Influence of blood haemoglobin concentration on renal haemodynamics and oxygenation during experimental cardiopulmonary bypass in sheep
Abstract
Aim
Blood transfusion may improve renal oxygenation during cardiopulmonary bypass (CPB). In an ovine model of experimental CPB, we tested whether increasing blood haemoglobin concentration [Hb] from ~7 g dL−1 to ~9 g dL−1 improves renal tissue oxygenation.
Methods
Ten sheep were studied while conscious, under stable isoflurane anaesthesia, and during 3 hours of CPB. In a randomized cross-over design, 5 sheep commenced bypass at a high target [Hb], achieved by adding 600 mL donor blood to the priming solution. After 90 minutes of CPB, PlasmaLyte® was added to the blood reservoir to achieve low target [Hb]. For the other 5 sheep, no blood was added to the prime, but after 90 minutes of CPB, 800-900 mL of donor blood was given to achieve a high target [Hb].
Results
Overall, CPB was associated with marked reductions in renal oxygen delivery (−50 ± 12%, mean ± 95% confidence interval) and medullary tissue oxygen tension (PO2, −54 ± 29%). Renal fractional oxygen extraction was 17 ± 10% less during CPB at high [Hb] than low [Hb] (P = .04). Nevertheless, no increase in tissue PO2 in either the renal medulla (0 ± 6 mmHg change, P > .99) or cortex (−19 ± 13 mmHg change, P = .08) was detected with high [Hb].
Conclusions
In experimental CPB blood transfusion to increase Hb concentration from ~7 g dL−1 to ~9 g dL−1 did not improve renal cortical or medullary tissue PO2 even though it decreased whole kidney oxygen extraction.
1 INTRODUCTION
Acute kidney injury (AKI) is a common and serious complication of cardiac surgery requiring cardiopulmonary bypass (CPB).1 There is evolving evidence that current standard perfusion practice during CPB does not adequately protect the kidney,2-4 particularly the renal medulla,5, 6 from hypoxia, thus increasing the risk of cardiac surgery-associated AKI. Experimental models of CPB in rats,7 pigs,8 and sheep5, 6 are all associated with renal medullary tissue hypoxia. Computational models of renal oxygenation also predict medullary hypoxia during CPB.9-12 Furthermore, risk of AKI is associated with indices of intra-operative renal hypoxia, as assessed by urinary oxygen tension (PO2)13-15 or near infrared spectroscopy.16, 17 Thus, strategies aimed at improving renal oxygenation during CPB have the potential to mitigate post-operative AKI.
The clinical perfusionist could improve renal oxygen delivery (DO2) during CPB by manipulating pump flow and/or blood haemoglobin concentration [Hb]. Findings from the Goal-Directed Perfusion Trial (GIFT) indicate that avoidance of a systemic DO2 of less than 280 mL min−1 m−2 during CPB can mitigate the risk of AKI.18 The primary intervention in this trial was to increase pump flow. However, if this was not sufficient to achieve a systemic DO2 of at least 280 mL min−1 m−2, or if the mixed venous oxygen saturation was <68% or systemic oxygen extraction was >40%, a unit of whole blood was given. Thus, the intervention was complex, involving variable combinations of increased pump flow and increased blood [Hb]. In an ovine model of CPB, we recently demonstrated that increasing pump flow or arterial pressure within a clinically achievable range improved renal medullary tissue oxygenation.5 However, the effects of varying blood [Hb] in this model remain to be determined. Therefore, in the current study, we tested the hypothesis that renal haemodynamics and oxygenation during experimental CPB are dependent on blood [Hb]. Using a cross-over design, blood [Hb] was varied between ~7 g dL−1 and ~9 g dL−1 during experimental CPB in sheep.
2 RESULTS
2.1 Variables in conscious sheep
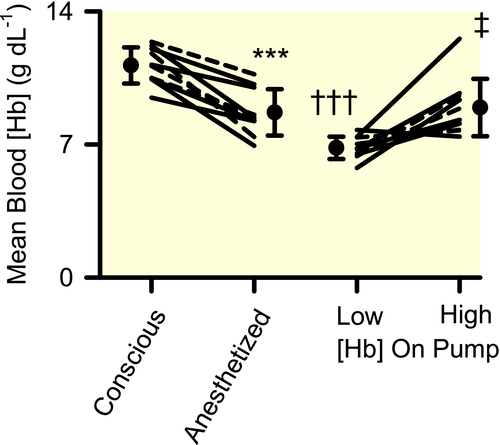
In conscious sheep, all variables were similar to those we have observed previously and were within the normal physiological range (Figures 1-3, Tables 1-4).5, 6
| Variable | n | 1. Conscious | 2. Anaesthetized | P1v2 | On bypass | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 3. Low Hb | P2v3 | 4. High Hb | P2v4 | P3v4 | |||||
| Body temperature (°C) | 10 | 39.5 ± 0.4 | 39.4 ± 0.4 | .63 | 10 | 35.9 ± 0.3 | <.001 | 35.8 ± 0.4 | <.001 | .99 |
| Central venous pressure (mmHg) | 9 | −0.2 ± 1.7 | 1.8 ± 2.5 | <.001 | 9 | 2.3 ± 2.7 | .02 | 2.3 ± 2.5 | .07 | >.99 |
| Systemic vascular conductance (mL kg−1 mmHg−1) | 10 | 1.02 ± 0.14 | 1.05 ± 0.14 | .12 | ||||||
| Systemic oxygen delivery (mL O2 kg−1 min−1) | 10 | 7.45 ± 1.23 | 9.61 ± 2.32 | .02 | ||||||
| Systemic oxygen consumption (mL O2 kg−1 min−1) | 10 | 2.48 ± 0.38 | 2.50 ± 0.53 | >.99 | ||||||
Note
- P values ≤ 0.05 are shown in bold. Data are expressed as mean ± standard deviation. Systemic vascular conductance is pump flow divided by the product of body weight and mean arterial pressure. Systemic oxygen delivery is the product of pump flow and arterial blood oxygen content. Systemic oxygen consumption is the product of pump flow and the difference in oxygen content of arterial and mixed venous blood. P values are the outcomes of Student's paired t test, with a Dunn-Sidak correction to account for the four planned comparisons in the analysis.
| Variable | n | 1. Conscious | 2. Anaesthetized | P1v2 | On bypass | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 3. Low Hb | P2v3 | 4. High Hb | P2v4 | P3v4 | |||||
| Renal vascular conductance (µL kg−1 min−1 mmHg−1) | 10 | 70.8 ± 18.8 | 39.7 ± 13.2 | <.001 | 10 | 28.7 ± 10.4 | .08 | 27.4 ± 12.3 | .03 | .93 |
| Cortical perfusion (units) | 10 | 2250 ± 1398 | 1672 ± 958 | .80 | 10 | 828 ± 610 | .15 | 710 ± 313 | .05 | .90 |
| Medullary perfusion (units) | 10 | 674 ± 566 | 644 ± 447 | >.99 | 10 | 272 ± 288 | .25 | 368 ± 386 | .36 | .73 |
| Urine flow (µL kg−1 min−1) | 10 | 15.4 ± 7.2 | 18.7 ± 13.1 | .97 | 10 | 41.6 ± 16.7 | .02 | 18.9 ± 11.0 | >.99 | <.001 |
| Sodium excretion (µmol kg−1 min−1) | 10 | 1.82 ± 1.66 | 2.54 ± 3.39 | .96 | 10 | 4.34 ± 3.07 | .63 | 1.43 ± 1.66 | .79 | .003 |
| CrCl (mL kg−1 min−1) | 10 | 2.49 ± 1.05 | 1.88 ± 1.08 | .70 | 10 | 1.12 ± 0.49 | .25 | 0.69 ± 0.40 | .01 | .05 |
| TNa+ (µmol kg−1 min−1) | 10 | 350 ± 169 | 271 ± 155 | .76 | 10 | 152 ± 70 | .15 | 98 ± 60 | .008 | .08 |
| Fraction Excretion of Sodium (%) | 10 | 0.55 ± 0.44 | 0.79 ± 0.59 | .74 | 10 | 2.62 ± 1.30 | .01 | 1.27 ± 0.78 | .40 | .01 |
| TNa+/renal VO2 (mol/mol) | 8 | 80.6 ± 34.4 | 87.0 ± 46.8 | >.99 | 7 | 83.7 ± 42.5 | >.99 | 42.2 ± 23.1 | .18 | .13 |
Note
- P values ≤ 0.05 are shown in bold. Data are expressed as mean ± standard deviation. Renal vascular conductance is renal blood flow divided by the product of body weight and mean arterial pressure. Creatinine clearance (CrCl) is the product of urine flow and urinary creatinine concentration divided by plasma creatinine concentration. Sodium reabsorption (TNa+) is the product of CrCl and plasma sodium concentration minus sodium excretion. P values are the outcomes of Student's paired t test, with a Dunn-Sidak correction to account for the four planned comparisons in the analysis.
| Variable | n | 1. Conscious | 2. Anaesthetized | P1v2 | On bypass | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 3. Low Hb | P2v3 | 4. High Hb | P2v4 | P3v4 | |||||
| PO2 (mmHg) | 10 | 97.3 ± 9.2 | 276.0 ± 24.8 | <.001 | 10 | 307.9 ± 31.6 | .08 | 317.1 ± 26.7 | .05 | .85 |
| SO2 (%) | 10 | 97.1 ± 1.7 | 99.5 ± 0.5 | <.001 | 10 | 99.3 ± 0.5 | .18 | 99.1 ± 0.4 | .09 | .12 |
| Hb (g/dL) | 10 | 11.3 ± 1.0 | 8.8 ± 1.2 | <.001 | 10 | 6.9 ± 0.6 | <.001 | 9.0 ± 1.5 | >.99 | .01 |
| Oxygen content (mL O2/dL) | 10 | 15.4 ± 1.2 | 12.8 ± 1.7 | .002 | 10 | 10.4 ± 0.8 | <.001 | 13.3 ± 0.7 | >.99 | .01 |
| PCO2 (mmHg) | 10 | 35.3 ± 3.1 | 29.2 ± 3.5 | .002 | 10 | 45.4 ± 6.1 | <.001 | 48.9 ± 7.1 | <.001 | .11 |
| pH | 10 | 7.52 ± 0.02 | 7.61 ± 0.04 | <.001 | 10 | 7.43 ± 0.06 | <.001 | 7.40 ± 0.07 | <.001 | .02 |
| Lactate (mmol/L) | 10 | 0.67 ± 0.39 | 1.01 ± 0.46 | .24 | 10 | 2.27 ± 1.12 | .04 | 2.41 ± 0.92 | .02 | >.99 |
| Sodium (mmol/L) | 10 | 137.6 ± 3.5 | 137.6 ± 3.5 | >.99 | 10 | 139.8 ± 2.8 | .54 | 141.0 ± 2.5 | .14 | .23 |
| Potassium (mmol/L) | 10 | 3.86 ± 0.30 | 3.53 ± 0.32 | .01 | 10 | 3.09 ± 0.30 | .001 | 2.99 ± 0.36 | .002 | .81 |
| Chloride (mmol/L) | 10 | 102.8 ± 4.0 | 103.8 ± 4.5 | .86 | 10 | 101.5 ± 3.3 | .29 | 102.6 ± 2.4 | .76 | .24 |
| Calcium (mmol/L) | 10 | 1.07 ± 0.10 | 1.03 ± 0.08 | .77 | 10 | 0.99 ± 0.09 | .84 | 1.05 ± 0.19 | .99 | .46 |
| Bicarbonate (mmol/L) | 10 | 28.7 ± 3.2 | 29.3 ± 2.5 | .85 | 10 | 29.6 ± 1.7 | >.99 | 29.5 ± 2.710 | >.99 | >.99 |
| Base-excess (mmol/L) | 10 | 5.84 ± 2.76 | 7.53 ± 2.10 | .08 | 10 | 5.20 ± 2.16 | .04 | 4.54 ± 3.21 | .18 | .88 |
Note
- P values ≤ 0.05 are shown in bold. Data are expressed as mean (standard deviation). Blood oxygen content was calculated as (0.0139 × [Hb] × SO2) + (0.003 × PO2). P values are the outcomes of Student's paired t test, with a Dunn-Sidak correction to account for the four planned comparisons in the analysis.
- Abbreviations: Hb, haemoglobin, PCO2, partial pressure of carbon dioxide, PO2, oxygen tension; SO2, saturation of haemoglobin with oxygen.
| Variable | n | 1. Conscious | 2. Anaesthetized | P1v2 | On bypass | P2v4 | P3v4 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 3. Low Hb | P2v3 | 4. High Hb | |||||||
| Mixed venous blood | ||||||||||
| PO2 (mmHg) | 10 | 39.6 ± 5.9 | 51.4 ± 11.1 | .04 | 10 | 46.9 ± 5.6 | .52 | 56.1 ± 12.8 | .77 | .07 |
| SO2 (%) | 10 | 64.5 ± 8.7 | 83.6 ± 8.1 | .003 | 10 | 70.1 ± 9.9 | .02 | 75.5 ± 11.7 | .17 | .23 |
| P50 (mmHg) | 10 | 31.4 ± 4.0 | 27.1 ± 4.8 | .02 | 10 | 33.8 ± 5.8 | .01 | 35.1 ± 6.0 | .004 | .25 |
| Hb (g/dL) | 10 | 11.1 ± 1.0 | 8.6 ± 1.3 | <.001 | 10 | 6.8 ± 0.6 | <.001 | 8.9 ± 1.6 | .99 | .01 |
| Oxygen content (mL O2/dL) | 10 | 10.2 ± 1.9 | 10.2 ± 1.6 | >.99 | 10 | 6.8 ± 1.2 | <.001 | 9.7 ± 2.7 | .97 | .03 |
| PCO2 (mmHg) | 10 | 40.5 ± 3.6 | 32.4 ± 3.6 | <.001 | 10 | 52.4 ± 6.7 | <.001 | 55.6 ± 8.5 | <.001 | .06 |
| pH | 10 | 7.46 ± 0.02 | 7.61 ± 0.13 | .02 | 10 | 7.38 ± 0.06 | .002 | 7.36 ± 0.07 | .002 | .36 |
| Lactate (mM) | 10 | 0.89 ± 0.42 | 1.10 ± 0.44 | .67 | 10 | 2.35 ± 1.22 | .06 | 2.54 ± 1.09 | .02 | .99 |
| Renal venous blood | ||||||||||
| PO2 (mmHg) | 8 | 57.5 ± 5.6 | 63.4 ± 7.2 | .25 | 7 | 54.8 ± 8.0 | .38 | 64.7 ± 12.0 | >.99 | .006 |
| SO2 (%) | 8 | 88.7 ± 2.3 | 90.9 ± 3.5 | .22 | 7 | 80.8 ± 7.0 | .10 | 83.3 ± 7.3 | .25 | .10 |
| Hb (g/dL) | 8 | 11.0 ± 1.2 | 8.5 ± 1.2 | .006 | 7 | 6.8 ± 0.7 | .01 | 9.1 ± 1.6 | .96 | .05 |
| Oxygen content (mL O2/dL) | 8 | 13.9 ± 1.3 | 11.0 ± 1.8 | .006 | 7 | 7.8 ± 0.5 | .01 | 10.7 ± 1.9 | >.99 | .03 |
| PCO2 (mmHg) | 8 | 36.2 ± 2.2 | 32.1 ± 3.9 | .02 | 7 | 50.3 ± 5.3 | .004 | 53.8 ± 8.8 | .008 | .23 |
| pH | 8 | 7.51 ± 0.03 | 7.58 ± 0.02 | <.001 | 7 | 7.41 ± 0.06 | .002 | 7.38 ± 0.08 | .002 | .19 |
| Lactate (mM) | 8 | 0.69 ± 0.44 | 0.96 ± 0.44 | .47 | 7 | 2.36 ± 1.33 | .16 | 2.14 ± 0.87 | .10 | .98 |
Note
- P values ≤ 0.05 are shown in bold. Data are expressed as mean (standard deviation). P50 is the partial pressure of oxygen at which haemoglobin is 50% saturated with oxygen. It was estimated from PO2 and SO2, in mixed venous blood, by the method of Doyle.50 Blood oxygen content was calculated as (0.0139 × [Hb] × SO2) + (0.003 × PO2). P values are the outcomes of Student's paired t test, with a Dunn-Sidak correction to account for the four planned comparisons in the analysis.
- Abbreviations: Hb, haemoglobin, PCO2, partial pressure of carbon dioxide, PO2, oxygen tension; SO2, saturation of haemoglobin with oxygen.
2.2 Effects of anaesthesia, prior to initiation of cardiopulmonary bypass
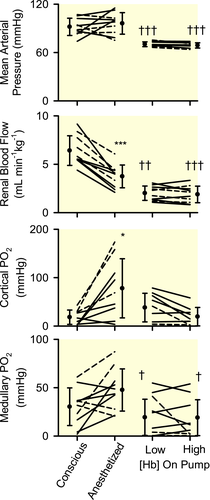
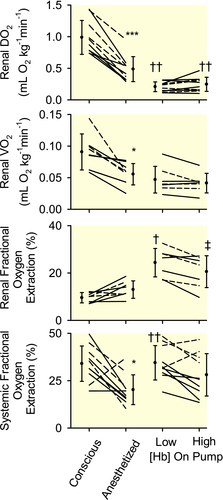
Isoflurane anaesthesia was accompanied by reduced blood [Hb] (−22 ± 6%, mean ± 95% confidence interval; Figure 1). Mean arterial pressure (MAP) did not change significantly, but there were marked reductions in renal blood flow (RBF, −42 ± 8%; Figure 2), renal vascular conductance (−46 ± 6%; Table 2), renal DO2 (−51 ± 8%; Figure 3) and renal oxygen consumption (VO2; −35 ± 12%; Figure 3). The PO2 at which haemoglobin was 50% saturated with oxygen (P50) in mixed venous blood fell by 4.4 ± 2.4 mmHg, indicating increased affinity of haemoglobin for oxygen (Table 4). Systemic fractional oxygen extraction fell by 35 ± 24% but renal fractional oxygen extraction tended to increase (by 41 ± 27%; P = .06). Neither cortical nor medullary perfusion were significantly altered (Table 2). Cortical oxygen tension (PO2) increased by 60 ± 36 mmHg but medullary PO2 was not significantly altered (Figure 2). Neither creatinine clearance nor total sodium reabsorption changed significantly (Table 2).
2.3 Effects of transition to cardiopulmonary bypass
Compared with the anaesthetized state, during CPB, MAP was 27 ± 7 mmHg less (Figure 2) and body temperature was 3.5 ± 0.3°C less (Table 1). RBF was 47 ± 10% less (Figure 2) and renal DO2 was 50 ± 12% less, but renal VO2 was not significantly altered (Figure 3). Medullary PO2 was 28 ± 17 mmHg less but cortical PO2 was not significantly altered (Figure 2).
Compared with the anaesthetized state, both systemic fractional oxygen extraction and renal fractional oxygen extraction were significantly increased during cardiopulmonary bypass at low, but not high, blood [Hb] (Figure 3). Calculated P50 of mixed venous blood was 7.3 ± 3.2 mmHg greater during CPB than during anaesthesia alone (Table 4), indicating reduced affinity of haemoglobin for oxygen during CPB.
2.4 Effects of altered blood [Hb]
Mean blood [Hb] was 6.8 ± 0.6 g dL−1 and 8.9 ± 1.5 g dL−1 respectively during the two experimental periods (Figure 1). Calculated mixed venous P50 did not differ significantly (Table 4), nor were any significant changes in MAP, systemic vascular conductance, RBF, or renal vascular conductance observed (Figure 2 and Tables 1 and 2). However, systemic DO2 increased by 29 ± 14% with increased blood [Hb], with no significant change in systemic VO2 (Table 1). Systemic fractional oxygen extraction tended to reduce, although this apparent effect was not statistically significant (P = .03 before and 0.12 after application of the Dunn-Sidak correction; Figure 3). Increased blood [Hb] was accompanied by a 9.9 ± 3.5 mmHg increase in renal venous PO2 and a 17 ± 10% reduction in renal fractional oxygen extraction (Figure 3). Nevertheless, trends for increased renal DO2 (18 ± 18%; P = .39; P = 12 before application of the Dunn-Sidak correction) and reduced renal VO2 (13 ± 14%; P = 0.58; P = .15 before application of the Dunn-Sidak correction) were not statistically significant. There was a 38 ± 21% reduction in creatinine clearance and 37 ± 21% reduction in total sodium reabsorption (Table 2). No significant changes in cortical or medullary tissue PO2 were observed (Figure 2). Furthermore, variations in cortical and medullary PO2 in the 5 sheep that commenced CPB at low [Hb] could not be distinguished from those in the 5 sheep that commenced CPB at high [Hb].
3 DISCUSSION
In a clinically relevant ovine model of CPB, we were unable to detect changes in renal cortical or medullary tissue PO2 as blood [Hb] was varied across a clinically relevant range, from an average of ~7 g dL−1 to ~9 g dL−1. This was despite the fact that increasing blood [Hb] increased both arterial blood oxygen content by 20% and systemic DO2 by 29% and reduced renal fractional oxygen extraction by 17%. Thus, our findings do not support the proposition that blood transfusion during CPB is an effective intervention to improve renal tissue oxygenation. In contrast, renal medullary hypoxia in this model can be mitigated by increasing renal DO2 through either increased pump flow, even in the absence of an associated increase in MAP, or by increasing MAP with the vasopressor, metaraminol.5 Thus, based on our findings using this model, manipulation of pump flow and arterial pressure appear to be more effective approaches to improve renal oxygenation during CPB than blood transfusion.
Pre-operative anaemia increases the risk of cardiac surgery associated AKI.19 This may at least partly be mediated by renal hypoxia, since experimental haemodilution can lead to renal tissue hypoxia,20-23 including during experimental CPB in the rat.7 However, for the most part, renal hypoxia in experimental models has been seen with relatively severe anaemia, resulting in reductions in arterial blood oxygen content in the order of 50%. A more clinically relevant question is whether renal tissue PO2 can be increased by blood transfusion, to increase blood [Hb] from a level which might trigger blood transfusion in the clinical setting during CPB (7-8 g dL−1), to a clinically achievable target of 9-10 g dL−1. Baseline blood [Hb] is slightly less in sheep than humans,24, 25 so we targeted blood [Hb] at the lower end of this range. We also applied a cross-over design rather than giving blood transfusions to all sheep after a period of haemodilution, to avoid potential time-dependent confounding. Our findings do not support the proposition that blood transfusion can improve renal tissue oxygenation during CPB. Thus, with regard to the GIFT trial,18 the critical component of the intervention may be increased pump flow rather than increased blood oxygen carrying capacity.
Our findings are also relevant to interpretation of the recent multicenter Transfusion Requirements in Cardiac Surgery III (TRICS III) Trial, which compared a restrictive (7.5 g dL−1) versus a liberal (9.5 g dL−1) threshold for blood transfusion during cardiac surgery.26 TRICS III provided compelling evidence for lack of inferiority of a restrictive threshold compared with a liberal threshold, for the primary composite endpoint of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis by hospital discharge or by day 28.27 A similar conclusion was drawn after follow-up of these patients 6 months after their operation.28 Importantly, in a pre-specified sub-study of TRICS III,29 no difference in the incidence of post-operative AKI was found between the restrictive and liberal thresholds.30 It is plausible that the lack of a reduction in the incidence of AKI when a more liberal transfusion threshold was used is at least partly attributable to the absence of a clinically significant impact of intra-operative blood transfusion on intraoperative renal medullary tissue hypoxia.
The failure of increased [Hb] to improve renal tissue oxygenation cannot be attributed to the potentially confounding effects of storage of donor blood, which increases the affinity of haemoglobin for oxygen.31 This effect can take between 5 days32 and two weeks33 to reach an equilibrium, but can be appreciable within the first 24 hours of storage.32 It is due in part to loss of erythrocyte cytoplasmic 2,3-diphosphoglycerate (DPG).31 Additional factors may also contribute, since even autologous salvaged blood, which is returned to the patient within a few hours using modern cell-saving techniques, had significantly greater haemoglobin affinity for oxygen than fresh blood, despite no significant difference in DPG concentration.34 However, calculated mixed venous blood P50 was not less at high [Hb] than low [Hb], indicating no appreciable effect of the donor blood on the affinity of haemoglobin for oxygen in vivo. This may reflect the relatively short period of cold storage of donor blood, which was 18 hours or less in all cases. Thus, it remains possible that longer storage of donor blood, as may occur in a clinical setting,35 could hinder tissue oxygenation.
The failure of increased [Hb] to improve renal tissue oxygenation is also unlikely to be due to increased local tissue oxygen consumption, since renal VO2 did not increase with increased blood [Hb]. Indeed, creatinine clearance was significantly less, and total sodium reabsorption tended to be less (P = .08), at high blood [Hb] than low blood [Hb]. But the impact of this on renal VO2 may have been offset by reduced efficiency of oxygen utilization for sodium reabsorption at higher blood [Hb], since there was at least a tendency for the ratio of sodium reabsorption to renal VO2 to be less (by 41 ± 29 mol/mol). This apparent effect was statistically significant without application of the Dunn-Sidak correction (P = .03) but not after the correction was applied (P = .13). Thus, our failure to detect a difference may represent a type 2 error.
Our results are consistent with the proposition that increasing blood [Hb] increases oxygenation of blood in the kidney. Renal fractional oxygen extraction was less (and renal venous blood PO2 was greater) at high than low [Hb], indicating improvement in the oxygen supply/demand relationship in the kidney. But this effect did not appear to lead to better tissue oxygenation. The failure of renal tissue PO2 to increase with increased blood [Hb], even though renal venous PO2 increased by ~10 mmHg, likely reflects reduced efficiency of oxygen extraction from blood to tissue. A similar phenomenon, of dissociation between changes in renal venous blood PO2 and renal tissue PO2, has been described in anaesthetized rabbits in response to changes in renal vascular resistance.36-38 The mechanisms underlying these phenomena remain unknown. However, arterial-to-venous (and in the medulla descending to ascending vasa recta) oxygen shunting12, 39 and increased heterogeneity of tissue perfusion40 and thus oxygenation41 could potentially lead to less efficient oxygen transport to tissue. Regardless, our current findings show that, during experimental CPB in sheep, renal venous blood PO2 can be increased by increasing blood [Hb] without appreciable changes in cortical or medullary tissue PO2.
CPB in humans is usually associated with the onset of a brisk diuresis.15 In our current experiment in sheep, low blood [Hb] during CPB was associated with approximately two-fold increases in creatinine clearance, fractional excretion of sodium, and urine flow, and a three-fold increase in sodium excretion. This phenomenon can likely be partly explained by the effects of reduced plasma oncotic pressure42 caused by haemodilution with crystalloid. A similar mechanism likely contributes to diuresis during CPB in humans, although other mechanisms, including the ability of mild hypothermia to inhibit renal metabolism,9 and of cold exposure to inhibit secretion of arginine vasopressin,43 may contribute.
The haemodilution we observed under anaesthesia could be attributable to two potential mechanisms. Firstly, urine flow under anaesthesia (18.7 ± 13.1 µL kg−1min−1) was only about half the infusion rate of compound sodium lactate (33 µL kg−1 min−1), so an increase in whole body water after induction of anaesthesia is likely (~1 mL per kg of body weight over an hour). However, we believe this is probably only a minor effect since the blood sampling during the experimental period under anaesthesia occurred within 30 minutes of induction of anaesthesia and thus the commencement of the infusion. The other potential mechanism is auto-transfusion. In our current study MAP under isoflurane anaesthesia was similar to that in the conscious state. But, based on our previous observations with isoflurane anaesthesia,44, 45 cardiac output is less and so total peripheral resistance must be greater in the present study to sustain MAP. We would therefore expect capillary hydrostatic pressure to be less under general anaesthesia than in the conscious state, leading to bulk flow of extracellular fluid into the vascular compartment. The level of haemodilution appears to be similar with isoflurane anaesthesia5 and propofol/fentanyl anaesthesia,6 although this comparison is confounded by the fact that propofol anaesthesia is associated with an obligatory fluid load due to the vehicle for the intravenous anaesthetic administration.
Strengths of our current study include the use of a clinically relevant large animal model of CPB. We targeted blood [Hb] relevant to recent clinical trials of blood transfusion protocols (TRICS III)30 and goal directed perfusion (GIFT)18 in cardiac surgery which have included the outcome of AKI. Nevertheless, the fact that we studied young and healthy sheep does limit our ability to generalize our findings to the population of patients who undergo cardiac surgery, who are often relatively old and suffer from multiple co-morbidities. We also cannot completely exclude the possibility that our failure to detect effects of altered blood [Hb] on cortical and medullary tissue PO2 represents a type II error. But post-hoc analysis of the current data indicate that our study was adequately powered. The standard deviations of the differences in cortical and medullary PO2 between the first and second 30 minutes period at each level of haemoglobin, reflecting within-subject variability, were both ~8 mmHg. Based on this, a sample size of 10 sheep would permit detection of a 12 mmHg change in cortical or medullary PO2, even after application of the Dunn-Sidak correction to account for multiple comparisons. By comparison, in a previous study we found that increasing pump flow from 80 to 100 mL kg−1 min−1 increased medullary PO2 by 9.5 mmHg, and increasing MAP with low dose metaraminol, from ~63 to ~90 mmHg, increased medullary PO2 by 20.2 mmHg.
In conclusion, our findings provide evidence that blood transfusion, to increase blood [Hb] from ~7 g dL−1 to ~9 g dL−1, is an ineffective intervention to increase renal tissue oxygenation during experimental CPB in sheep. Our findings cannot be explained by the impact of donor blood on haemoglobin-oxygen affinity in vivo. They provide a potential explanation for the lack of efficacy of a liberal transfusion strategy to reduce the incidence of AKI in the TRICS III trial.30 They also provide insight into the potential mechanisms underlying the success of goal directed perfusion, as demonstrated by the GIFT trial; namely that increasing pump flow and/or arterial pressure during CPB, rather than blood transfusion to increase blood oxygen carrying capacity, best protects the kidney from tissue hypoxia, thus mitigating the risk of post-operative AKI.
4 MATERIALS AND METHODS
4.1 Ethics
Experiments were approved by the Animal Ethics Committee of the Florey Institute of Neuroscience and Mental Health under guidelines of the National Health and Medical Research Council of Australia. All studies fulfilled the Animal Research: Reporting of In Vivo Experiments (ARRIVE) criteria.46 Prior to experimentation the 10 sheep, of body weight 37.0 ± 5.7 kg (mean ± standard deviation, range 31.5-51.0 kg) were housed in individual metabolic cages with free access to water and 800 g of oaten chaff daily. The samples size was based on findings from a prior study in which we detected improved renal medullary tissue PO2 during CPB, in response to either increased pump flow or vasopressor therapy to increase arterial pressure, with observations from 10 sheep with working oxygen probes in the renal medulla.5 These probes were operable in all 10 sheep we studied, so the experiment was terminated once the tenth sheep was studied. The authors confirm that this work conforms with good publication practice in physiology.47
4.2 Surgical preparation
As described in detail previously,6 sheep underwent a preliminary operation under isoflurane anaesthesia to implant a transit-time flow probe around the renal artery for measurement of RBF, fibre-optic probes in the renal cortex and medulla for measurement of tissue PO2, tissue perfusion by laser Doppler flowmetry, and tissue temperature,48, 49 and catheters were inserted in the renal vein, carotid artery, jugular vein and bladder. The experiment was performed five days later.
4.3 Experimental measurements
MAP, RBF (mL min−1), and cortical and medullary perfusion (arbitrary units), tissue PO2 (mmHg) and temperature (°C) were recorded digitally.48 Arterial, mixed venous and renal venous blood samples were collected, at the mid-point of each experimental period, for oximetry and blood chemistry (ABL systems 625, Copenhagen, Denmark). P50 was calculated by the method of Doyle.50 Plasma and urine concentrations of creatinine and sodium were measured in a hospital pathology laboratory.
4.4 Experimental protocol
Prior to the experiment, 600-900 mL of blood was obtained from two donor sheep (300-500 mL from each). The blood was heparinized (12.5 IU/mL) and stored at 4°C until use. Blood was warmed to room temperature and used within 18 hours of collection.
Each animal was first studied while conscious and unrestrained in its home-cage (30 min). Anaesthesia was then induced with sodium thiopental (15 mg kg−1), and after intubation, maintained on isoflurane (2.0%-2.5%) delivered through artificial ventilation (before CPB) or through the membrane oxygenator (during CPB). Once anaesthesia was induced, sheep received a continuous infusion of compound sodium lactate at 2 mL kg−1h−1 until termination of the experiment. This equates to a constant fluid input of 33.3 µL kg−1min−1 and sodium input of 4.35 µmol kg−1 min−1. Once stable anaesthesia was achieved, a second 30 minutes experimental period commenced.
CPB was established, as previously described in detail,6 at a flow rate of either 80 mL kg−1 min−1 (n = 6) or 60 mL kg−1 min−1 (n = 4), a target arterial pressure of 70 mmHg, and a target body temperature of 36.5°C. Metaraminol was administered in boluses of 0.1 mg to achieve target MAP in 3 of the 10 sheep (total doses of 20.5, 30.0 and 14.0 mg respectively). We reasoned that use of two rates of pump flow would provide a range of levels of renal oxygenation across the 10 sheep in the study, thus optimizing the conditions for observing differences in renal tissue PO2 at high and low [Hb]. The dataset was not stratified by pump flow.
A within-subject cross-over design was deployed. Five sheep were placed on CPB at ‘high [Hb]’ for 90 minutes followed by ‘low [Hb]’ for 90 minutes. The other five sheep were randomized to low [Hb] followed by high [Hb].
Therefore, within each pump flow rate, sheep were randomized to either low or high [Hb] as described below:
- For five sheep, the priming solution for CPB comprised 600 mL of blood from donor sheep, 1 g cefazolin (AFT Pharmaceuticals, NSW, Australia), 50 mL mannitol (20% w/v Osmitrol, Baxter, NSW, Australia) and 10 000 IU heparin (Heparin Injection, Pfizer, NSW, Australia) with the volume made up to 1.25 L with PlasmaLyte 148® (Baxter, NSW, Australia). Once CPB was established, the heart was fibrillated with a 9 V direct current across the ventricle. A total of 3 hours of CPB then followed. After 30 minutes of stable CPB, there were two 30 minutes experimental periods at high [Hb], with a target of ~9 g dL−1. We then removed blood from the pump reservoir and added PlasmaLyte, to target a blood [Hb] of ~7 g dL−1. Across the five sheep in this arm of the study, the total volume of blood removed from the pump reservoir ranged from 500 mL to 2.5 L. The total volume of PlasmaLyte® added ranged from 800 mL to 2.5 L. Target [Hb] was achieved over a 30 minutes run-in period, after which there were two 30 minutes experimental periods at low [Hb].
- These 5 sheep were treated identically to those described above, except that (i) the donor blood was not added to the CPB priming solution, so the first two experimental periods on CPB for these sheep were at low [Hb]. After these periods, excess blood was removed from the pump reservoir (0-1.0 L) and 800-900 mL of donor blood was added. A 30 minutes run-in period was followed by two 30 minutes experimental periods at high [Hb].
4.5 Statistical analysis
Absolute levels of variables are expressed as between-sheep mean ± standard deviation. Changes are expressed as mean ± 95% confidence interval. Nearly all variables remained stable across the two 30 minutes periods at either high or low [Hb]. Therefore, for analytical purposes these 30 minutes periods were combined. Specific contrasts were made using Student's paired t test with a Dunn-Sidak correction.51 Two-sided P ≤ .05 was considered statistically significant.
ACKNOWLEDGEMENTS
The authors thank Mr Tom Vale and Mr Tony Dornom for their technical assistance.
CONFLICT OF INTEREST
None. The results presented in this paper have not been published previously in whole or part, except in abstract format.
AUTHORS’ CONTRIBUTIONS
CNM, YRL, RGE, ADC and RB contributed to study concept and design. YRL, SGH, ADC, CNM, BM, PRM, RGE and NO contributed to acquisition, analysis and interpretation of data. RGE contributed to drafting of the manuscript. YRL, CNM, RB, ADC, BM, PRM, SGH and NO contributed to critical revision of the manuscript for intellectual content. RGE, CNM, ADC and RB contributed to obtaining funding. RGE contributed to statistical analysis.
Funding information
This work was supported by the National Health and Medical Research Council of Australia (GNT1122455; GNT1185777), the Victorian Government Operational Infrastructure Support Grant, and the National Heart Foundation of Australia (101853).
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.




