Endogenous flux of nitric oxide: Citrulline is preferred to Arginine
Abstract
Both arginine (Arg) and its precursor citrulline (Cit) have received much interest in the past two decades because of their potential effects on whole-body nitric oxide (NO) production and augmentation of NO-dependent signalling pathways. However, the usefulness of Arg supplementation for NO production is questionable because of its high splanchnic first pass metabolism (FPM), which limits its systemic availability. Both hepatic- and extrahepatic arginases critically limit the availability of Arg for the NO synthase enzymes (NOSs) and therefore, a limited amount of oral Arg can reach the systemic circulation for NO synthesis. Arg also has some undesired effects including induction of arginase activity, an increase of urea levels, a decrease of cellular uptake of Cit and decrease of recycling of Arg from Cit. In contrast, Cit has more availability as an NO precursor because of its high intestinal absorption, low FPM and high renal reabsorption. At the cellular level, co-localization of Cit transport systems and the enzymes involved in the Cit-Arg-NO pathway facilitates channelling of Cit into NO. Furthermore, cells preferably use Cit rather than either intra- or extracellular Arg to improve NO output, especially in high-demand situations. In conclusion, available evidence strongly supports the concept that Cit leads to higher NO production and suggests that Cit may have a better therapeutic effect than Arg for NO-disrupted conditions.
1 INTRODUCTION
Arginine (Arg), a conditionally essential amino acid, has received significant research interest over the last two decades, because of its involvement in several metabolic pathways.1 Although Arg has key roles in synthesis of proteins, creatine, polyamines, agmatine and urea, as well as in the metabolism of proline and glutamate in the body,1 the focus has been on its capability as a unique precursor of nitric oxide (NO).2 This property has led to its wide use as a complementary treatment in various NO-disrupted conditions such as, hypertension,3-5 pre-eclampsia6 and endothelial dysfunction.7 Despite lack of direct evidence, some effects of Arg supplementation on NO-related functions, for example, regulation of blood pressure and vascular function, have led to a dominant paradigm that oral administration increases its systemic availability and promotes NO production. This notion has now been challenged because there is strong evidence indicating limited bioavailability of orally ingested Arg,8, 9 its plasma and cellular compartmentalization and the competitive pathways utilizing Arg for synthesis of urea and ornithine.10, 11
Citrulline (Cit) is a neutral, non-essential, non-protein amino acid, which is an intermediary in the urea cycle and also a precursor of Arg de novo synthesis and NO production.12 Currently, Cit is receiving much attention as a natural NO precursor, especially for its potential cardiovascular and anti-hypertensive benefits and also for enhancing exercise performance and recovery.13-17 This is because Arg supplementation is not as effective as Cit for NO production18 and has some undesirable effects including induction of arginase activity,19, 20 increased urea levels,21 a decrease in cellular uptake of Cit,22 decrease in recycling of Arg from Cit,20 suppression of eNOS expression and activity23 and induction of cellular oxidative stress.23 In addition, there is some evidence suggesting the existence of cellular ‘Arg tolerance’ following long-term Arg exposure, a phenomenon that reduces the expected beneficial effects of long-term Arg supplementation.23 Furthermore, there is some debate regarding the efficacy24 and safety25 of long-term Arg supplementation, for example, an increased risk of myocardial infarction and mortality rate have been reported following supplementation of 9 g/d of Arg for 6 months.25 There are also numerous reports supporting the efficacy and safety of Cit13, 26, 27 thus highlighting the importance of Cit as a precursor of Arg and NO.
In this review, we summarize the evidence suggesting that supplementation with Cit is superior over Arg itself to improve Arg systemic bioavailability and NO production. If Cit is unequivocally shown to be superior over Arg as a safe and effective precursor for endogenous NO production, then its supplementation will receive further justification in medical practice.
2 ARG AND CIT BIOSYNTHESIS
Arg is provided by both exogenous dietary intake and endogenously through protein breakdown and de novo synthesis (Figure 1A). In healthy adult humans, Arg is considered to be a non-essential dietary amino acid because of its sufficient endogenous synthesis.28 Dietary proteins are a major source of Arg; seafood, nuts, seeds, alga, meats, rice protein concentrate and soy protein are considered to be Arg-rich foods.12
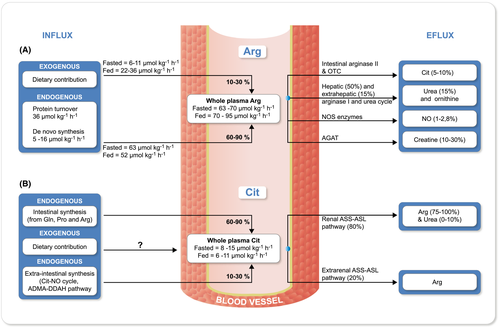
Plasma Arg level ranges 80-120 µmol L−1 in healthy adults.1, 29 Fluxes of plasma Arg in healthy humans on a normal diet have been estimated to be 63-70 and 70-95 µmol kg−1 h−1 in fasted and fed states respectively.8, 11 Depending on Arg content, the contribution of diet to plasma Arg flux is as low as ~8%-20% in the fasted state (6-11 µmol kg−1 h−1) to as large as ~35%-40% in postprandial state (~22-36 µmol kg−1 h−1).30-32
In healthy adults ~10%-15%12, 33 of the plasma Arg flux originates from de novo synthesis (~5-16 µmol kg−1 h−1),11, 30, 34 which mainly occurs in the proximal tubules of the kidneys. About 50%-80% of circulating Cit is taken up by the kidneys,35, 36 of which 75%37 to 100%35 is converted to Arg, by the enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). Other cells such as activated macrophages38 and the endothelial cells39 can take up Cit and use it as a substrate to synthesize Arg and NO.
Following a 7-day Arg-free diet, plasma flux of Arg (~52 µmol kg−1 h−1) is enriched entirely by protein turn over (~36 µmol kg−1 h−1) and de novo synthesis (16 µmol kg−1 h−1).30 Following an Arg-rich diet, the absolute values for the contribution of protein turnover and de novo synthesis in the whole plasma Arg flux remain constant; however, because of increased whole plasma Arg value, the percent contribution for protein turnover is decreased from 70% to 40% and for de novo synthesis from 30% to 18%.30
Plasma Cit is mainly supplied through endogenous synthesis with limited amounts obtained from usual dietary intake as its food source is relatively limited.14 Although watermelon is a rich source of Cit (~1.6-3.5 g kg−1 fresh weight), it would take about 1-1.5 kg of fresh watermelon to provide the minimum effective amount of Cit, which is 3 g per day.14 Cit content is higher in watermelon rind compared to its flesh, ~ 24.7 vs 16.7 mg g−1 dry weight.40 There is no recommended dietary allowance for Cit.12
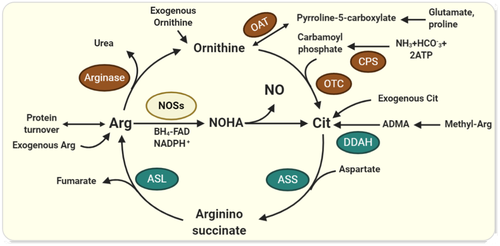
Mean plasma Cit concentration has been reported to be about 20-40 µmol L−134, 41-44 and whole-body Cit flux is estimated to be roughly about 8.9 μmol kg−1 h−1 (6-10 μmol kg−1 h−1) in healthy adults in the fasted state.41 The intestine is the principal site of Cit biosynthesis37 and 60%-90% of plasma Cit flux comes from this source45 (Figure 1B). Glutamine via glutaminase and pyrroline-5-carboxylate synthase pathway is the main precursor of Cit biosynthesis (60%-80%) in humans.33, 35 Proline via proline oxidase and ornithine aminotransferase (OAT), and Arg through arginase activity, are also involved in supplying the ornithine that is used for Cit biosynthesis in the enterocytes.46, 47 The ornithine is finally converted into Cit by the action of ornithine transcarbamylase (OTC) and carbamoyl phosphate synthetase I (CPS-I).44 High-protein diets and excess Arg intake downregulate CPS-I and OTC, reducing the conversion of ornithine to Cit.20, 48
Another potential source of systemic Cit is the Arg-NO pathway in NO-producing cells (Figure 2), which accounts for the release of Cit into the systemic circulation at a rate of 1 µmol kg−1 h−1 (~10% of plasma Cit flux, which is 9.5 µmol kg−1 h−1).11 Cit can also be synthesized and released into circulation through degradation of asymmetric dimethylarginine (ADMA) via dimethylarginine dimethyloaminohydrolase (DDAH)49, 50 (Figure 2). The relative contribution of these potential sources to the supply of circulating Cit has not been quantified; however, a comparison of the net intestinal Cit release to that of the net renal uptake in the fasted state (ie 2.4 ± 0.4 vs 3.0 ± 0.5 µmol kg−1 h−1),45 implies that the extra-intestinal tissues contribute about 20% (10%-30%) of the plasma Cit. The extra-intestinal Cit biosynthetic pathways (ie citrulline-NO cycle and ADMA-DDAH pathway) seem to be crucial in supporting the local requirements of Cit in NO-producing cells rather than supplying to the systemic pool.
3 SYSTEMIC BIOAVAILABILITY OF EXOGENOUS ARG AND CIT
The main evidence justifying preference for Cit over Arg supplementation comes from preliminary isotope studies addressing the gut-liver metabolism of amino acids,8, 9, 11, 30-32, 51, 52 and more recent investigations evaluating pharmacokinetics of exogenous doses (from low to high doses, ie 2-15 g per day) of Arg compared to that of Cit.18, 53 All evidence indicates a limited systemic bioavailability of oral Arg, alongside a more efficient and robust metabolism of Cit to elevate Arg flux.
3.1 Gut-liver metabolism of Arg
3.1.1 Arg absorption
Arg is absorbed in the small intestine, mainly from the jejunum, via a transport system that is shared with lysine, ornithine and cysteine,36 however, Arg has relatively poor absorption compared to the other amino acids.54 Intestinal Arg absorption has saturable kinetics (Km or Kt = 3.8 mmol L−1; Vmax = 46.3 μmol min−1 30 cm−1); jejunal perfusion of high doses of Arg ceases its intestinal absorption and results in excessive secretion of water and electrolytes into the lumen, this is considered to be a non-specific toxic reaction of Arg on the mucosa.54 When intestinal segments of rats were perfused with Arg at a dose of 20 mmol L−1, the observed absorption rate was 9.8 µmol min−1 g−1.55
3.1.2 Splanchnic uptake of Arg
Arg is largely extracted by the splanchnic region, intestine and liver51, 56 and mainly converted to urea and ornithine through first pass metabolism (FPM)10, 11 (Figure 3). Since arginase II activity in the intestine and arginase I activity in the liver are relatively high, 40%-60% of exogenously absorbed Arg is removed by FPM within the intestinal–liver bed.10, 11, 51 The rate of splanchnic uptake of dietary Arg is estimated to be 2.8-3.5 µmol kg−1 h−1, where the rate of its dietary input is ~4.7 µmol kg−1 h−1 (obtained by intragastric doses of 5.3 µmol kg−1 [13C]arginine or [M4]arginine, over 3 minutes).51 Arg FPM is not a fixed fraction and the habitual dietary intake of proteins,57 body protein/Arg demand and the amount of ingested Arg are all important modifying factors.20, 58 Increased ingested doses of Arg substantially promote FPM by ~2-fold (from 32.5% in control diet providing 75 µmol kg−1 h−1 Arg to 60.4% in supplemented diet providing 375 µmol kg−1 h−1 Arg).58
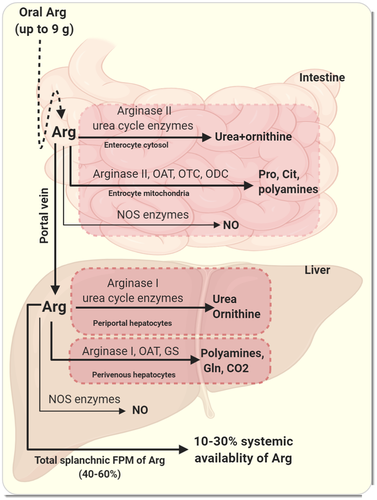
Relative contribution of intestine and liver in Arg FPM has not been exactly quantified; however, existence of zone-specified hepatic utilization of Arg and well-structured compartmentalization of Arg metabolism within the hepatocytes,57, 59-61 suggest that the contribution made by the liver is more important than that of the intestine. Animal experiments also indicate that the liver is the main site of Arg removal during FPM since lack of arginase II (the intestinal isoform of arginase) causes only a 10% reduction in Arg FPM from 75% to 65%.58
3.1.3 Intestinal and hepatic metabolism of Arg
After entering the absorptive epithelial cells of the small intestine, Arg is mainly degraded by arginase II that is located in both the cytosol and the mitochondria of the enterocytes, and to a much lesser extent by the nitric oxide synthase (NOS) enzymes.62 The intestinal arginase II is co-localized with ornithine-utilizing enzymes [ie OAT, OTC and ornithine decarboxylase (ODC)] in the mitochondria, and the produced ornithine can therefore contribute to the synthesis of proline, polyamines and Cit.9, 51, 63 A minor fraction of Arg is converted to NO in the enterocytes.9
The Arg presented to the liver is mainly converted to urea and ornithine within the periportal hepatocytes,61 where arginase I is co-localized with the urea cycle enzymes.59 Because hepatic arginase I activity is high and there is tight channelling of metabolites of the urea cycle to the next enzymes within the pathway,64, 65 there is little or no net production and export of Arg by the liver.2 The fraction of orally ingested Arg that is converted to urea during FPM is anything between 15% to 60%.10, 11 In healthy adults, the hepatic Arg flux within the urea cycle is estimated to be about 239 µmol kg−1 h−1 in the fed state,11 which is mainly (~95%) obtained by dietary rather than plasma Arg.10, 11 The conversion of ingested Arg into urea occurs rapidly within early catabolic stage and reaches its maximum value within 30 minutes after ingestion; 90% of the whole-body urea production from dietary Arg occurs almost entirely during its first pass, that is within the first 2-hour post-ingestion period.10 In normal situations, the NO pathway as well as synthesis of glutamine and polyamine from ornithine do not substantially contribute to the hepatic catabolism of Arg.66, 67 As a result of the co-existence of arginase I with OAT and glutamine synthetase (GS) in the perivenous cells (hepatocytes in zone 3 of the hepatic acinus60), the complete Arg catabolic pathway resulting in CO2 production occurs in these cells.61
3.1.4 Systemic availability of oral Arg
As a result of substantial splanchnic FPM, limited amounts of intact ingested Arg (~10%-30%) reach the circulation.51, 56, 68 However, in a recent study, the postprandial contribution of orally ingested Arg (acute doses of 1.8 and 4.9 g) to the total plasma pool of Arg was estimated to be 57% and 70%, respectively10; this estimation was obtained based on the [15N-15N-(guanido)]-arginine that appeared in the plasma and did not differentiate intact ingested Arg from that was recycled, i.e. resynthesized from Cit by the kidney.10 The Arg-Cit-Arg cycle via the intestinal-renal axis contributes to the fraction of Arg reaching the systemic circulation. The contribution of recycled-Arg to the total postprandial increased plasma Arg levels is estimated to be about 15%,10 which is close to the percentage of Cit produced by the intestinal trapped Arg within FPM (~17%).9
Taken together, oral intake of Arg is not an efficient strategy to potentiate NO synthesis. Multi-complex catabolic pathways that are activated upon exogenous Arg intake limit its accessibility to the systemic circulation and thus a large amount of oral Arg is required to provide a therapeutic plasma level; such high doses may increase blood nitrogen urea levels especially in cases where renal function is insufficient, as a major amount of ingested Arg is metabolized into urea and ornithine.
3.2 Gut-liver metabolism of Cit
3.2.1 Cit absorption
Orally ingested Cit can effectively be absorbed by the enterocytes, especially in the middle to the lower ileum,69 and transported to the portal circulation using a Na+-dependent transport system (B0+) and Na+-independent saturable transport (L and b0,+) systems.22 Such a broad set of transporters involved in intestinal Cit transport may be a compensation for the low Cit content in foods, and may explain why there is better bioavailability of Cit compared to Arg. In addition, it may also explain as to why high doses of Arg but not Cit cause osmotic diarrhoea.22 Km of Cit uptake in a model of human intestinal epithelial cell was 0.46 and 0.67 mmol L−1 for the Na+-dependent and Na+-independent mechanisms; Vmax was 3.58 and 2.16 nmol mg−1 min−1 in the Na+-dependent and Na+-independent uptake respectively.22
3.2.2 Splanchnic FPM and systemic availability of Cit
In contrast to Arg, Cit seems to effectively bypass the intestinal and liver metabolism15, 37 since absorbed Cit appears not to be metabolized by the enterocytes and hepatocytes.37 Extraction of Cit is not significantly different from zero across the portal drained viscera (PDV) following intravenous infusion of L-[ureido-13C–2H2]Citrulline (infusion rate of 0.14 µmol kg−1), indicating that net PDV Cit flux equals total Cit production.33 Our initial understanding was that Cit passes through the liver without being taken up37; however, further studies showed that indeed the liver can take up about 55% of the Cit that is released by the intestine33 or around 8%-12% of the whole flux.45, 70 Nevertheless, the net hepatic exchange of Cit remains close to zero, because of the similar rates in its active hepatic uptake and active hepatic release, estimated to be about 5 µmol kg−1 h−1.33 How the Cit taken up by the liver is metabolized is not fully understood, however, its only fate appears to be conversion into Arg, thus serving as a substrate for NOS.45
In summary, because of lack of significant splanchnic FPM, exogenous Cit appears almost entirely in the plasma.58 In contrast to Arg, the effective net splanchnic exchange of Cit, that is low uptake and high release, bypasses intestinal-hepatic FPM and facilities down-stream Cit-Arg recycling. Thus, exogenous Cit is more efficient in increasing systemic Arg availability and NO production.
3.3 Pharmacokinetics of oral doses of Cit and Arg
An overview of the pharmacokinetics of Arg vs Cit provides further evidence indicating how Cit results in a greater and sustained systemic level of Arg. About 1 hour after an oral dose of 10 g of Arg, its peak plasma concentration is about 287 µmol L−1, which is threefold greater than that of the baseline.68 Lower oral doses of Arg (up to 10 g) are eliminated from the plasma via non-renal clearance rate of 5 mL min−1 kg−1, whereas higher doses are eliminated biphasically with a rapid renal clearance within 90 minutes (with a rate of 1.17 mL min−1 kg−1) followed by a slower non-renal clearance (with a rate of 5 mL min−1 kg−1).68 Comparison of the pharmacokinetic behaviour of oral vs intravenous doses of Arg provides further evidence to support the importance of FPM on its systemic bioavailability. Plasma concentration of Arg after an acute oral dose of 10 g was threefold higher than the baseline vs a 90-fold increase in plasma concentration after an intravenous infusion of 30 g over 30 minutes indicating a limited systemic bioavailability after oral ingestion.68 Peak plasma concentrations of Arg were about threefold higher following an infusion of 6 g of Arg compared to an equivalent orally ingested dose (822 μmol L−1 at Tmax = 22 minutes vs 310 μmol L−1 at Tmax = 90 minutes).71 Similar findings in animals underscore the complex pharmacokinetic nature of oral compared to intravenous doses of Arg.72
Following oral doses of Cit (2-15 g), its plasma concentration rapidly (Tmax = 38-56 minutes) reaches a maximum level (Cmax = 515-3849 µmol L−1).53 In contrast to high doses of Arg that exceed the renal threshold for its reabsorption resulting in Arg overflow into the urine,68, 71 renal Cit reabsorption is extremely powerful,53, 73 and its urinary excretion remains as low as 5%, even at the highest dose of 15 g (fractional Cit reabsorption rate ~99.0%-91.9% for doses of 2-15 g).53 The prompt appearance and quantitatively massive increased plasma Cit following its oral consumption (~10-fold at the dose of 2 g to 100-fold at the dose of 15 g) alongside its gradual disappearance within 5-8 h post-loading, support a more efficient absorption and retention of orally ingested Cit.
Comparison of the pharmacokinetics of Cit and Arg implies that Cit is at least as efficient as Arg in increasing plasma Arg concentrations. Following oral Cit ingestion (an acute dose of 3 g) de novo Arg synthesis is substantially stimulated from 12.0 ± 1.0 to 66.3 ± 4.8 μmol kg−1 h−1.74 The peak plasma concentrations of Arg following oral doses of 2-15 g of Cit is 146-303 µmol L−1, which was observed at times of 1.17-2.29 hours.53 Compared with the same dose of Arg,68 an acute dose of 10 g of Cit resulted in a similar maximum Arg concentration, ie about 280 µmol L−1.53 Furthermore, the actual body exposure to Arg, which was estimated using the area under the curve of baseline-adjusted plasma Arg vs time, was about twofold higher following ingestion of Cit compared to Arg (AUC = 1043 vs 557.2 µmol L−1 h−1).53, 68 Such data indicate that the same dose of Cit supports systemic availability of Arg more efficiently than Arg itself. Cit at one-half dose of Arg resulted in similar plasma concentrations of Arg after oral dosing18; 0.75 g of Cit and 1.6 g of Arg administered twice daily increased Cmax (54 vs 49 µmol L−1) and AUC (271 vs 289 µmol h L−1) to the same extent.18 Plasma concentration of Arg reached its maximum level more gradually following Arg administration compared to Cit (Tmax = 3.7 vs 2.3 h)18; this delayed time to peak may indicate that plasma appearance of the ingested Arg is secondary to the Arg-Cit-Arg interorgan cycle. The plasma half-life of Arg (for an oral dose of 6 g) was estimated to be around 1 hour.71 For Cit this value is dose-dependent and ranges from 0.65-1.14 hours (for oral doses of 2-15 g).53 Kinetic parameters of Arg and Cit in both steady-state and post-ingestion of the amino acids are summarized in Table 1.
| Arg | Cit | |
|---|---|---|
| Steady-state | ||
| Plasma concentrations (µmol L−1) | 80-1201, 29 | 20-4034, 41-44 |
| Net splanchnic exchange (µmol min−1) | 18-1956, 115 | –12, –1356, 115 |
| Renal clearance (ml min−1) | 0.1673 | 0.2773 |
| Urinary excretion (%) | 0.1173 | 0.2073 |
| Urinary concentrations (µmol/mmol of Cr) | 1.273 | 0.973 |
| Post-prandial | ||
| Intestinal absorption | ||
| V max | 46.3 μmol min−1 30 cm−154 | 2.16-3.58 µmol min−1 g−122 |
| Km (mmol L−1) | 3.8 | 0.46-0.67 |
| Renal clearance (ml min−1 kg−1) | 1.1768 | 0.00573 |
| Systemic availability (%) | 10-3051, 56, 68 | 100 |
| Half-life (h) | 171 | 0.65-1.1453 |
| Cmax (μmol L−1) | 310 (a dose of 6 g)71 | 515-3849 (doses of 2-15 g)53 |
| Tmax (min) | 90 (a dose of 6 g)71 | 38-56 (doses of 2-15 g)53 |
Note
- Cmax, maximum concentration; Km, the Michaelis-Menten constant; Tmax, time to reach maximum concentration; Vmax, the maximum rate of absorption.
In summary, compared to Arg, ingested doses of Cit have more efficient intestinal absorption, relatively no FPM and high renal re-absorption. The body seems, therefore, adopting a set of different economic approaches to trap and retain orally ingested Cit. These pathways enable Cit to be more efficient than Arg itself for increasing Arg flux.
4 CONTRIBUTION OF ARG VS CIT TO THE WHOLE-BODY NO FLUX
4.1 Role of Arg in whole-body NO synthesis
The fraction of plasma Arg that contributes to circulatory NO is estimated to be as low as 0.5%-2.8% in either fasted or postprandial conditions.10, 11, 52, 74, 75 The conversion rate of plasma Arg to NO (estimated by the rate of conversion of the [15N]guanidino nitrogen of Arg to plasma [15N]ureido citrulline) has been estimated to be about 0.36 to 0.96 µmol kg−1 h−1 in healthy adults.11, 74 This contribution is independent of the amount of exogenous Arg reaching the systemic circulation. That is, regardless of increased whole-body Arg flux and increased plasma concentrations by an Arg-rich diet, the fraction of plasma Arg pool used for NO synthesis remains unchanged under both Arg-rich and Arg-free diets (about 0.55% and 0.58%).52 Such data suggest that most of the plasma Arg is metabolized by other enzymatic pathways including arginase and arginine:glycine amidinotransferase, producing ornithine, urea and creatine.76
Contribution of dietary or exogenous Arg utilized as a substrate for NOS is negligible (ranged from zero to ~1%). Dietary Arg (40 g daily for 6 days) did not alter the urinary nitrate excretion, its plasma concentration, nor the conversion rate of [15N]arginine to NO.77 Less than 0.1% of an ingested dose of [15N]-arginine (85 mg kg−1) is recovered as urinary nitrate in 24 hours78 and only 1% of the ingested doses of Arg, either as an acute dose or a 4-week supplementation, are converted into urinary 15N-labelled nitrate without any change in urinary cGMP level.79 It has been reported that the fraction of an oral dose of Arg that contributes to the plasma NO flux is very small indeed, for example 0.087% of 1.8 g and 0.068% of 4.9 g.10 Quantitatively, that translates to about 10 and 20 µmol of NO over a period of 12 hours.10
Whether intravenous or oral/intragastric Arg administration contributes more efficiently to the synthesis of NO within the whole body is not well understood. Using intravenous bolus injection of L-[15N]arginine and subsequent analysis of L-[15N]arginine in plasma and [15N]nitrate in the urine, the contribution of Arg doses to NO production was estimated to be ~1.7%-3.8% (average: 2.8%, 0.019 µmol h−1 kg−1),75 which is more than those estimated from oral doses.10, 77-79 Some evidence however indicates that intragastric but not intravenous doses, are likely to be associated with an increased NO generation, because a significant fraction of trapped Arg within the FPM is diverted towards the NO synthesis pathway.8 Contribution of Arg to the whole-body NO production is ~fourfold higher by the intragastric rout compared to intravenous administration (15.1% vs 3.7% of 15NO3 excreted in the urine over 24 hours; 1.95 vs 0.6 µmol 15NO3).8 About 0.34% of the dietary Arg trapped by the FPM within the splanchnic bed turns into NO, which accounts for ~16% of the whole daily endogenous NO synthesis.8 This splanchnic NO production is mainly attributed to the intestine rather than the liver.80 There is a substantial difference between the amount of NO produced from dietary Arg compared to that produced from plasma dietary Arg over 12 hours, either at low (AUC = 10 vs 0.15 µmol) or high (AUC = 20 vs 0.35 µmol) doses of ingested Arg.10 This may also provide further evidence on the importance of splanchnic Arg FPM for NO production. These observations appear to be in contrast with the common assumption regarding the effects of FPM on the capability of dietary Arg for NO production.
4.2 Role of Cit in whole-body NO synthesis
In contrast to Arg, Cit intake appears to provide more systemic available NO. An acute dose of 3 g of Cit effectively stimulates NO production rate by about 10-fold, from 0.36 to 3.57 μmol h−1 per kg of body weight or from 0.48 to 4.81 μmol h−1 per kg of fat free mass in young adults.74 More quantitatively, 3 g of Cit resulted in 1340 µmol of NO produced over a period of 6 hours (where the mean body weight of the subjects were 69.6 kg and the rate of NO synthesis attributed to Arg-derived citrulline was 3.2 μmol h−1 per kg of body weight).74 When comparing the amount of NO produced from 5 g of ingested Arg (~20 µmol over 12-h),10 it becomes apparent that Cit is about 200-fold more efficient in inducing NO production compared to Arg. Such estimations however need to be confirmed in well-designed isotopic studies investigating the contribution of similar doses of Arg vs Cit in NO production simultaneously.
In healthy humans, following one week of oral ingestion of Cit (3.0 g twice daily) and Arg (either as sustained-release 1.6 g twice daily or immediate-release 1.0 g three times daily), only Cit significantly increased the markers of systemic NO production ie urinary nitrate excretion (from 92 to 125 µmol L−1) and urinary cGMP concentrations (from 38 to 50 nmol mmol−1 creatinine).18
5 CELLULAR METABOLISM AND PREFERENTIAL UTILIZATION OF ARG VS CIT FOR NO SYNTHESIS
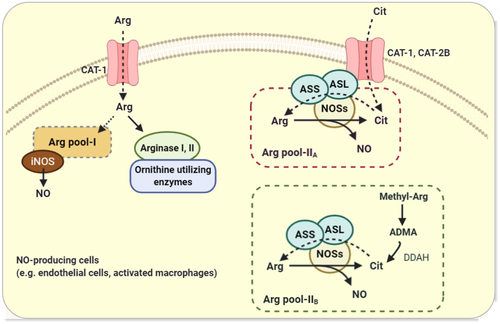
Different patterns of cell-specific expression and subcellular localization of the enzymes involved in Arg and Cit metabolism, coupling among the enzymes, compartmentalization of Arg and Cit metabolism, the presence of various inter- and intracellular transport systems, as well as fluxes of substrates determine how cells metabolize these amino acids.1, 12 These factors limit utilization of extracellular Arg or make it conditionally-dependent available for NO production.81 In contrast, NO-producing cells (eg endothelial cells and macrophages) have been well equipped by compartmentalized Cit-NO pathways,82, 83 enabling them to prefer Cit over Arg for NO production regardless of Arg's source, intracellular or imported extracellular. This ability to be independent of extracellular Arg for NO production, is more critical in activated macrophages, which need to produce large amounts of NO to enhance immunity.81 In such situations, exposing cells to Arg results in more production of ornithine (because of channelling of Arg toward arginase) rather than NO, whereas Cit effectively is directed to the NO pathway.81 Figure 4 illustrates the proposed cellular compartments (Arg pools, Cit transporters and NOSs, with ASS-ASL) and mechanisms that enable the cells to preferentially utilize Cit rather than Arg for NO production.
5.1 Cellular Arg pools and NO production
Two intracellular pools of Arg, named pool I and pool II, are detected in endothelial cells and macrophages (Figure 4). Pool I is exchangeable with extracellular cationic amino acids through the CAT system, whereas pool II does not rely on extracellular sources.84 The iNOS activity in macrophages is suggested to be dependent on pool I and extracellular Arg when the pool I cannot provide sufficient intracellular Arg.84 Pool II is supported by Cit recycling pathways 39 and is accessible to eNOS in the endothelial cells (but not to iNOS in macrophages), this enables eNOS to be independent of extracellular Arg.84 Pool II has two components; pool II-A participates in recycling of Cit to Arg and can be replenished by Cit, pool II-B is supplied by protein breakdown and is likely to accumulate the methyl-Arg and ADMA (as the main intracellular precursor of Cit).39 Taken together, Arg pool II (both II-A and II-B), which seems to be the main source of NO production under normal condition, is tightly dependent on Cit and its recycling into Arg. Such precise intracellular compartments make freely distributed Arg, if any, unavailable for NO production especially by eNOS. It is speculated that lack of efficient Cit recycling and alterations in pool II or an impaired access of eNOS to pool II make cellular NO production dependent to extracellular Arg, a situation that well explains the “Arg paradox”.39
5.2 Cellular compartments of Cit-utilizing enzymes and NO production
Cell-specific compartmentalization of intracellular transport systems and enzymes involved in Cit-Arg-NO pathway facilitates channelling of Cit into NO synthesis instead of using Arg. For example, co-localization of ASS and ASL with all NOS isoforms, ie inducible NOS (iNOS),82 endothelial NOS (eNOS), and neuronal NOS (nNOS)85 as well as their co-induction in NO-producing cells may enable Cit to be more efficient than Arg to support NO production. ASL is the essential component of the compartment, maintaining the enzymes together to make a dynamic ‘NOS multi-protein complex’, while lack of ASL results in cellular NO insufficiency, a situation that cannot be compensated with exogenous Arg.85
Previously, it was speculated that the caveolar complex between the CAT-1 and eNOS provides a mechanism for the directed delivery of extracellular Arg to eNOS.86 However, further studies have indicated that this compartment enhances NO production independent of Arg transport.87 Caveolar co-localization of cationic amino acid transporters, CAT-1 and CAT-2B, with ASS-ASL and eNOS, which effectively couples endothelial NO production to the Cit-NO cycle,83 provides further evidence for preferential use of Cit but not Arg for NO production.
5.3 The economic approaches of the cell to preserve Cit for NO
Recycling of Cit from Cit-NO cycle, which is dependent on the compartment of NOS, ASL and ASS, effectively retains Cit upon synthesis of NO from Arg.11 Intracellular Cit can also be recycled through degradation of asymmetric dimethylarginine (ADMA), an Arg metabolite produced upon hydrolysis of intracellular proteins containing methylated-arginine via arginine methyltransferases (PRMTs).49, 50 Since about 250 μmol intracellular ADMA is degraded daily by dimethylarginine dimethyloaminohydrolase (DDAH) into Cit and dimethylamine, cells can obtain a substantial amount of Cit through this way.88 Existence of such a Cit-recycling pathway may imply that the cell prefers Cit rather than Arg for NO production.
5.4 Arg and the challenge of arginase
Cit is also suggested to be a more effective NO precursor than Arg, since Arg induces intracellular arginase, the enzyme that competes with NOS for the intracellular Arg pool.34 In humans, orally ingested Arg (7 g three times daily for 3 days) significantly increases plasma urea levels (from 5.2 to 6.7 mmol L−1).21 Besides intestinal and hepatic arginases that substantially decrease systemic Arg bioavailability and compartmentalize it into urea and ornithine production, Arg entered into the other cells may also be confined into the urea cycle (either completely or partially), which make it unavailable for NOS.
Arginase has emerged as an important regulator of NO bioavailability because of its competition with NOSs for Arg as their common substrate.89 Although the affinity of the NOS enzymes for Arg is about three orders of magnitude greater than the arginases (Km = 2-20 µmol L−1 vs Km = 1-5 mM), the Vmax (the maximum activity) of the arginases is also about three orders of magnitude greater than that of the NOSs, resulting in a similar rate of substrate utilization within physiologic concentrations of Arg.2 In endothelial cells, the amount of intracellular Arg catabolized by the arginase is ~200-fold more than those catabolized by NOSs.90 In addition, in RBCs, which are considered to be as important as endothelial cells for supplying plasma NO levels, the predominant activity of arginase vs eNOS (RBC-NOS) is well documented.91, 92
Upregulated arginase expression and activity is involved in vascular dysfunction because of decreased Arg supply for NO production.93 Therefore, excessive arginase activity is associated with pathological cardiovascular conditions such as hypertension, atherosclerosis, myocardial ischaemia, congestive heart failure and vascular dysfunction in patients with diabetes mellitus.94, 95 Evidence indicates that arginase is co-expressed with NOS in NO-producing cells in the vasculature and a modest level of arginase is constitutively expressed in endothelial cells93, 96; however, arginase activity is increased by inflammatory factors (eg lipopolysaccharides, tumour necrosis factor alpha) or by ageing.88
Arg is an allosteric activator of arginase and accelerates the urea cycle; regulatory effects of Arg on ureagenesis are dependent on its nutritional status rather than on its local de novo synthesis through argininosuccinate synthase activation.19, 28 Induction of arginase limits Arg as a substrate for NOSs, and therefore affects cellular NO production.97 In contrast, inhibition of arginases has been suggested to be an efficient way to maintain NO production and to prevent adverse effects related to impaired NO production in peripheral tissues.98 Cit is a potent inhibitor of arginase in the liver, macrophages20 and endothelial cells99; ingestion of Cit does not increase urea levels and instead improves nitrogen balance.73
5.5 Arg and ADMA metabolism
ADMA, an endogenous competitive inhibitor of NOSs, antagonizes NO production, therefore increased availability of Arg seems theoretically to be an effective strategy to restore NO formation. Targeting ADMA is of great interest because of its association with endothelial dysfunction and cardiovascular diseases.100, 101 Short-term use of Arg to reverse the competitive ADMA-induced inhibition on NOS has provided conflicting results. Following Arg supplementation, plasma ADMA concentration (~0.5 µmol L−1102) remains unchanged24 or even increases.103 Theoretically, Arg regulates intra- and extracellular levels of ADMA at the level of synthesis or its cellular transport, therefore the higher the Arg concentration, the higher the ADMA concentration would be. Arg competitively inhibits DDAH and ADMA catabolism104 and may saturate the y+ transport system (CAT transporters) and limit cellular export of ADMA, resulting in increased intracellular ADMA levels and inhibition of NO production.105 The antagonistic effect of ADMA on eNOS activity is suggested to be offset by increasing intracellular levels of Cit-derived Arg.106
6 METABOLISM OF ARG VS CIT IN NO-DISRUPTED CONDITIONS
Further evidence supporting the preference for Cit over Arg for NO production comes from focusing on Cit, Arg and NO metabolism in pathologic conditions. For example, in patients with MELAS syndrome (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes), decreased NO synthesis is associated with decreased Cit flux, de novo Arg synthesis rate and the percentage of Arg flux derived from de novo synthesis rather decreased Arg flux itself.34 The argininosuccinic aciduria (ASA), the second most common human urea cycle disorder, caused by congenital ASL deficiency, is related to decreased NO production and severe systemic hypertension despite high extracellular Arg flux.107, 108 Arg administration to ASA subjects (100 mg kg−1 per day, as a standard treatment) increases Arg and urea fluxes as well as Cit flux while 15N transfer from infused 15N2-guanidino-labelled-Arg to 15N-Cit, a marker of NO production, remains near zero (in healthy controls, the administered Arg resulted in ~5 µmol L−1 kg−1).85 Such observations provide further evidence implying that recycling intracellular Cit into Arg is more critical than Arg availability for endogenous synthesis of NO.85, 107
In conditions with pathogenic elevated arginase activity (eg sickle cell disease, endotoxaemia),109-111 Arg supplementation is not suitable and enhances arginase activity.112, 113 In contrast, Cit supplementation is suggested as a promising alternative, because of its capability to bypass the arginase activity.111, 114 Intravenous infusion of similar doses (6.25 mg h−1, during 6 hours) of Cit was superior over Arg in a mice model to increase plasma and tissue concentrations of Arg and Cit, and to restore intracellular NO production in the intestine.110 Cit was also more effective than Arg in enhancing plasma (> twofold) and tissue (> 10-fold in liver and renal tissues) concentrations of Arg and NO production in arginase-injected mice.111 Cit (100 µmol L−1) completely prevents glucose-induced arginase activity in bovine aortic endothelial cells and restores NOS activity.99 Supplementation with a dose of 2 g/d Cit for 1 month also decreases arginase activity by 30% in patients with type 2 diabetes and elevates NO production.99 Superiority of exogenous Cit over Arg may be extrapolated to other conditions characterized by elevated arginase activity including haemolysis, sepsis, asthma and liver diseases.
7 CONCLUSION
As summarized in Table 2, current evidence indicates the preference for Cit over Arg administration for NO production. Because of more efficient intestinal absorption, low FPM and high renal reabsorption, oral Cit compared with same dose of Arg provides more systemic available Arg in a sustainable manner. Cit is a more powerful NO precursor than Arg because: (a) exogenous Cit increases intracellular Arg pools, (b) Cit transporters and ASS-ASL enzymes are co-localized and co-induced with NOS enzymes, and (c) Cit recycling pathway is found in NO-producing cells. In contrast, imported Arg seems to be more available for arginase rather than the NOSs, which effectively limits its accessibility for NO synthesis and shuttles Arg into other cellular pathways (eg production of ornithine and polyamines). The evidence strongly supports the hypothesis that extracellular sources of Arg can be utilized for other cellular requirements than NO production. On the other hand, side-effects (eg induction of arginase, disruption of ADMA metabolism and inhibition of Cit recycling pathway), inhibit the usefulness of Arg supplementation for NO production. Since Cit is superior over Arg as a safe and effective precursor of NO production, its supplementation has further justification in medical practice.
| Cit | Arg | Reference | |
|---|---|---|---|
| Intestinal absorption | More efficient | — | 22 |
| FPM (bypassing liver-intestine metabolism) | Limited or no | High | 15, 37 |
| Renal reabsorption | More efficient | — | 53, 73 |
| Providing systemic available Arg | High | — | 18, 51, 66, 116 |
| Effect on arginase | Inhibition | Induction | 20, 99 |
| Utilization by NO-producing cells | More efficient | — | 11, 47, 48 |
| Contribution to NO production | More efficient | — | 11, 47, 48 |
| Supply intracellular Arg pools in the subcellular compartments associated with NO production | More efficient | — | 39 |
| Utilization by the cells at a high-demand NO conditions | More efficient | — | 81 |
| Potentiating NO synthesis in pathologic conditions with elevated arginase activity | More efficient | — | 105, 106, 110, 111 |
| Safety and tolerability even at high doses | High | — | 13, 24, 25 |
Note
- Arg, arginine; Cit, citrulline; FPM, first pass metabolism; MELAS syndrome, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; NO, nitric oxide; NOSs, nitric oxide synthases.
ACKNOWLEDGMENT
This study has been supported by Shahid Beheshti University of medical Sciences (Grant Number 15142), Tehran, Iran.
CONFLICT OF INTEREST
None.




