MicroRNA-133 suppresses ZFHX3-dependent atrial remodelling and arrhythmia
Funding information
This work was supported by the Ministry of Science and Technology, Taiwan [grant numbers MOST 105-2628-B-038-012-MY3, MOST 106-2811-B-038-013, MOST 107-2811-B-038-507, MOST 107-2314-B-038-101-MY3 and NSC 102- 2628-B-038-002-MY3] and the Wan Fang Hospital, Taipei Medical University [grant numbers 104-wf-eva-01, 104swf01, 105swf09 and 106-swf-02].
Abstract
Aim
Atrial fibrillation (AF) is an important cause of morbidity and mortality in the modern world. Loss-of-function mutation in the zinc finger homeobox 3 gene (ZFHX3) is associated with increased risk of AF. MicroRNAs (miRNAs) participate in arrhythmogenesis, and thus miRNA modulators may be applicable as therapeutic modalities for AF. However, the altered miRNA profiles after ZFHX3 knockdown (KD) remain unclear. This study aimed to analyse the changes of miRNA expression in loss-of-function of ZFHX3 and the effect of miRNA modulation on atrial arrhythmias in this model.
Methods
We performed small RNA deep sequencing on ZFHX3-KD and control HL-1 mouse atrial myocytes. The effect of miRNAs on ZFHX3-dependent atrial arrhythmia was evaluated through in vitro and in vivo assays in mice.
Results
Among the differentially expressed miRNAs, 11 were down-regulated and 6 were up-regulated after ZFHX3 KD. Quantitative real-time PCR analysis confirmed that after ZFHX3 KD, miR-133a and miR-133b were significantly down-regulated, whereas miR-184 was the most significantly up-regulated. DIANA-miRPath analysis suggested that miR-133a/b down-regulation increases the targeted signalling of miR-133 (ie, adrenergic, Wnt/calcium and fibroblast growth factor receptor 1 signalling), which could contribute to pathological remodelling of cardiomyocytes. These results were confirmed through Western blotting. After transfection of miR-133a/b mimics in ZFHX3-KD cells, miR-133a/b levels increased, accompanied by the inhibition of their target signalling. Treatment with miR-133a/b mimics diminished ZFHX3 KD–induced atrial ectopy in mice.
Conclusion
ZFHX3-KD promotes distinct miRNA expressional changes in atrial myocytes. MiR-133a/b mimics may reverse signalling of ZFHX3 KD-mediated cardiac remodelling and atrial arrhythmia.
1 INTRODUCTION
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia; it is also a critical contributor to morbidity and mortality.1, 2 AF is associated with alterations in the electrophysiological and contractile properties and structural architecture of the atria.3, 4 Furthermore, calcium regulation has a significant role in AF pathophysiology.5, 6 Current accumulating evidence has highlighted the importance of connexin expression and regulation in AF pathophysiology and management.7
Genetic variants of the zinc finger homeobox 3 (ZFHX3; also known as ATBF1) loci are strongly associated with AF progression.8-13 The ZFHX3 SNP rs2106261(G/A) has been identified a risk marker for AF and AF-related phenotypes.14-16 Furthermore, the missense mutation in ZFHX3 damages its ZFHX3 structure in patients with AF.17 ZFHX3 is a corepressor of signal transducer and activator of transcription 3 (STAT3) and inhibits its DNA-binding activity.18 ZFHX3 also protects cells from oxidative stress by activating the platelet-derived growth factor receptor β/ATM cascade.19 STAT3–induced increases in oxidative stress have been implicated in AF pathogenesis.20, 21 Moreover, ZFHX3 mediates the SUMOylation of misfolded and damaged proteins, which contribute to AF.22 Dysregulation of the ZFHX3-Wnt-miRNA signalling may alter calcium homeostasis.23, 24 ZFHX3 has been shown to cooperate with myocyte enhancer factor 2 to promote miRNA expression.25, 26 Taken together, the loss of function of ZFHX3 is critical in AF pathophysiology. However, knowledge regarding the mechanisms of ZFHX3 loss-of-function that contribute to atrial arrhythmogenesis is limited.
Numerous micro-RNAs (miRNAs) have been implicated in the control of AF; these miRNAs can provide novel insights into the molecular processes leading to AF, signifying targeting miRNAs as a potential approach for treating cardiac arrhythmia.27, 28 Elucidating the mechanisms of ZFHX3-regulating miRNAs on controlling myocyte remodelling may aid in understanding AF progression. Thus, in this study, we systematically analysed the differential miRNAs expression profile of stable ZFHX3-KD and control HL-1 cells and explored the potential underlying signalling.
2 MATERIALS AND METHODS
2.1 Cell culture and transfection
HL-1 cells derived from mouse atrial cardiac muscle cells (kindly provided by Dr Claycomb, Louisiana State University Health Sciences Center, New Orleans, LA, USA) were cultured and maintained in a humidified atmosphere of 5% of CO2 at 37°C in Claycomb medium (Sigma, St Louis, MO, USA). The short hairpin RNA (shRNA) vectors for negative control or ZFHX3 knockdown (KD; ZFHX3 SureSilencing shRNA insert sequence: TCAACAGCATATCTCCAAAGT, KM32862P, Qiagen, Germany) were transfected into the HL-1 cells using the TransIT-X2 reagent (Mirus Bio LLc, Madison, WI, USA) and stably selected using puromycin.
2.2 RNA isolation and deep sequencing
RNA was isolated from ZFHX3-KD or control HL-1 cells using TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. Small RNA sequencing libraries were created using Illumina TruSeq Small RNA Sample Preparation protocol (Illumina, San Diego, CA, USA). First, 3′ and 5′ RNA adapters, specifically modified to target the ends of small RNA molecules, were ligated to 1 µg of total RNA. Subsequently, the ligation products are reverse transcribed and amplified through polymerase chain reaction (PCR) to generate a cDNA library suitable for high-throughput sequencing. Amplified cDNA construction was size fractionated (145-160 bp) and purified by 6% of Novex TBE PAGE gel (Invitrogen). Small RNA libraries quantified through the reverse transcription quantitative PCR (qPCR), and size distribution was validated on a 2100 Bioanalyzer (Agilent) using a high-sensitivity DNA chip. The obtained libraries were then sequenced on a HiSeq 2500 (Illumina) through single-end sequencing with a 50-bp read length. Unique sequences aligned with known miRNAs (according to miRBase, version 21) were quantified as absolute expression values for each known miRNA. The read counts of each known miRNA were normalized to the library size and presented as reads per million mapped reads (RPM). MiRNAs (≥10 RPM in at least 1 library) were subjected to further analysis. Differential expression between the ZFHX3-KD and control HL-1 cells was calculated using edgeR, followed by false discovery rate (FDR) correlation.29 The miRNAs with values of log2 ratio (ZFHX3 KD/control HL-1) > ±1, along with an FDR of <0.01, were considered up-regulated and down-regulated after ZFHX3 KD respectively.
2.3 Real-time reverse transcription PCR
Among differentially expressed miRNAs, we selected 6 that were expressed abundantly in the HL-1 atrial myocytes and validated their expression patterns through real-time reverse transcription-PCR (qPCR). MiRNAs were reverse transcribed using TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems). PCR was performed according to the manufacturer's protocol. The Ct values were collected in triplicate and then averaged, and the relative target miRNA expression was determined using the ΔΔCt method.30
2.4 Kyoto encyclopaedia of genes and genomes pathway analysis of miRNA target genes
We used DIANA-miRPath (version 3.0) for the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway analysis of miRNA pathway.31 We selected differentially expressed miRNAs for further analysis, the source of interactions (Targetscan and Tarbase), choose the merging algorithm (genes) and the statistics engine. DIANA-miPath combined all the selected miRNA target genes and computed P-values that describe KEGG pathway enrichment of the target genes.
2.5 Small RNA transfection
Equal amounts (30 nM) of a negative control (NC) miRNA mimic, mmu-miR-133a-3p mimic or mmu-miR-133b-3p mimic (Thermo Fisher Scientific) was transfected into ZFHX3-KD HL-1 cells using the RNAiMAX (Invitrogen), and the cells were harvested for protein after 48 hours.
2.6 Western blotting
Equal amounts of total protein from each treated HL-1 cell were subjected to gradient SDS PAGE (5%-12%) and then transferred onto an equilibrated polyvinylidene difluoride membrane.32 All these blots were probed with primary antibodies against ZFHX3 (1:2000, MBL, Nagoya, Japan PD010), dishevelled segment polarity protein 2 (1:1000, DVL2; Cell Signalling, #3216), CaMKII (1:5000, GeneTex, GTX111401), phosphorylated CaMKII at Thr 286 (pCaMKII; 1:5000, Abcam, ab32678) c-Jun N-terminal protein kinase (JNK; 1:1000, Cell Signalling, #9258), pJNK (1:1000, Cell Signalling, #4668), connexin43 (CX43; 1:1000, BD Bioscience, 610062), fibroblast growth factor receptor 1 (FGFR1; 1:1000, Cell Signalling, #9740), sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a; 1:5000, Santa Cruz, sc-8095), ryanodine receptor (RyR; 1:1000, Thermo, MA, USA, MA3-916), phosphorylated RyR at Ser 2814 (RyR-pS2814, 1:1000, Badrilla, A010-31AP), inositol 1,4,5-trisphosphate (IP3) receptor (IP3R; 1:1000, Cell Signalling, #8568) and β-actin (1:5000, Abcam, ab6276). All targeted bands were normalized to β-actin to confirm equal protein loading.
2.7 Luciferase reporter assay
pEZX-MT05, containing the mouse DVL2 3′ untranslated region (UTR; catalogue no.: MmiT026970-MT05) and mouse FGFR1 3′-UTR (catalogue no.: MmiT088636-MT05) constructs, were obtained from GeneCopoeia (Rockville, MD, USA). The pEZX-MT05 vector also contains secreted Gaussia luciferase (GLuc) open reading frame, driven by the SV40 promoter as a reporter of the 3′-UTR expression, and a secreted alkaline phosphatase (SEAP) reporter, driven by a CMV promoter as an internal control. Further, ZFHX3-KD cells were used for the 3′-UTR luciferase assay. Cells were seeded (1 × 105 cells/well) in 12-well plates. In total, 100 ng well−1 of pEZX-MT05 vector containing mouse DVL2 3′-UTR or mouse FGFR1 3′-UTR containing mmu-miR-133a-3p or mmu-miR-133b-3p, using GenJet Plus DNA in vitro transfection reagent (SignaGen, MD). After 48 hours of transfection, GLuc and SEAP activities were measured through luminescence in conditioned medium using the Secrete-Pair™ Dual Luminescence Assay Kit (GeneCopoeia). GLuc activity was normalized to SEAP activity (GLuc/SEAP). The statistical differences were analysed using one-way analysis of variance, followed by post hoc Tukey testing. (P < 0.05) of normalized data.
2.8 Sarcoplasmic reticulum Ca2+-ATPase activity
Sarcoplasmic reticulum (SR) Ca2+ATPase activity (SERCA activity) was assessed by a colorimetric ATPase assay kit (catalogue no. 601-0120, Novus Biologicals, Littleton, CO), as described previously.33 The homogenates were centrifuged for 10 min at 1000 g, followed by a centrifugation of consecutive supernatants first for 10 min at 10,000 g, and then for 1 hour at 100,000 g.34 The incubation solution contained 50 µg of microsomal extract with 1 µg/mL of ionophore A23187, 1 µg/mL of ETGA, 0.1 M of Tris (pH7.5), MgCl2 (5 mM) and CaCl2 (10 μM) at 37°C for 5 minutes. The ATPase activity rates were read at 650 nm.
2.9 Measurement of sarcoplasmic reticulum Ca2+ contents
After achieving steady-state Ca2+ transients with repeated pulses from −40 to 0 mV (1 Hz for 5 seconds), the total amount of charge crossing the membrane SR Ca2+ content was estimated by integrating the sodium Na+–Ca2+ exchanger (NCX) current rapid application of 20 mM of caffeine during rest with the membrane potential clamped to −40 mV to cause SR Ca2+ release. The NCX current was measured by whole cell patch clamp experiments on an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, USA) at 35 ± 1°C, with the micropipettes filled the solution containing 20 mM of NaCl, 110 mM of CsCl, 0.4 mM of MgCl2, 1.75 mM of CaCl2, 20 mM of TEACl, 5 mM of BAPTA, 5 mM of glucose, 5 mM of MgATP and 10 mM of HEPES (at pH 7.25 adjusted with CsOH). The external solution contained 140 mM of NaCl, 2 mM of CaCl2, 1 mM of MgCl2, 5 mM of HEPES and 10 mM of glucose at pH 7.4 and contained 10 μM of strophanthidin, 10 μM of nitrendipine and 100 μM of niflumic acid. The time integral of the NCX current was converted to amol of Ca2+ released from the SR.35
2.10 Telemetry electrocardiography in siZFHX3–injected mice
We studied the effects of ZFHX3-KD on electrocardiography (ECG) in vivo in BLTW:CD1 (ICR) mice (aged 8 weeks). This investigation was approved by the Institutional Animal Care and Use Committee of Taipei Medical University (LAC-2017-0461). Implantation of the telemetry transmitter (ETA-F10; Data Sciences International, St. Paul, MN, USA) was performed according to the manufacturer's protocol. Transmitters were placed intraperitoneally, and ECG leads were placed in a lead II configuration. The ECG data were collected and processed using LabChart (version 8; Data Sciences International). At 9 weeks of age, the mice were tail vein–injected with ZFHX3 siSTABLE siRNA (siZFHX3, 20 μg time−1 mice−1; Dharmacon Research, Lafayette, CO) 4 times in 12 days. The sense and antisense sequences of ZFHX3 were designed as follows: sense, GGAUAAACCAAACGAGUUA and antisense, UAACUCGUUUGGUUUAUCC. After siZFHX3 injection, mice were tail vein–injected with mmu-miR-133a-3p/133b-3p mimics (1 nmol time−1 mice−1) 2 times in 6 days. In vivo-jetPEI was used for delivering siZFHX3 or mmu-miR-133a-3p/133b-3p mimics to ICR mice. Baseline data were obtained 10 days after implantation for 2 hours. Heart rhythm was monitored for 2 hours by implanted device at day 9, 10, 12 and 18. Ectopic atrial beats were defined as the occurrence of the P wave with shapes different from those in sinus beats. Ventricular premature captures were defined as the occurrence of QRS complex earlier than the regular normally conducted beat without the preceding P waves. Atrial or ventricular ectopy was measured from hourly occurrence (beats/hr) of atrial ectopic beats or premature ventricular complex in the detection interval.
2.11 Immunohistochemistry
For the confirmation of effects of siZFHX3 in mice, control group (n = 5) or siZFHX3/miR-133a/b mimics–injected group (n = 5) ICR mice were euthanized with isoflurane inhalation. The hearts were removed and immediately fixed in 4% of paraformaldehyde in phosphate-buffered saline (pH 7.4), embedded in paraffin and sectioned 5 μm thick. These sections were incubated with an anti-ZFHX3 antibody (PD011; MBL, Nagoya, Japan) and then analysed on the Polink-2 HRP Plus Rabbit DAB Detection System (D39-6; GBI Labs). The images were acquired using TissueFAXS system (TissueGnostics, Austria).36, 37
2.12 Statistical analysis
All results are expressed as the means ± standard error of the mean. Student paired t testing was used to compare control and ZFHX3-KD HL-1 cells. Multiple comparisons were analysed using one-way analysis of variance, followed by post hoc Tukey testing. The data were analysed using GraphPad Prism (version 5); a P value < 0.05 was considered significant.
3 RESULTS
3.1 RNA sequencing of ZFHX3-KD and control HL-1 cells
To investigate the changes in miRNA expression after ZFHX3 KD, we performed small RNA sequencing for identifying differentially expressed miRNAs between ZFHX3-KD and control HL-1 cells. Figure 1A demonstrates that ZFHX3 expression was significantly repressed (0.67-fold) in ZFHX3-KD cells compared with control. Four small RNA libraries from ZFHX3-KD and control HL-1 cells were constructed (n = 2 for each stable cell line). Small RNA sequencing reads were then mapped to the mouse genome (mmp10). From the sequences, 936 mature miRNAs were identified and represented as RPM in the 4 libraries (Supplemental Table 1). The comparable RPM between ZFHX3-KD and control libraries revealed that 29 miRNAs were differentially expressed (FDR ≤ 0.01; Figure 1B). Of these 29 miRNAs, 21 were down-regulated and 8 were up-regulated in ZFHX3-KD cells compared with control cells. The 29 differentially expressed miRNAs were further filtered for those with providing high signals (average of 4 libraries > 100 RPM) and the changes in expression are greater than twofold difference (log2 fold change ≥ ±1). Next, 17 selected miRNAs were visualized in a heatmap (Figure 1C and Table 1). Among these, mmu-miR-465a-3p, mmu-miR-465b-3p and mmu-miR-465c-3p shared the sample mature sequence. Therefore, we identified 17 differentially expressed miRNAs after ZFHX3 KD in HL-1 cells.
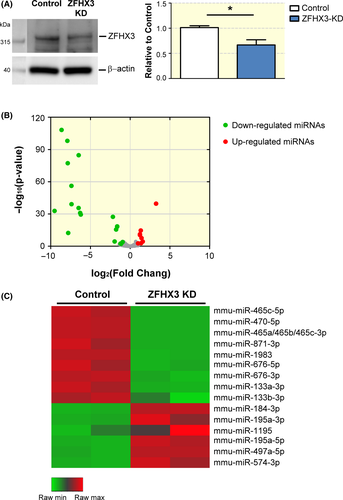
| miRNAs | Average RPM in 4 libraries | log2 (FC) | P-value <0.01 | FDR |
|---|---|---|---|---|
| Down-regulated miRNAs | ||||
| mmu-miR-465c-5p | 216.72 | −8.66 | 1.95E-101 | 1.81E-98 |
| mmu-miR-470-5p | 188.70 | −7.92 | 9.44E-93 | 4.39E-90 |
| mmu-miR-465a-3p & mmu-miR-465b-3p & mmu-miR-465c-3p | 126.06 | −7.88 | 1.50E-78 | 3.50E-76 |
| mmu-miR-871-3p | 180.25 | −6.50 | 1.12E-83 | 3.46E-81 |
| mmu-miR-1983 | 243.60 | −2.20 | 1.35E-26 | 1.05E-24 |
| mmu-miR-676-5p | 169.61 | −1.79 | 6.44E-17 | 4.00E-15 |
| mmu-miR-676-3p | 737.58 | −1.66 | 3.04E-20 | 2.02E-18 |
| mmu-miR-133a-3p | 1957.37 | −1.66 | 3.14E-23 | 2.25E-21 |
| mmu-miR-133b-3p | 147.65 | −0.98 | 9.35E-06 | 0.000334651 |
| Up-regulated miRNAs | ||||
| mmu-miR-184-3p | 196.50 | 3.23 | 3.12E-41 | 4.15E-39 |
| mmu-miR-195a-3p | 121.82 | 1.40 | 5.11E-10 | 2.38E-08 |
| mmu-miR-1195 | 10590.77 | 1.37 | 9.15E-05 | 0.002660737 |
| mmu-miR-195a-5p | 468.32 | 1.31 | 2.97E-13 | 1.62E-11 |
| mmu-miR-497a-5p | 1313.78 | 1.21 | 1.57E-12 | 8.13E-11 |
| mmu-miR-574-3p | 277.86 | 1.20 | 2.54E-10 | 1.24E-08 |
- Abbreviations: FC, fold change; FDR, false discovery rate; miRNA, microRNA; RPM, reads per million mapped reads.
3.2 qPCR validation
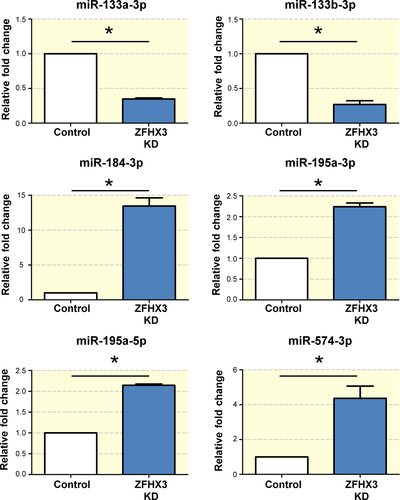
We used qPCR to validate the expression of differentially expressed miRNAs between ZFHX3-KD and control cells. Among the 17 differentially expressed miRNAs, 11 (mmu-miR-465c-5p, mmu-miR-470-5p, mmu-miR-465a-3p, mmu-miR-465b-3p, mmu-miR-465c-3p, mmu-miR-871-3p, mmu-miR-1983, mmu-miR-676-5p, mmu-miR-676-3p, mmu-miR-133a-3p and mmu-miR-133b-3p) were significantly down-regulated and 6 (mmu-miR-184-3p, mmu-miR-195a-3p, mmu-miR-1195, mmu-miR-195a-5p, mmu-miR-497a-5p and mmu-miR-574-3p) were significantly up-regulated after ZFHX3 KD (Table 1). Among them, 6 miRNAs (mmu-miR-133a-3p, mmu-miR-133b-3p, mmu-miR-184-3p, mmu-miR-195a-5p, mmu-miR-195a-3p and mmu-miR-574-3p) have known human homologues, it seemed reasonable to assume that their miRNA targets sequence would also be conserved. Thus, we selected the 6 potential miRNAs for further validation and investigation. The qPCR validation demonstrated that 6 selected miRNAs were dramatically different between ZFHX3-KD and control cells; the results were supported by our sequencing results (Figure 2, n = 3). Moreover, these 6 miRNAs were reported for the first time to be differentially expressed after ZFHX3 KD, whereas miR-133a-3p (0.34-fold change) and miR-133b-3p (0.27-fold change) were significantly down-regulation and miR-184-3p has the highest up-regulation (13.39-fold change) compared with control cells (Figure 2).
3.3 Pathway analysis of 6 differentially expressed miRNAs
We used DIANA-miRPath (version 3.0) algorithm to gain insights into the functional pathways of the miRNAs that were altered after ZFHX3 KD.31 The miRNAs vs pathway heatmaps show that 22 pathways were significantly enriched with down-regulated miRNAs (mmu-miR-133a-3p and mmu-miR-133b-3p), and 5 pathways were significantly enriched with 4 up-regulated miRNAs (Figure 3A,B). As shown in Figure 3A, the target signalling of the miR-133a-3p/miR-133b-3p are mostly involved in “Adrenergic signalling” “Wnt signalling pathway” “Hippo signalling pathway” “Regulation of actin cytoskeleton” “Extracellular matrix (ECM)-receptor interaction” “Adherens junction” “Notch signalling pathway” and many others signalling (Figure 3A and Table 2). In addition, the target genes of the up-regulated miRNAs are mostly involved in “Wnt signalling pathway” and “Fatty acid metabolism” (Figure 3B and Table 2). Accordingly, the expressed ZFHX3-miRNAs pathways may play a vital role in controlling cardiac biofunctions by mediating its target signalling.
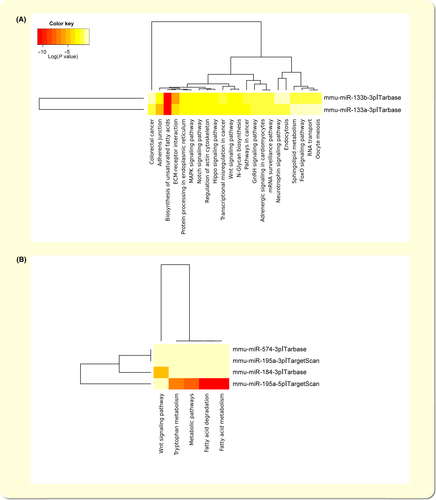
| miRNAs | Targeted signalling | Target genes |
|---|---|---|
| Down-regulated miRNAs enriched signallings | ||
| mmu-miR-133a-3p & | Adrenergic signalling | CaMKII |
| mmu-miR-133b-3p | in cardiomyocytes | CACNB1 (LTCC subunit) |
| PRKX | ||
| NFACT4 | ||
| mmu-miR-133a-3p & | Wnt signalling pathway | LRP5 |
| mmu-miR-133b-3p | DVL2 | |
| CaMKII | ||
| mmu-miR-133a-3p & | Hippo signalling pathway | CTGF |
| mmu-miR-133b-3p | MOB1A | |
| STK3 | ||
| mmu-miR-133a-3p & mmu-miR-133b-3p | Regulation of actin cytoskeleton | ITGB5 ACTN4 |
| mmu-miR-133a-3p & | ECM-receptor interaction | ITGA3 |
| mmu-miR-133b-3p | ITGAV | |
| COL4A1 | ||
| mmu-miR-133a-3p & | Adherens junction | FGFR1 |
| mmu-miR-133b-3p | CDC42 | |
| EP300 | ||
| mmu-miR-133a-3p & mmu-miR-133b-3p | Notch signalling pathway | NOTCH3 |
| Up-regulated miRNAs enriched signallings | ||
| mmu-miR-184-3p | Wnt signalling pathway | FZD4 |
| CTBP2 | ||
| mmu-miR-195a-5p | Fatty acid metabolism | ACOX1 |
| EHHADH | ||
| mmu-miR-195a-5p | Tryptophan metabolism | MAOB |
| EHHADH | ||
| IDO2 | ||
3.4 Validation of potential miR-133 target signalling in ZFHX3-KD and control HL-1 cells
Among miR-133a-3p and miR-133b-3p targeting signalling, “Adrenergic signalling” “Wnt signalling pathway” were closely involved calcium homeostasis, whereas “Regulation of actin cytoskeleton” “Adherens junction” “ECM-receptor interaction” played a crucial role in the controlling the myocyte structure remodelling (Figure 3 and Table 2). A study demonstrated that miR-133 can control multiple components of the β1-adrenergic receptor transduction cascade and is cardioprotective during heart failure.23 We further verified the expression levels of miR-133 target signalling, such as Adrenergic signalling (CaMKII), Wnt/calcium signalling (DVL2, CaMKII and JNK) and Adherens junction signalling (FGFR1) between ZFHX3-KD and control, and discovered the expressions of DVL2, pCaMKII, pJNK, JNK and FGFR1 were significant up-regulated after ZFHX3 KD (Figure 4A,B). Previous results recognized that JNK activation can contribute to reduce CX43 and development of atrial arrhythmias.38, 39 In this study, we also identified that the expression level of CX43 was reduced after ZFHX3 KD (Figure 4A,B).
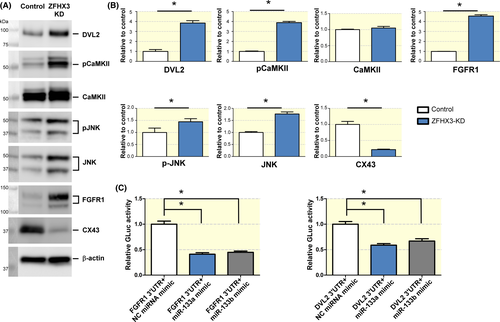
To confirm direct binding of miR-133a-3p and miR-133b-3p to the mouse FGFR1 or DVL2 3′UTR, we performed luciferase reporter assays in ZFHX3-KD cells. The 3′-UTR of DVL2 or FGFR1 were cloned downstream of GLuc and the interaction of the miR-133a-3p and miR-133b-3p with their targets in these regions were detected as a decrease in the luminescence measurements (Figure 4C). These experiments confirmed direct binding of miR-133a-3p and miR-133b-3p to both 3′-UTR of FGFR1 and DVL2 (Figure 4C).
3.5 MiR-133a-3p and miR-133b-3p can partially rescue myocyte defects after ZFHX3 KD
We investigated whether adding miR-133a-3p and miR-133b-3p mimic can recover ZFHX3/miR-133 signalling dysfunction in HL-1 cells. The transfection efficiency of miR-133a-3p and miR-133b-3p in ZFHX3-KD cells was confirmed by qPCR (Figure 5A). We performed miR-133a-3p and miR-133b-3p mimic transfection in the ZFHX3-KD cells and analysed the expression of their target signalling (eg, CaMKII, Wnt/calcium and FGFR1 signalling). MiR-133a-3p and miR-133b-3p mimics can significantly inhibit the increase in the expression of DVL2, CaMKII, pJNK and FGFR1 after ZFHX3 KD and reverse the decrease in the expression of CX43 in ZFHX3-KD cells (Figure 5A). MiR-133a-3p and miR-133b-3p mimics can significantly inhibit the increase in the expression of SERCA2a (SR calcium uptake protein) and IP3R (SR calcium leak channel) after ZFHX3 KD (Figure 5A). As compared to control, ZFHX3 KD cells did not significantly change the expression of total RyR, but enhance the expression of RyR-pS2814 (Figure 5A). Moreover, MiR-133a-3p or miR-133b-3p mimics-treated ZFHX3 KD cells had greater expressions of RyR than ZFHX3 KD cells. However, MiR-133a-3p or miR-133b-3p mimics-treated ZFHX3 KD cells had lower ratio of RyR-pS2814 to total RyR than ZFHX3 KD cells (Figure 5A). Additionally, ZFHX3-KD cells had larger SERCA activity and SR Ca2+ contents than did the control cells (Figure 5B,C). Correspondingly, miR-133a-3p and miR-133b-3p treatment could block ZFHX3 KD–enhanced SERCA activity and SR Ca2+ content (Figure 5B,C).
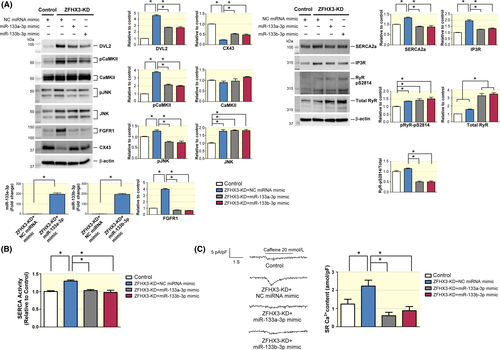
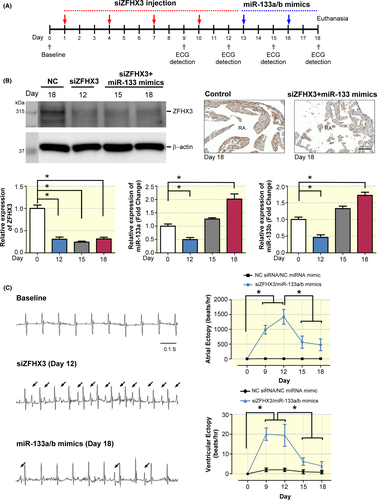
3.6 Treatment with miR-133a-3p and miR-133b-3p mimics on ZFHX3 KD–induced atrial and ventricular ectopy
To examine whether siZFHX3 tail vein–injected mice develop spontaneous atrial arrhythmias, lead II telemetry ECG recordings were performed (Figure 6A). As shown in Figure 6B, the expression of the ZFHX3 in the atrium of siZFHX3–injected mice was significantly reduced at day 12, 15, and 18 after the initiation of the experiment. We also identified that the expression of both miR-133a and miR-133b were significantly down-regulated after siZFHX3 injection (day 12), but increased after miR-133a/b mimics injection (day 18) in atrium compared with negative control (NC) siRNA. We observed increase in atrial ectopy (atrial ectopic beats) and ventricular ectopy (ventricular ectopic beats) in the siZFHX3–injected mice (Figure 6C). But atrial ectopy and ventricular ectopy were not found in NC miRNA mimic or NC siRNA-treated mice. Moreover, siZFHX3–injected mice receiving miR-133a-3p and miR-133b-3p mimics exhibited reduced siZFHX3–induced atrial ectopy and ventricular ectopy at days 15 and 18 (Figure 6C), indicating that treatments with the miR-133a-3p and miR-133b-3p mimics partially reversed siZFHX3–induced cardiac arrhythmia in mice.
4 DISCUSSION
In this study, we verified that 6 miRNAs were altered after ZFHX3 KD. Among them, miR-133a-3p and miR-133b-3p were significantly down-regulated and miR-184-3p, miR-195a-5p, miR-195a-3p and miR-574-3p were significantly up-regulated in ZFHX3-KD cells compared with control cells. Similarly, studies have shown that miR-133 was down-regulated, whereas miR-574-3p was up-regulated in the AF patients relative to the sinus rhythm patients.40, 41 In our study, we identified that the reduction of ZFHX3/miR-133 axis activity contributed to the induction of targeted adrenergic, Wnt/calcium, and FGFR1 signalling (Figure 7A,B), which participated in controlling cardiac remodelling.42-45 Based on DIANA-Tarebase, the DIANA-miRPath analysis predicted that miR-133 potentially inhibits the expression of CACNB1, PRKX, ITGA3/ITGB5 and TGFB2 (Figure 7B). NFATC4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression.46 After ZFHX3 KD, we noted JNK activation and CX43 down-regulation. Notably, miR-133a-3p and miR133b-3p mimic delivery in to ZFHX3-KD cells led to a significant reduction in its target signalling. ZFHX3 may promote miR-133 expression through interaction with myocyte enhancer factor 2.25, 26 MiR-133a-3p and miR-133b-3p mimic transfection could reduce the ZFHX3 KD–mediated up-regulation of pCaMKII, SERCA2a and IP3R, SERCA activity and SR Ca2+ content. Previous results indicated that miR-133a controls the abundance of IP3R, thereby regulating Ca2+ release from the ER.47 The deregulation of calcium homeostasis could contribute to cardiac arrhythmia pathogenesis.48 Moreover, telemetry ECG recordings revealed that the miR-133a/b mimics reduced ZFHX3 KD–induced atrial arrhythmia in ICR mice. Thus, combined with bioinformatics analysis and our results revealed that ZFHX3/miR133 signalling play a pivotal role in controlling cardiac calcium homeostasis and rhythms.
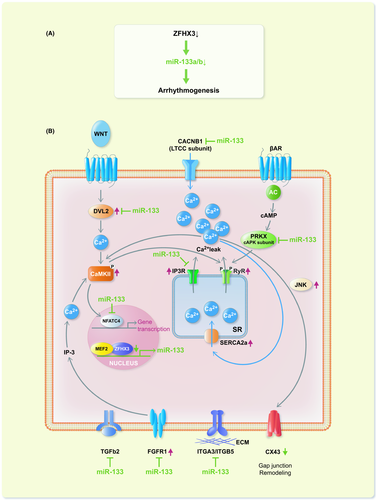
A decrease in miR-133 levels was identified in the patients with age-associated AF.49 Reduced miR-133 levels can contribute to nicotine–induced canine atrial remodelling.50 A study established that miR-133 is crucial in controlling structural changes in canine chronic AF.51 MiR-133 modulates β1-adrenergic receptor signal cascade and is cardioprotective during heart failure.23 In addition, miR-133 can inhibit the activation of the JNK2/STAT3 signalling pathway.52 JNK activation can reduce CX43 expression, which favours the occurrence of cardiac arrhythmia.38, 39 A study indicated that the axis, consisting of DVL1, CaMKII, histone deacetylase 4 and myosin enhancer factor 2, is an attractive therapeutic target for preventing cardiac remodelling.53 By the reporter assay, this study confirmed the direct binding of miR-133a-3p and miR-133b-3p to both 3′-UTR of FGFR1 and DVL2. DVL2 is necessary to mediate CaMKII function in the Wnt/Ca2+ pathway; this Wnt pathway promotes intracellular calcium transients.54 Moreover, FGFR1 signalling activation promotes cardiac remodelling.55 Therefore, the down-regulation of the ZFHX3/miR-133 axis was accompanied by the activation of DVL2/CaMKII/JNK signalling pathway and down-regulation of CX43 expression. Thus, miR-133 mimic treatment can reverse ZFHX3 defects in HL-1 cells.
Extensive studies suggest that alterations of SR Ca2+ channels including SERCA2a, RyR2 and IP3Rs contribute to changed intracellular Ca2+ transients and SR Ca2+ release that in turn lead to Ca-triggered ventricular and atrial arrhythmogenesis.56 Our previous study demonstrated that ZFHX3-KD cells have increased SERCA2a expression and activity, which may result in a larger SR Ca2+ content.32 Similarly, this study found that miR-133a-3p or miR-133b-3p mimic could block ZFHX3 KD–increased expression of SERCA2a, IP3R, SERCA activity and SR Ca2+ content. Therefore, our findings suggest that the recovery of arrhythmia by miR-133a/b mimics in siZFHX3 injected mice may be caused by reversing the ZFHX3 KD's effect on regulation of intracellular calcium homeostasis. However, miR-133a-3p or miR-133b-3p mimic did not reduce the up-regulation of RyR in ZFHX3 KD cells, suggesting that the enhanced expression of RyR by ZFHX3 KD was independent of miR-133 pathway. The decreased ratios of RyR p2814 to total RyR in miR-133 mimics-treated ZFHX3 KD cells may arise from their inhibitory effects on pCaMKII.
Our in vivo experiments indicated that ZFHX3 KD could induce atrial ectopy in ICR mice, suggesting that the loss-of-function of ZFHX3 increased atrial arrhythmia. Atrial remodelling and atrial ectopic triggers may increase AF incidence.57-59 Calcium handling abnormalities can promote ectopic activity, conduction abnormalities facilitating re-entry and AF-related remodelling.6 In the present study, we indicated that curative treatment with miR-133 mimics can reverse siZFHX3–induced atrial ectopic rhythm in ICR mice.
We noted that miR-184, the miR-195a family and miR-574 were up-regulated after ZFHX3 KD. MiR-195 is a cardio-miRNA that can elicit hypertrophic cardiomyopathy, dilated cardiomyopathy and heart failure.60 ZFHX3 can regulate the redox homeostasis in cardiomyocytes.61 In the present study, we also noted JNK expression after ZFHX3 KD. JNK signalling initiates physiological changes, resulting in the amplification of reactive oxygen species (ROS) generation.62 Studies have indicated that ROS induces miR-195 in cardiomyocytes, and JNK activates miR-184 signalling.63, 64 Accordingly, ZFHX3/JNK/ROS cascade may induce expression of stress response miRNAs, such as miR-195 and miR-184.
An earlier study also showed that miR-195 expression was up-regulated in myocardial ischaemia/reperfusion injury.65 KD of miR-195 could reduce diabetic cardiomyopathy in mice.66 Our bioinformatics analysis indicated that miR-184-3p, miR-195a-5p, miR-195a-3p and miR-574-3p target gene sets were involved in controlling the Wnt signalling cascade and fatty acid metabolism (Figure 3). Thus, ZFHX3 miRNAs signalling on regulatory fatty acid metabolism may contribute to cardiac dysfunction.
In conclusion, we proposed that ZFHX3/miR-133 targeted signalling is associated with cardiac arrhythmogenesis though mediated atrial myocyte remodelling. In particular, we noted that miR-133 mimic transfection could reverse ZFHX3 KD–mediated activation of cardiac remodelling signalling, SR Ca2+ content increase and atrial arrhythmia induction. Therefore, the increased miR-133 levels might be a potential therapeutic approach for treating AF patients with ZFHX3 dysfunction.
ACKNOWLEDGEMENTS
The authors acknowledge the High-throughput Genome and Big Data Analysis Core Facility of National Core Facility Program for Biotechnology, Taiwan (MOST 105-2319-B-010-001) for small RNA sequencing and thank the Taiwan Animal Consortium (MOST 106-2319-B-001-004, MOST 107-2319-B-001-002) Taiwan Mouse Clinic, funded by the Ministry of Science and Technology (MOST) of Taiwan for technical support in telemetry ECG experiments.
CONFLICT OF INTEREST
The authors report no commercial or proprietary interest in any product or concept discussed in this article.




