Natriuretic peptides relax human intrarenal arteries through natriuretic peptide receptor type-A recapitulated by soluble guanylyl cyclase agonists
Abstract
Aim
Natriuretic peptides, BNP and ANP increase renal blood flow in experimental animals. The signalling pathway in human kidney vasculature is unknown. It was hypothesized that BNP and ANP cause endothelium-independent relaxation of human intrarenal arteries by vascular natriuretic peptide receptor-A, but not -B and -C, which is mimicked by agonists of soluble guanylyl cyclase sGC.
Methods
Human (n = 54, diameter: 665 ± 29 µm 95% CI) and control murine intrarenal arteries (n = 83, diameter 300 ± 6 µm 95% CI) were dissected and used for force recording by four-channel wire myography. Arterial segments were pre-contracted, then subjected to increasing concentrations of BNP, ANP, phosphodiesterase 5-inhibitor sildenafil, sGC-activator BAY 60-2770 and -stimulator BAY 41-2272. Endothelial nitric oxide synthase (eNOS) dependence was examined by use of L-NAME and eNOS knockout respectively. Molecular targets (NPR A-C, sGC, phosphodiesterase-5 and neprilysin) were mapped by PCR, immunohistochemistry and RNAscope.
Results
BNP, ANP, sildenafil, sGC-activation and -stimulation caused concentration-dependent relaxation of human and murine intrarenal arteries. BNP responses were independent of eNOS and were not potentiated by low concentration of phosphodiesterase-5-inhibitor, sGC-stimulator or NPR-C blocker. PCR showed NPR-A and C, phosphodiesterase-5, neprilysin and sGC mRNA in renal arteries. NPR-A mRNA and protein was observed in vascular smooth muscle and endothelial cells in arteries, podocytes, Bowmans capsule and vasa recta. NPR-C was observed in tubules, glomeruli and vasculature.
Conclusion
Activation of transmembrane NPR-A and soluble guanylyl cyclase relax human preglomerular arteries similarly to phosphodiestase-5 inhibition. The human renal arterial bed relaxes in response to cGMP pathway.
1 INTRODUCTION
The natriuretic peptides (NPs) atrial-, brain- and c-type natriuretic peptide (ANP, BNP and CNP) are synthesized and released predominantly by myocardial cells upon extracellular fluid volume stimuli or atrial/ventricle wall stress. In humans, NPs exert effects through natriuretic peptide receptors. They are membrane-bound with two types coupling to guanylyl cyclase (GC) activity (GC-A or NPR-A and NPR-B) and the C-type, which is truncated and without GC activity and mediates internalization and degradation of NPs. In kidney, ANP exhibited differential vascular effects such that rat afferent arterioles relaxed while efferent arterioles constricted which explained stimulatory effects on GFR.1, 2 In nanomolar range, ANP dilated arcuate and afferent but not efferent vessels in rat juxtamedullary nephron preparation.3 Similar effects were observed in hydronephrotic rat kidney with efferent constriction that translated into increased GFR and filtration fraction in intact kidney4, 5 and this effect was caused by NPR-A.5 Papillary blood flow was increased by ANP infusion in rat.6 Such hemodynamic effects are less well recognized for BNP and not characterized in human intrarenal vessels. In patients with acute kidney failure, ANP infusion increased renal blood flow, filtration fraction and GFR,7, 8 but there are no available data whether this effect was by direct interaction with vascular NP receptors. Expression and localization of NPR-C and the neutral endopeptidase neprilysin in human kidney are unknown. The phosphodiesterase that degrades cGMP in human renal vessels has not been demonstrated functionally, but phosphodiesterase (PDE) type-5 immunoreactive protein was present in human kidney vasculature9 Both NPs7, 8, 10-13 and drugs that protect against NP degradation (neprilysin inhibitors) and drugs acting to promote the cGMP pathway by other targets (soluble GC activators/stimulators, PDE inhibitors) have renoprotective effects, found mostly in preclinical studies, but also in humans.14 Based on these observations, the questions arise whether beneficial renal effects of cGMP pathway stimulation are fully or partially mediated through a vascular action and, in that case, what is the role for endothelial vs smooth muscle cells. In mice, vascular but not endothelial natriuretic peptide receptors yielded relaxation15, 16 The present study was designed to test the hypotheses that the human intrarenal arterial vasculature relaxes in response to NPs, sGC stimulators/activators and PDE5 inhibitors. It was hypothesized that relaxation to NPs is mediated through receptors on smooth muscle and that PDE-5 in smooth muscle is a predominant pathway for cGMP degradation. The hypotheses were tested first by recordings of isometric tension in a four-channel myograph setting with artery rings dissected and mounted ex vivo from human cancer nephrectomy tissue specimens and compared with murine counterparts. Subsequently, studies of mRNA and protein localization were performed by PCR, RNAscope, immunofluorescence and -histochemistry analyses on human kidney sections, isolated intrarenal artery segments and glomeruli.
2 RESULTS
In total, 183 artery rings from 54 patients and 162 artery rings from 83 mice were mounted (Table S1). Baseline characteristics of human and murine vascular rings, results from viability and functional test are presented in supplement expanded results section.
2.1 Prestimulation of artery rings and force responses
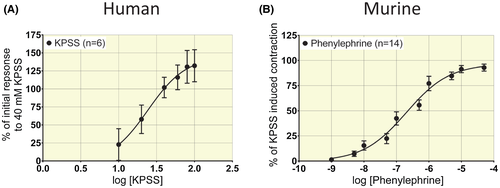
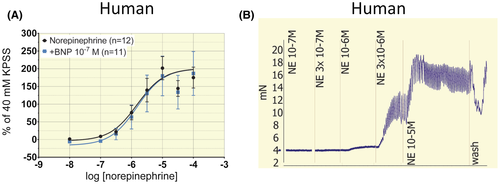
In human intrarenal segments from n = 6 patients, increasing concentrations of K+ yielded EC50 = 24.3 ± 2.2 mmol/L (Figure 1A).Subsequent experiments were conducted with 40 mmol/L K+ (~EC70) which yielded the most consistent responses. In artery segments from n = 12 patients, cumulative concentrations of norepinephrine (NE) produced log EC50 (mol/L) = −5.8 ± 0.2 (Figure 2A). Human artery rings exposed to NE immediately started oscillating and did not maintain stable tension (Figure 2B).This precluded the use of NE to obtain stable tone to study relaxation. In murine renal segmental artery rings from n = 14 animals, cumulatively increasing concentrations of phenylephrine (PE) yielded log EC50 (mol/L) = −6.7 ± 0.1 (Figure 1B). Subsequent experiments were conducted with 10−6 mol/L PE (~EC70) in murine artery rings.
2.2 Response of pre-stimulated artery rings to natriuretic peptides
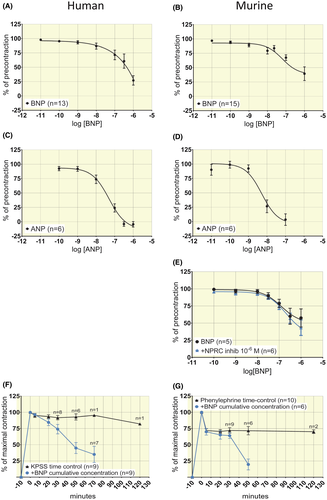
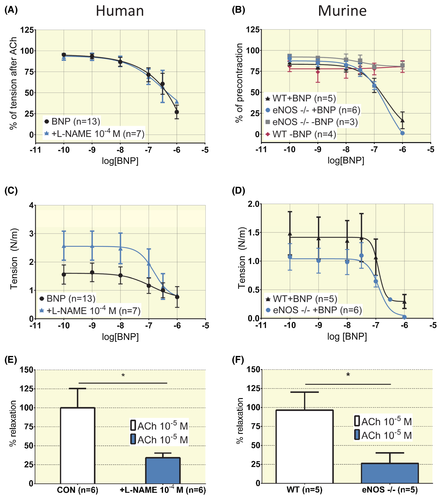
In both human and murine pre-stimulated arterial rings, BNP and ANP induced concentration-dependent relaxation (Figure 3). In human segments from n = 13 patients, a full concentration-response curve to BNP could not be generated. The potency was estimated at log IC50 (mol/L) = −6.3 ± 1.1 assuming a maximal relaxation corresponding to 0% of pre-stimulation (Figure 3A). The response to cumulative concentrations of NE in human arteries was not impacted by incubation with a concentration of BNP below IC50 (10−7 mol/L, Figure 2A, P ˃ .05). For ANP log IC50 (mol/L) was −7.3 ± 0.2 (n = 6) and Emax = 107 ± 16% (Figure 3C). In murine segments, the concentration-response curve for BNP yielded a log IC50 (mol/L) = −7.2 ± 0.2 and Emax = 55 ± 8% relaxation (Figure 3B, n = 15). For ANP, murine segments displayed a log IC50 (mol/L) = −8.3 ± 0.2 (n = 6) and Emax = 105 ± 11% relaxation (Figure 3D). Time-control experiments documented that K+-induced tone in human artery rings and PE-induced tone in murine rings was stable over time (Figure 3F,G, only 1-2 control rings incubated with agonists beyond the time used to obtain concentration responses). Involvement of eNOS in the relaxation to BNP was tested in human segments by incubating rings with 10−4 mol/L L-NAME and by using segments from eNOS-/- mice. In human rings from n = 7, L-NAME had no effect on Emax or IC50 for BNP (Figure 4A, P > .05), but attenuated acetylcholine-induced relaxation (Figure 4C (Emin) and 4E (Emax), P < .05). In murine segments from eNOS-/-, BNP elicited a response that was not different from the one observed in rings from wildtype animals for Emax or IC50 (n = 6, Figure 4B, P > .05). The tension observed after phenylephrine precontraction was attenuated in eNOS-/- (Figure 4D (Emin), P < .05) as were the responses to 10−5 mol/L acetylcholine (Figure 4F, P < .05).
2.3 Effect of a PDE-5-inhibitor on vascular reactivity and impact on BNP response
The PDE-5-inhibitor sildenafil induced concentration-dependent relaxation both in human and murine precontracted arterial rings. In human segments, sildenafil yielded an effect at log IC50 (mol/L) = −5.1 ± 0.3 and Emax = 126 ± 59% relaxation (Figure 5E, n = 6) and in murine segments at log IC50 (mol/L) = −6.2 ± 0.2 and Emax = 100 ± 11% relaxation (Figure 5F, n = 11). Incubation with sildenafil for 10 minutes at a concentration which had no independent effect on relaxation (10−7 mol/L) did not augment the subsequent response to BNP in human rings (n = 5, P > .05) or murine rings (n = 8, P > .05) by comparison of Emax and IC50 by F test and by Bonferroni's multiple comparisons test (Figure 5A,B show relative effect and Figure 5C,D show effect on absolute tension, all P > .05).
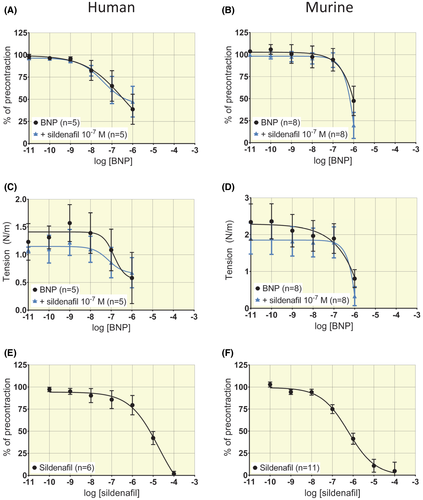
2.4 Vascular responses to soluble Guanylyl Cyclase (sGC)-activator and -stimulator and impact on BNP response
sGC-activator BAY 60-2770 induced concentration-dependent relaxation in both human and murine arterial segments. In human segments, log IC50 (mol/L) = −5.3 ± 0.4 and Emax = 105 ± 42% relaxation (Figure 6A, n = 6). In murine segments log IC50 (mol/L) = −7.5 ± 0.3 and Emax = 74 ± 13% relaxation (Figure 6B, n = 6). sGC-stimulator BAY 41-2272 induced concentration-dependent relaxation in both human and murine arterial segments. In human segments, a full concentration-response curve to BAY 41-2272 could not be generated (Figure 6C). The potency estimated by assuming a maximal corresponding to 0% of pre-stimulation yielded log IC50 (mol/L) = −5.1 ± 12.6 (Figure 6C from n = 4 patients). In murine segments, log IC50 (mol/L) = −6.5 ± 0.2 and Emax = 93 ± 13% relaxation (Figure 6D, n = 5). In murine rings, the application of 10−8 mol/L sGC-stimulator BAY 41-2272 did not significantly impact the response to increasing concentrations of BNP (Figure 6E, log IC50 (mol/L) = −7.2 ± 0.3 for BNP and −7.4 ± 0.4 for BAY with BNP, n = 8, P > .05).
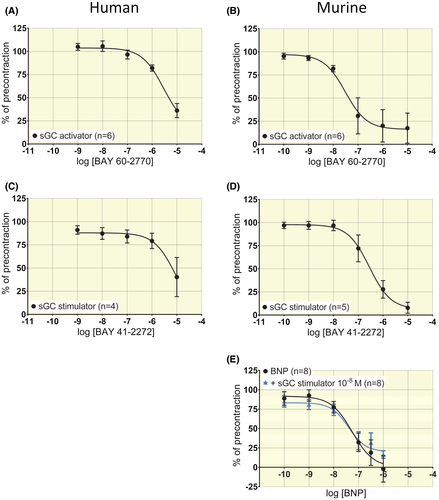
2.5 Impact of NPR-C inhibitor on BNP response
In murine arterial segments, the NPR-C inhibitor AZ12107657 (= M372049, 10−6 mol/L)17, 18 had no effect on force development in response to phenylephrine. The response to increasing concentrations of BNP was similar with or without NPR-C blocker (Figure 3E, n = 6, P > .05).
2.6 Localization of NPRs, PDE5 and sGC in human kidney tissue
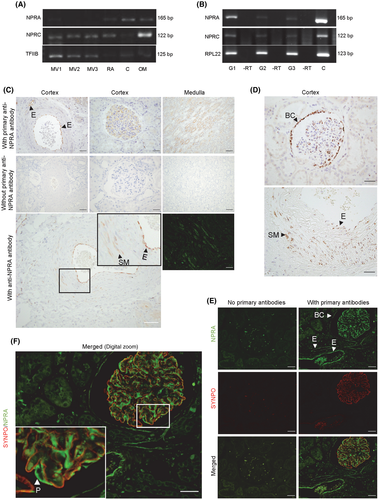
NPR-A was detected by PCR in kidney cortex and outer medulla and in isolated human glomeruli, but less consistently in microdissected small-calibre intrarenal arteries (Figure 7A,B, Figure S1). In kidney sections, mRNA and protein for NPR-A was associated with glomeruli by RNAscope and immunohistochemistry, which also showed NPR-A in vasa recta and the parietal layer of Bowman´s capsule (Figure 7C,D, Figure S2). RNAscope and immunohistochemistry both demonstrated NPR-A in endothelial cells in arteries and arterioles and in scattered smooth muscle cells in intrarenal artery media (Figure 7C,D and Figure S2). NPR-A immunoreactivity in glomeruli, arteries and vasa recta was a consistent finding in n = 10 human kidney sections across sex, diagnoses and age (Figure S2). In glomeruli, NPR-A co-localized with synaptopodin by fluorescence-double labelling (Figure 7E,F), but not with integrin α-8 (Figure S4). NPR-A immunoreactivity was found both in descending and ascending vasa recta in the outer medulla (Figure S6). NPR-B was not observed in human kidney tissue by PCR or RNAscope (Figure S5). mRNA for NPR-C was detected in cortex and outer medulla and was associated with arteries and glomeruli by PCR (Figure 7A,B, Figure S1). RNAscope showed that the mRNA for NPR-C was located in glomeruli, proximal convoluted tubules and tubules in the outer medulla and in arterial smooth muscle cells (Figure S3). PDE-5 mRNA was detected by PCR in renal artery and intrarenal arteries and enriched compared to cortex/outer medulla tissue (Figure S1). Soluble guanylyl cyclase (sGC) was detected in renal and intrarenal arteries and was enriched compared to cortex/medulla tissue (Figure S1). Neprilysin was not detected consistently in the intrarenal vasculature but amplified consistently in cortex and medulla tissue (Figure S1).
3 DISCUSSION
The present study finds that BNP and ANP concentration dependently relaxed intrarenal arteries from humans and mice, in agreement with vascular smooth expression of natriuretic peptide receptor type A, NPR-A. This may account for stimulatory effects on renal blood flow in animals and patients.4-8 Relaxation was induced by the sGC-stimulator and -activators BAY 41-2272 and BAY 60-2770, in accordance with vascular expression of sGC. Significant contribution to metabolism of cGMP by phosphodiesterase-5 (PDE-5) was revealed by relaxation to the PDE-5 blocker sildenafil. The NPR-C receptor was detected in tubules and vasculature, but an NPR-C blocker did not alter vascular responsiveness to NPs. NPR-A responses were independent of endothelial NO synthesis in mice and human intrarenal vessels in agreement with observations in mice of dispensable endothelial NPRA.15, 19 In human cutaneous microvasculature, BNP responses were partially dependent on eNOS activity,20 so at least in humans, endothelial dependence of BNP responses differ between vascular beds. The relaxing effect of NPs on intrarenal arteries was in agreement with NPR-A immunolabelling in smooth muscle in arterioles and in media of larger arteries. In medulla of human kidney, both ascending and descending vasa recta exhibited immunoreactive NPR-A consistently across age, gender and diagnoses (n = 10). In rat kidney, isotope labelled ANP bound to structures of the outer medulla compatible with vasa rectae.21, 22 Efferent arterioles in rodents contract in response to ANP1 but, to our knowledge, there are no data from isolated vasa recta. Medullary blood flow increased by ANP in pig and rat.6, 10 Based on the present immunolabelling and preclinical observations, it is an intriguing possibility that natriuretic peptides relax human vasa recta and increase medullary blood flow.
In healthy humans, plasma concentration of natriuretic peptides23 is lower than the effective in vitro concentrations in the present study and previous studies.23, 24 This difference could be due to the reduced ex vivo preparation and it could also relate to the size of the vascular segment. The present study used segments of ~600 µm and the much smaller glomerular arterioles are sensitive to picomolar levels of ANP in rat1 and in smaller intrarenal rings, ANP displayed an IC50 of 9 nmol/L.24 Other explanatory factors could be prolonged incubation for wash-out of anaesthetics and different NPR-A receptor density (increasing with decreasing artery calibre).1 The potency of BNP and ANP in murine preglomerular arteries exceeded that of human arteries by one order of magnitude and ANP was more potent than BNP. This difference between ANP and BNP was observed for NPR-A expressed in vitro.25 The species difference was likely caused by the different agonist used to induce baseline tension. The human artery rings showed regular oscillation of vascular tension in response to norepinephrine (Figure S5-C), a well-known response ex vivo.26 This phenomenon urged the use of potassium to generate stable tension. Vasomotion was not observed with potassium-induced tension as with norepinephrine which confirms dependence on membrane potential fluctuations also in human renal vascular bed.26 Relaxation in response to NPs depends on potassium channel activation and hyperpolarization in animal vasculature.27 This dependence on membrane potential changes may account for the reduced potency of NPs in K+-pre-stimulated human rings compared to murine rings. The consequent use of human natriuretic peptides, and not murine, could have contributed to different sensitivity.
The present study supports that both ANP and BNP exert effects through the NPR-A since NPR-B expression was not detected in human kidney. NPR-C expression was observed in vascular smooth muscle cells, tubular epithelium and intraglomerular cells in agreement with previous studies.21, 22 The responsiveness of isolated rings to BNP was not altered by high concentrations of an inhibitor of NPR-C. This confirms previous data where ANP effect did not depend on NPR-C in mesenteric vessels.17 The absence of NPR-A in smooth muscle in mice abolished relaxation of renal vessels to ANP.15, 19 It can be concluded that NPR-C does not contribute to renal vascular relaxation to ANP/BNP. The present results support NPR-A as common receptor for ANP and BNP in the renal vasculature. No labelling of tubular compartment for NPR-A was detected in human kidney while there was abundant NPR-C in proximal tubules and distal nephron parts. Because NPR-C clears and commits ANP/BNP for degradation, the data indicate that NPR-A signalling is not likely in human kidney epithelium. A new observation was abundant NPR-A in podocytes and in parietal layer of Bowman´s capsule in accord with ANP binding data from rat.21, 22 NPR-A in podocytes exerted significant protection against hypertensive injury in a DOCA-salt murine kidney injury modul.28 The wide vascular and epithelial expression of NPR-C in human kidney may account for the significant ~50% renal extraction of ANP29 that is larger than for BNP. Neprilysin was detected in human kidney tissue but not consistently in vasculature. BNP depends to a larger degree than ANP on renal clearance and impaired renal function is associated with elevated plasma BNP also in the absence of left ventricular dysfunction.30, 31 The present data showed function and expression of phosphodiesterase type 5 (PDE-5) and sGC in human intrarenal arteries in accord with previous data.9, 32, 33 Thus, both transmembrane and soluble guanylyl cyclase are functional in human renal vascular smooth muscle. The present study has several limitations; it was ex vivo and under isometric condition and thus the multiple inputs that converge on the vessels in vivo (flow-mediated NO, sympathetic nerve input and transmural pressure) are absent which may have affected sensitivity and reactivity. Vessels were predominantly from kidney cancer patients and although harvested from normal tissue, the presence of tumour tissue in the kidney could have affected results. Finally, vessels were from aging patients and therefore may exhibit variable degrees of endothelial dysfunction, exaggerated stiffness and altered perivascular adipose tissue. With these limitations in mind it is safe to conclude on the presence of NPRA in preglomerular artery smooth muscle in human kidney. In pathophysiological settings, drugs are available that increase ANP/BNP half-life and bioavailability (neprilysin inhibitors), stimulate sGC and inhibit cGMP degradation (PDE-5 inhibitors). The present study shows that such drugs may all exert direct vascular effects at the level of intrarenal arteries in human kidneys. Such effects may contribute to observed, beneficial, renal effects of ANP in acute renal failure7, 8 and cardio-pulmonary surgery10 patients and of sildenafil in preclinical studies of transplantation34 and warm ischemia.35
4 MATERIALS AND METHODS
Detailed description of the methods and materials used is given in the supplement section. Patients who underwent unilateral total nephrectomy for renal cancer were recruited. The use of tissue was approved by the ethics committee of the Region of Southern Denmark (#S-2014-0159) and used only after informed written and oral consent from each patient. Tissue was transferred to physiological buffer for dissection (see protocol in supplement) 30-45 minutes after extirpation, while samples of renal cortex, outer and inner medulla were snap frozen in liquid nitrogen and stored at −80°C. Wedge-shaped tissue blocks were cut along the cortical-papillary axis and subsequently paraformaldehyde-immersion fixed for 48 hours and then embedded and stored in paraffin. Subsequently, 5 µm sections were cut and mounted on slides for histochemical labelling.
4.1 Experiments
All series I-XIV are described in detail in the supplement. Human and murine (wildtype and eNOS-/-) intrarenal arterial segments were suspended in physiological saline solution aerated with 5% CO2 in air in a Multi Wire Myograph (model 610M, Danish Myo Technology, Hinnerup, Denmark) for real-time measurement of changes in tension during pharmacological manipulation. Active tension in human and murine arterial segments was achieved by elevation of potassium through isosmotic exchange with sodium to induce depolarization (EC70 ~ 40 mmol/L) and by phenylephrine (EC70 ~ 10−6 mol/L) respectively (Figure S1). Cumulative concentration-response curves were generated to BNP (including series with sildenafil, sGC activator, NPR-C inhibitor17, 18 and L-NAME), ANP, sildenafil, a sGC stimulator and activator (BAY 60-2770 and 41-2272, respectively). PCR, RNAscope and immunohistochemistry were performed in cortical and medullary human kidney tissue and intrarenal arteries as described in supplementary methods.
4.2 Data analysis and statistics
Isometric tension recordings were expressed relative to the tension level induced by precontraction or as absolute values (N/m). Concentration-response curves were compared by two-way analysis of variance or for potency (log EC50/IC50) and efficacy (Emax) by F test in Graph Pad Prism 6.07 (GraphPad Software Inc, San Diego, CA, USA). Bonferroni's multiple comparisons test was applied where appropriate. For Figure S4, comparisons were made by paired two-tailed Student's t test. P-values < .05 were considered significant.
ACKNOWLEDGEMENTS
Authors thank Kenneth Andersen, Kristoffer Rosenstand, Inger Nissen and Gitte Kitlen for expert technical assistance. Peter Sandner at Bayer AG is thanked for providing sGC stimulator and activator substances. Dr. Mark Lal at AstraZeneca is thanked for sharing the NPR-C blocker. This work was supported by the Novo Nordisk Foundation with grant numbers NNF17OC0028972 and NNF19OC0058780; the Karen Elise Jensen Foundation (no number) and the Danish Research Council for Independent Research with grant number 6120-00067B to Andreas Frees; The Region of Southern Denmark and Zealand (no number).
CONFLICT OF INTEREST
Authors report no conflicts of interest.




