly altered in exercise sEVs, our result showed that only four proteins were significantly increased in unfit individuals. It is unclear if the modest response from unfit individuals is due to a lower immune competency, or if the immune modulation via sEVs is attenuated in unfit people. We observed that ANXA6, EEF1A1, and ITGB2 are among the proteins that were increased only in fit individuals after exercise. Exercise can induce plasma membrane disruptions in skeletal muscle,35 and ANXA6 as a calcium-dependent membrane-binding protein has been shown to participate in membrane repair and protect against membrane injury in skeletal muscle.36, 37 EEF1A1 is an important component of protein translation machinery, and has been found to protect cells during heat shock response and to prevent protein misfolding in neurodegenerative diseases.38 ITGB2 is a leucocyte-specific adhesion molecule that is essential for leucocyte trafficking and function.39 In fit and young individuals, LCP1 was also found to be increased. This leucocyte-specific protein is identified to play critical roles in the regulation of immune cell processes, including antigen receptor signalling, adhesion, and motility.40 Together, the findings suggest the beneficial effects of exercise are correlated with the release of sEVs that contain an increased abundance of proteins in fit individuals compared to unfit individuals. The pathways contributing to the variation in exercise sEV protein cargo remain unclear; however, our results suggest that it may be influenced by factors such as age and aerobic fitness of an individual.
Further evidence of immune-related protein release in exercise sEVs is shown by the increased abundance of LYZ and LTF after exercise. Both LYZ and LTF are antimicrobial proteins that have been shown to increase in the blood and saliva during exercise.41-43 LTF was previously shown to be enriched in exercise-derived sweat EVs to regulate immunity.44 Despite the different sources of EVs, our data support the release of proteins with antimicrobial and immunological properties in sEVs during exercise. We also observed that LYZ, MPO, and S100A8 are significantly increased in all four participant subsets, in which the increase in MPO and S100A8 was further confirmed using Western blot. Similar to DAMPs, LYZ, MPO, and S100A8 are among the proteins that have been suggested to regulate the innate immune response.45 Collectively, these findings indicate that exercise can potentially regulate innate immune response, including antimicrobial activity, through the release of immune-specific proteins in sEVs.
Previously, a single bout of exercise has been shown to evoke a transient leucocytosis by mobilizing leucocyte subsets into the blood compartment including neutrophils, lymphocytes, and monocytes.46, 47 In pooled data, we observed the increase in several proteins that have been found earlier in neutrophil-derived EVs such as ANXA1, CTSG, MPO, LTF, and S100A8.48 LCP1 has also been previously identified in EVs from NK-cell-enriched lymphocytes.49 While the exact origin of the exercise sEVs described here remains to be determined, it is becoming increasingly clear that they are at least in part originating from cells in the circulatory system, including platelets, endothelial cells, and leucocytes. In support of this notion, Brahmer and colleagues observed a significant increase in surface markers for platelets (CD41b, CD42a, and CD62P), endothelial cells (CD31, CD105, and CD146), lymphocytes (CD4 and CD8), and leucocytes (CD40) in sEVs secreted during exercise, suggesting the potential origins of exercise sEVs in blood and immune cells.16 Currently, the mechanism for how immune proteins are secreted in exercise sEVs remains unclear, but our results suggest a pathway for the release of these proteins via sEVs derived from immune cells in the peripheral blood during exercise. This raises the potential that sEVs from acute moderate-to-vigorous intensity exercise are an important immune system adjuvant to stimulate the known ongoing exchange of leucocyte information between the circulation and tissues.50
Habitual exercise is known to improve immunity by lowering the risk of infection, reducing chronic low-grade inflammation, and enhancing immune responses to vaccination.51, 52 The exact mechanism of how exercise enhances immune function is currently unknown, but our results here suggest that exercise sEVs can play a role in this pathway. We propose that acute exercise stimulates circulating immune cells to release proteins in sEVs, which can have a role in immune system modulation. Importantly, it has been shown that immune cells are redeployed with every bout of exercise, and the summation of this transient increased mobilization of immune cells to peripheral tissues is thought to improve immune surveillance.45, 46, 51, 53 It is thus conceivable that exercise stimulates the release of sEVs from blood immune cells to increase overall immune surveillance, improving body function against infections. Of note, our findings are in contrast to the analysis of sEVs from a high-intensity exercise study that reported significant changes in >300 proteins.22 Interestingly, high-intensity exercise has previously been shown to limit immune response and dampen immune signalling.45, 54-56 The large increase in sEV proteins after high-intensity exercise may be an increased signalling response, or alternatively, an increase in the removal of waste products in sEVs during intense exercise. Although not identical methodologies, it is clear from our combined studies that changes in age, aerobic fitness, and exercise intensity can have a significant effect on the release of exercise sEVs and their cargo.
A limitation in all plasma EV studies is the technical challenges in isolating sEVs due to the complex mixture of components in plasma. Similar to other available plasma EV isolation techniques, we observed the presence of lipoproteins in our sEV samples, suggesting the co-isolation of lipoprotein particles. While the co-isolation of lipoproteins is unpreventable, the abundance remained unaltered in our studies here between pre- and post-exercise. It is therefore likely that the protein differences described in this study between resting and exercise can be attributed to the changes observed in response to exercise. Other than ApoA-1, other non-EV components such as other lipoproteins (e.g., VLDL and chylomicrons) and plasma proteins are likely to co-isolate with any of the plasma EV isolation techniques. Although GM130 was not detected, non-EV proteins such as albumin and other lipoproteins may also be present. It should be noted, however, that we only observed a small number of proteins that were significantly changed from pre- to post-exercise. In support of our EV isolation method, several recent studies have demonstrated that differential ultracentrifugation can isolate a greater purity of sEVs from blood plasma, as compared to other emerging techniques, including size exclusion chromatography57 and other commercial isolation kits.58, 59 Importantly, the majority of the immune-related proteins identified in the current study were also detected in plasma sEVs isolated using size exclusion chromatography,22 supporting the presence of these proteins in sEVs purified using differential ultracentrifugation in the current study.
Although we identified a number of immune proteins in our sEV preparations, it is not clear if they were associated with the sEVs or localized to the surface of the sEVs.60 Further experimentation (e.g., proteinase K digestion) would potentially determine the topology of these proteins. There is also the chance that proteins could be co-sedimented with EVs during the purification protocol; however, this is highly unlikely given the difference in size and density of EVs compared to proteins. Nevertheless, proteins either within or on the surface of EVs could potentially be involved in signalling, and all have been identified in multiple past experiments submitted to the ExoCarta database.25 A final limitation of our study was the age brackets selected for the participants, with the relatively close age gap between the young and mature groups potentially diminishing the ability to detect the effect of age on the release of sEVs after exercise. Moreover, a complete blood count analysis before and after exercise could provide information to delineate the link between modulation of immune cells and the release of immune proteins in small EVs during exercise.
4 MATERIALS AND METHODS
4.1 Participants
Twenty-eight healthy men who were part of a larger study17 were randomly selected from young, mature, and unfit and fit categories for additional proteomic investigation of sEVs, using an online random number generator available at https://randomchoicegenerator.com/. The inclusion criteria included aged between 18–35 and 50–70 years, healthy with no known clinical disease, and free from lower body injury 2 months prior to participation. All participants were informed of the procedures and possible risks associated with the experiments and provided written consent to participate in the study. All experimental procedures were approved by the Swinburne Human Research Ethics Committee (SHR project 2019/016) and conducted in accordance with the standards of the Declaration of Helsinki of the World Medical Association.
4.2 Experimental protocol
Participants were asked to attend two experimental trials on separate days, as previously described.17 In brief, during the first trial, participants were asked to perform a YMCA submaximal cycle ergometer test to estimate their maximum oxygen consumption (VO2peak). The YMCA submaximal cycle ergometer test was conducted as described.61 VO2peak was estimated according to the ACSM's guidelines.62 During the second trial, participants were asked to perform a 20-min continuous cycling exercise at a power output that elicited approximately 70% of individual estimated VO2peak. Heart rate and rating of perceived exertion (RPE) were recorded every 4 min during the cycling exercise. Before and immediately after the cycling exercise, 34 mL of venous blood samples was withdrawn from the medial cubital vein by venipuncture using a 21-gauge BD Vacutainer® Safety-Lok™ needle (BD Biosciences, Oxford, UK) into Acid Citrate Dextrose Vacutainer® (ACD-A, BD Biosciences, Oxford, UK). Prior to both experimental trials, participants were told to abstain from strenuous physical activity for 24 h, caffeine for 8 h, and food for 2 h.
4.3 EV isolation from blood plasma
Plasma was collected from blood after the first centrifugation at 2000g for 10 min and subjected to a second centrifugation at 2000g for 10 min at 4°C to obtain platelet-free plasma. The isolation of sEVs was achieved by a three-step differential centrifugation protocol with two ultracentrifugation steps. Equal volume (8 mL) of platelet-free plasma from pre- and post-exercise were centrifuged at 20 000g for 20 min at 4°C in a fixed-angle rotor (Type 70Ti, Beckman Coulter, Krefeld, Germany) to separate large EVs. The supernatant was then diluted with ice-cold phosphate-buffered saline (PBS) and centrifuged at 100 000g for 60 min at 4°C to collect sEVs. After careful removal of the supernatant, the sEV pellet was washed in ice-cold PBS at 100 000 g for 60 min at 4°C. Small EV pellets resulting from 100 000 g centrifugation were resuspended by repeated pipetting in the appropriate buffer. For Western blot analysis, pre- and post-exercise sEV pellets were resuspended in equal volume of Laemmli buffer and denatured at 95°C for 5 min. For mass spectrometry analysis, sEV pellets were resuspended in equal volume of PBS. As such, all pre- and post-exercise sEV samples were standardized with respect to equal starting volume of plasma. All samples were stored at −80°C until further analysis.
4.4 EV particle analysis
EV size and particle count analysis were performed using the nCS1 Instrument (Spectradyne, Torrance, CA, USA). In brief, pre- and post-exercise sEV samples were diluted to 1:10 in 20 nm filtered PBS containing 0.2% polysorbate and 5 μL of diluted samples was loaded onto TS-400 cartridges that allowed measurement for particles between 65 and 400 nm. Particle acquisition was carried out with the loaded cartridges using the Spectradyne nCS1 instrument and software. The acquired results were analysed using the Data Viewer software. Final particle counts were calculated by determining the diameter factor using standard-sized beads and the concentration factor after subtracting the blank.
4.5 Sample preparation for mass spectrometry (MS)
Small EV samples were lysed in 100 mM HEPES and 1% (w/v) sodium deoxycholate (SDC), pH 8.0. The samples were sonicated, and the protein concentration was determined using a BCA kit (Thermo Fisher, Waltham, MA, USA). Two-hundred micrograms of proteins from sEV lysate was reduced with 10 mM Tris(2carboxyethyl) phosphine (TCEP), alkylated with 40 mM 2-chloroacetamide (CAA), and then digested overnight with sequencing-grade trypsin (Promega) with the ratio of 1:100 (trypsin to total protein, w/w) at 37°C. Digestion of protein was stopped using 1% formic acid and desalted using in-house-made SDB-RPS (3 M Empore) tips. The eluted peptides were concentrated using a vacuum concentrator and reconstituted in loading buffer (0.1% formic acid and 2% acetonitrile).
4.6 Liquid chromatography–tandem MS (LC–MS/MS) analysis
For analysis of the global sEV proteome, the peptide samples were analysed by LC–MS/MS using a Dionex Ultimate 3000 RSLCnano equipped with a QExactive HF mass spectrometer (Thermo Fisher, Waltham, MA, USA). Samples were loaded onto an Acclaim PepMap 100 trap column (100 μm × 2 cm, nanoviper, C18, 5 μm, 100 Å; Thermo Fisher, Waltham, MA, USA) and separated on an Acclaim PepMap RSLC analytical column (75 μm × 50 cm, nanoviper, C18, 5 μm, 100 Å; Thermo Fisher, Waltham, MA, USA). The peptides were separated using a 120 min linear gradient at a flow rate of 250 nL min−1 using increasing concentrations of buffer B (0.1% formic acid and 80% acetonitrile) added to buffer A (0.1% formic acid). MS data were acquired with the mass spectrometer operated in data-dependent acquisition mode. MS1 spectra were acquired in the Orbitrap at 120 000 resolution (m/z 375–1575) using an automatic gain control (AGC) target of 3 × 106 with a maximum injection time of 54 ms. The top 12 intense precursor ions were isolated in the quadrupole using an isolation window of 1.4 m/z, and fragmented by higher-energy collisional dissociation (HCD) with a normalized collision energy of 27. MS2 spectra were acquired at 30 000 resolution using an AGC target of 2 × 105 with a maximum injection time of 54 ms.
4.7 Bioinformatic analysis
The acquired MS data files were analysed using MaxQuant (version 1.6.5.0) coupled with the Andromeda search engine to obtain protein identifications and their respective label-free quantitation (LFQ) intensities. Database searching was performed against the human SwissProt database, downloaded in June 2020 with the following parameters: carbamidomethylation as fixed modification; methionine oxidation and N-terminal acetylation as variable modifications; and 1% protein false discovery rate (FDR) for protein and peptide identification. The lists of proteins with LFQ values were analysed using LFQ-Analyst available at https://bioinformatics.erc.monash.edu/apps/LFQ-Analyst/. The data processing workflow was described previously.63
Comparison of sEV proteome in the current study versus manually curated EV databases in Venn diagram was carried out using FunRich tool (version 3.1.4) available at http://www.funrich.org/. Functional enrichment analyses were carried out on all significantly altered proteins using DAVID Bioinformatics Resources tool (version 6.8) available at https://david.ncifcrf.gov/tools.jsp with four databases including Gene Ontology biological process (GOBP), Gene Ontology molecular function (GOMF), Gene Ontology cellular component (GOCC) and Reactome Pathway database.64 Protein–protein interaction (PPI) network analysis was carried out using Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) tool (version 11.5) available at https://string-db.org/ with the confidence level set to 0.5.
4.8 Western blot analysis
Thirty microlitres (30 μL) of sEV pellets resuspended in Laemmli buffer were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. Samples were separated by SDS-PAGE, and transferred to polyvinylidene difluoride membrane (PVDF, Bio-Rad, Hercules, CA, US). Membranes were blocked with 5% milk powder in Tris-buffered saline containing 0.05% Tween-20 (TBST) and incubated sequentially with primary antibodies overnight at 4°C and HRP-coupled secondary antibodies for 60 min at room temperature. Proteins were detected using enhanced chemiluminescence (ECL) reagents (GE Healthcare, Chicago, IL, USA) on X-ray films. Commercially available antibodies were used, including rabbit anti-Tsg101 (Sigma-Aldrich, T5701, 1:1000), rabbit anti-syntenin (Abcam, ab133267, 1:1000), mouse anti-ApoA-1 (Santa Cruz, sc-376818, 1:1000), mouse anti-GM130 (BD Biosciences, #610822, 1:1000), rabbit anti-Annexin-6 (Abcam, ab199422, 1:1000), rabbit anti-BASP1 (Abcam, ab214322, 1:500), rabbit anti-plastin L (Abcam, ab109129, 1:10000), rabbit anti-Myeloperoxidase (Abcam, ab208670, 1:1000), and mouse anti-Calgranulin A (Santa Cruz, sc-48352, 1:100). Secondary antibodies that were used are horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Upstate Biotech, #12-348, 1:5000) and HRP-conjugated goat anti-mouse antibody (Upstate Biotech, #12-349, 1:5000). ImageJ software (version 1.52a, National Institutes of Health (NIH), Bethesda, MD, USA) was used for the semi-quantitative analysis of WB signals. WB signals with high background noise were excluded as data points in the quantitative analysis. WB signals of the proteins of interest were normalized to TSG101 signals. Post-exercise fold change of protein abundance was expressed in relation to pre-exercise.
4.9 Statistical analysis
Statistical analyses and the processed MS data were performed and visualized using GraphPad Prism 9 (GraphPad Software, CA, USA). Statistical outliers were detected and removed using ROUT method, hence were excluded and not represented as data points in the statistical analysis. Student's paired t-tests were used to compare the differences in sEV particle count and Western blot band densitometry from pre- to post-exercise. Linear mixed-effects models were used to compare the differences in Western blot band densitometry for four participant subsets from pre- to post-exercise. Fixed effects (group (young, mature, unfit, or fit), time (pre- and post-exercise), and random effect (participant)) were fitted for each dependent variable. A Bonferroni post hoc pairwise comparison was performed if a significant main effect and/or interaction effect was present. A 95% confidence interval was used to define the level of significance. Data are presented as mean ± SD, unless otherwise stated. Sample sizes and other statistical parameters are indicated in the figure legends.
5 CONCLUSION
There is a growing consensus among exercise immunologists that moderate-intensity exercise bouts of less than 60 min can enhance immune surveillance, compared to prolonged or intensive exercise that can lead to immune suppression.45 Here, we present evidence that further supports this notion, showing for the first time that moderate-to-vigorous intensity exercise results in an increased immune system response via sEVs. Overall, our results suggest the involvement of sEVs in modulating innate immune system and antimicrobial activity during exercise, via the transport of immune proteins in the systemic circulation. Our findings suggest a mechanism for how exercise-derived sEVs can potentially promote immune modulation to reduce risk of infection and chronic diseases as we age. Further studies are now required to confirm the role of exercise-derived sEVs in immune system signalling, which could play an important role in immune surveillance pathways promoted through exercise.
AUTHOR CONTRIBUTIONS
Jason Howitt: Conceptualization; writing – original draft; writing – review and editing; funding acquisition; supervision. Mee Chee Chong: Conceptualization; methodology; data curation; formal analysis; investigation; writing – original draft; writing – review and editing; project administration; validation. Anup D. Shah: Methodology; writing – review and editing; formal analysis. Ralf B. Schittenhelm: Methodology; formal analysis; writing – review and editing. Anabel Silva: Methodology; writing – review and editing. Patrick F. James: Methodology; writing – review and editing. Sam Shi Xuan Wu: Conceptualization; writing – review and editing.
ACKNOWLEDGMENTS
We are grateful to the volunteers for participating in the study. We thank Ms Michelle Bouman (Swinburne University of Technology) and Ms Iresha Hanchapola (Monash University) for assistance with some experiments. This study used BPA-enabled (Bioplatforms Australia)/NCRIS-enabled (National Collaborative Research Infrastructure Strategy) infrastructure located at the Monash Proteomics and Metabolomics Facility. Open access publishing facilitated by Swinburne University of Technology, as part of the Wiley - Swinburne University of Technology agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
No third-party funding was received for the submitted work.
CONFLICT OF INTEREST STATEMENT
A.S. and P.F.J. are employees of Exopharm Ltd. P.F.J is a shareholder of Exopharm. J.H. is a scientific consultant to Exopharm Ltd.
Open Research
DATA AVAILABILITY STATEMENT
All data generated in this study are included in this published article and its supplementary information. All relevant experimental parameters were submitted to the EV-TRACK knowledgebase (EV-TRACK ID: EV220329).65 The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository66 with the dataset identifier PXD038401.




