Patients with central serous chorioretinopathy not only have pachychoroidal disorders but also altered retinal metabolic function
Margarita G. Todorova was partially supported by unrestricted grant from OPOS (Stiftung Ostschweizerische Pleoptik- and Orthoptik-Schule). The sponsor had no role in the design or conduct of this research.
Abstract
Purpose
The aim of our study was to compare metabolic (oxygen saturation; %) and anatomical (diameter; μm) retinal vessel parameters of patients with central serous chorioretinopathy (CSC) to those of controls.
Methods
In this prospective cross-sectional cohort study, 72 eyes of patients with CSC were compared with 21 eyes of healthy controls. Of the 72 patients, 52 had chronic, nonactive CSC (subgroup nCSC) and 20 had active CSC (subgroup aCSC), according to activity on fluorescein angiography. Retinal vessel oximetry (RO) was performed using the Oxymap T1 oximeter. Oxygen saturation in all major peripapillary retinal arterioles (A-SO2) and venules (V-SO2) was measured, and their difference (A-V SO2) was calculated. In addition, we evaluated the corresponding diameter in retinal arterioles (D-A) and venules (D-V). For statistical evaluation, ANOVA-based linear mixed-effects models were calculated (SPSS®; p < 0.05).
Results
Central serous chorioretinopathy (CSC) patients had significantly higher A-SO2 and V-SO2 compared to that of controls (p = 0.031 and p = 0.018 respectively). Especially, the subgroup of aCSC patients showed significantly higher A-SO2 and V-SO2 values (p = 0.027 and p = 0.034, respectively). In addition, superotemporal and superonasal quadrant location showed significant interactions with A-SO2 and V-SO2 (p ≤ 0.03). Diameter in retinal arterioles (D-A), an venules (D-V) and A-V SO2 findings showed no significant differences (p > 0.096).
Conclusion
These data indicate that patients with CSC have altered metabolic function. The presence of disease activity showed the greatest influence on RO measurement, both compared to controls and to those with inactive chronic CSC disease.
Introduction
Central serous chorioretinopathy (CSC), a common disease that predominantly affects men of working age, has not been fully understood despite extensive research. The disease usually presents with visual symptoms caused by a subretinal fluid at the posterior pole. There are several explanations for how the subretinal fluid evolves, but the primary site of development is most likely the choroid. In this study, we did not want to focus on the choroid but on the metabolic function of the retina in this disease. For this purpose, we examined the retinal vessel oxygen saturation of patients with CSC and compared the results to those of healthy subjects.
Multiple pathophysiological mechanisms are discussed in the literature regarding the development of CSC. Mostly the cause is thought to be found in the choroid, but there are also explanations that look for the cause in the retinal pigment epithelium. The following risk factors have been identified so far: age 30–44 years, male gender, increased stress level, endogenous and exogenous hypercortisolism, sleep disorders, pregnancy and a genetic predisposition, as well as increased choroidal thickness and an increased choroidal vascularity index (CVI) (Kaye et al. 2020).
Central serous chorioretinopathy (CSC) is often classified in the pachychoroid spectrum. All diseases of this spectrum have a thickened choroid with attenuation of the choriocapillaris in common without a known common cause. A possible approach for explanation is the theory of venous overload choriopathy (Spaide et al. 2021a).
Studies on the retinal vessels of patients with CSC are scarce in literature. One study examined the capillary vessel density (VD) of the superficial and deep capillary plexus as well as the avascular zone of patients with CSC without finding a significant difference compared with healthy eyes (Han et al. 2020). This was partially confirmed by a study by Yu et al. (2018), who found no difference in the superficial macrovasculature but did find a difference in the superficial microvasculature. In contrast, an Italian study showed significantly reduced VD in patients with CSC compared to that of healthy controls. This study also showed that under exertion, patients with CSC have significantly increased blood flow compared to that of healthy subjects (Cardillo Piccolino et al. 2018). Another study of the retinal vessels in patients with CSC using dynamic vessel analysis showed a reduced dilation of the venous vessels to a flicker stimulus, the authors speculated that increased sympathetic tone may be responsible for this (Tomasso et al. 2018).
Given the known dysfunction of superficial microvessels in CSC (Yu et al. 2018; Han et al. 2020), it is to be expected that choroidal and retinal vasculature may also reflect metabolic dysfunction.
The retinal vessel oximetry (RO) is a noninvasive, noncontact, painless, reproducible and timely examination that measures the oxygen saturation of the retinal arterioles and venules. The technique is simple and has been extensively studied with a low variability on test–retest studies (Stefánsson et al. 2019). The arterio-venous difference of oxygen saturation allows conclusions to be drawn about the metabolic activity of the retina.
The main objective of this prospective observational study was therefore to investigate the RO parameters and retinal vessel diameters of eyes affected by CSC and to compare these measurements with healthy control eyes. We aimed to find both similarities and differences between affected and healthy eyes. Furthermore, the activity of the CSC disease and its relation to the retinal metabolic function has not been studied before. Therefore, our secondary objective of the study was to link the presence of CSC activity to retinal vascular abnormalities and metabolic function parameters.
Materials and Methods
Prospective cross-sectional cohort study of 121 eyes of 68 subjects was performed: 100 eyes of 53 patients diagnosed with CSC and 21 eyes of 15 healthy control subjects were examined from September 2019 to August 2020 in one center for ophthalmology (Department of Ophthalmology, Cantonal Hospital St. Gallen, Switzerland).
All patients and controls underwent all required standard evaluations by experienced specialists for acquired retinal diseases (M.G.T, C. V. and S.T). All CSC patients presented with a typical clinical picture of the disease. The clinical phenotype of CSC patients was characterized according to diagnostic imaging (including colour fundus imaging, fundus autofluorescence imaging, fluorescein angiography and optical coherence tomography; OCT). Central serous chorioretinopathy (CSC) patients were divided into two subgroups (active: aCSC and nonactive: nCSC) according to the presence or absence of activity on fluorescein angiography. The CSC was labelled as active (aCSC) in the presence of serous detachment of the neurosensory retina associated with a focal leak or leaks on the level of the RPE on fluorescein angiography (FAG). The nonactive (nCSC) or chronic CSC was defined based on the presence of widespread tracks ‘crying retina sign’ on fundus autofluorescence imaging (Handtke et al. 2018). In addition, the monolaterality, bilaterality or mulitfocality were taken into consideration for each CSC patient.
Retinal vessel oximetry acquisition
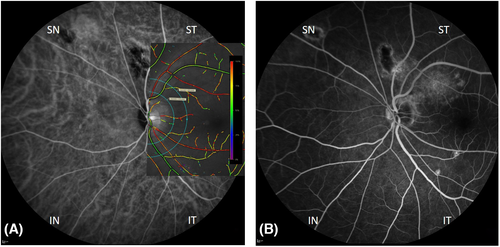
Retinal vessel oximetry (RO) measurements were performed with the spectrophotometric oximetry unit for retinal vessel oximetry (Oxymap T1 ehf., Reykjavik, Iceland). Fundus images were recorded using a camera system, Digital Camera Topcon TRC 50DX (Topcon TRC-50DX; Topcon Corporation, Tokyo, Japan). The software operating the system (Oxymap ehf., Reykjavik, Iceland) differentiates simultaneously between oxygenated and deoxygenated haemoglobin based on the different light-imaging characteristics at various wavelengths, measuring the oxygen saturation level in the examined retinal vessel. In summary, RO was performed at two different wavelengths: at the green (isobestic) channel (586 ± 10 nm) to record the oxygen-insensitive image and at the red (nonisobestic) channel (605 ± 10 nm) to record the oxygen-sensitive image (Hardarson et al. 2006). An optic disc-centred image protocol was used where two concentric rings were created in the peripapillary area: one with a radius of 2 optic disc diameters, and the other with a radius of 3 optic disc diameters. The region between these two circles defined the area of interest where all measurements were obtained (Fig. 1). Two test–retest fundus images for each eye were performed (Todorova et al. 2014; Türksever et al. 2014; Türksever et al. 2015; Todorova et al. 2016). One image with optimal illumination, at least 8% the scale was further selected for analysis. For oximetry analyses, all main arterioles and venules were selected manually within the area of interest. The software (Oxymap ehf.) then calculates the optical density ratio (ODR) of the two images and, thus, the mean oxygen saturation of the evaluated retinal vessel. The global mean oxygen saturation in retinal arterioles (A-SO2) and venules (V-SO2) was measured, and their difference, the A-V SO2, was calculated. In addition, we determined the diameters of the corresponding retinal arterioles (D-A) and venules (D-V).
We determined the inclusion criteria for all patients and controls to be as follows: Stable fixation and refractive error (spherical equivalent) of <6 diopters. Patients and controls who had previous ocular surgery or ocular and systemic pathology (as for instance, diabetes mellitus or neurological disease) that may influence the FAG, OCT and RO measurements were not included in this study. Additional exclusion criteria were images with inadequate quality, or an expressed unwillingness to participate in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional/national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All participants underwent a detailed ophthalmic examination, which included among others refraction, best-corrected visual acuity obtained under standardized ETDRS conditions (usina a Sloan Early Treatment Diabetic Retinopathy Study), intraocular pressure examination by applanation, biomicroscopy and funduscopy. Prior to diagnostic imaging, pupils were maximally dilated (eye drops prepared in our institutional pharmacy as a combination of phenylephrine HCI 25 mg/ml and tropicamid 5 mg/ml in 3-ml container-labelled MIX).
Statistics and mathematical analyses
For statistical evaluation, ANOVA-based linear mixed-effects models were performed with SPSS® (IBM SPSS Statistics®, Version 25.0.0.0; Armonk NY; IBM Corp.), which allows for taking the dependency of the left and right eyes in the same subject into account and is suitable for repeated measurements also when taking both eyes into the analysis. A linear mixed-effects model was performed for each pair of the tested methods, where one parameter of the tested pair is a dependent variable.
The mean SO2 parameters (A-SO2, V-SO2, their difference: A-V SO2), as well as the mean of the vessel diameter measurements (D-A and D-V), were taken as independent variables.
In the present study, in order to predict the effect of the underlying disease on RO measurements, ‘subject’ was taken as a random factor, and the ‘group’ and the ‘eye’ were taken as fixed factors. In order to exclude the effect on age, location (quadrants) or refraction, we took into account the age, the location and the spherical equivalent as covariates.
In addition, in order to evaluate the effect of the activity of the CSC disease, the data were evaluated also in subgroups, divided as aCSC (active) and nCSC (nonactive). Results are presented as adjusted mean values and standard error for controls and the corresponding mean diference in patients' subgroups with the respective p-values. p < 0.05 was defined as statistically significant.
Results
In total, 121 eyes of 68 subjects were enrolled in the study: 20 eyes of 17 patients diagnosed with active CSC (aCSC), 52 eyes of 38 patients with nonactive CSC (nCSC) were compared with 21 eyes of 15 age-matched controls. Note, that two patients were diagnosed with aCSC in one eye and nCSC in the other eye, so the number of subjects is not cumulative. In 72 of the 100 eyes of the CSC patients, the disease could be detected. In 28 eyes of the CSC patients neither active nor chronic CSC disease could be detected and therefore were excluded from further statistical analyses. All demographic characteristics of our participants are summarized in Table 1.
| Clinical details | Number of subjects | Eyes evaluated | Age, year; Mean (±SD) | Gender (♀:♂) |
Mean best-corrected visual acuity, (±SD) (Snellen charts) |
|
|---|---|---|---|---|---|---|
| RE | LE | |||||
| Controls | 15 | 10 | 11 | 47 (21) | 9:6 | 0.98 (0.16) |
| aCSC | 17* | 11 | 9 | 46 (9) | 6:11 | 0.86 (0.25) |
| nCSC | 38* | 23 | 29 | 54 (12) | 9:29 | 0.72 (0.34) |
- Note, that two patients (*) were diagnosed with aCSC in one eye and nCSC in the other eye, so the number of subjects is not cumulative. RE-right eye; LE-left eye.
Increased A-SO2 and V-SO2 values in CSC patients
In general, patients with CSC had higher average A-SO2 and V-SO2 and unaffected A-V SO2 values when compared to controls (p ≤ 0.03, ANOVA based on mixed-effects models). In CSC patients, mean retinal A-SO2 and V-SO2 values were significantly increased compared to controls with 97.97% and 68.51% versus 96.70% and 66.09%, respectively, while A-V SO2 showed no statistically significant difference with 29.74% versus 29.99% (Table 2).
Adjusted mean values [standard error], based on linear mixed-effects models Controls/CSC averaged |
p-values 2 groups |
Mean difference in subgroups (p-values) Controls/aCSC/nCSC | p-values 3 groups |
|||
|---|---|---|---|---|---|---|
| Controls | CSC | Group effect | Controls versus aCSC | Controls versus nCSC | aCSC versus nCSC | Group effect |
| A-SO2, % | ||||||
| 96.70 [0.53] | 97.97 [0.26] | 0.03 | −1.27 (0.03) | −0.32 (0.63) | 0.95 (0.07) | 0.04 |
| V-SO2, % | ||||||
| 66.09 [0.93] | 68.51 [0.40] | 0.02 | −2.04 (0.034) | −1.03 (0.34) | 1.01 (0.22) | 0.08 |
| A-V SO2, % | ||||||
| 29.99 [1.10] | 29.74 [0.50] | 0.72 | 0.46 (0.72) | 0.44 (0.76) | −0.02 (0.99) | 0.93 |
| D-A, μm | ||||||
| 97.12 [2.35] | 101.45 [1.14] | 0.097 | −2.13 (0.39) | −1.61 (0.57) | 0.52 (0.81) | 0.69 |
| D-V, μm | ||||||
| 127.49 [4.07] | 124.66 [1.76] | 0.52 | 3.61 (0.372) | 1.79 (0.70) | −1.82 (0.60) | 0.64 |
- Both are the significant p-value differences of the examined subgroups.
- aCSC = active CSC, CSC = central serous chorioretinopathy, nCSC = nonactive CSC.
- Statistical sifnificance was defined as p-values <0.05 (bold) and statistical trend as p-values between 0.05 and 0.10 (in italic).
Retinal vessel diameters in CSC patients
In general, the average retinal vessel diameters, both the D-A and D-V were not significantly affected in the CSC patients than in controls (p ≥ 0.097). Noticeably, the CSC group showed a trend for an average peripapillary D-A dillatation at 101.45 μm when compared to the control group at 97.12 μm. Nevertheless, this value did not reach a statistically significant difference (p = 0.097; Table 2).
Subgroup analysis based on the activity of CSC
Since patients suffering from CSC often present with bilateral involvement and assymetric activity of the disease, we divided the CSC group, according to the presence or absence of activity on fluorescein angiography, into two subgroups of active: aCSC and nonactive: nCSC.
Comparison of retinal oximetry (RO) parameters in CSC subgroups against controls
Evaluated in subgroups, eyes with aCSC showed significant differences when the parameters A-SO2 and V-SO2 were considered. In the presence of active CSC disease, A-SO2 and V-SO2 were significantly elevated compared to controls (p = 0.03). Oxygen saturation in retinal arterioles (A-SO2), which even tended to increase in aCSC compared to nCSC, did not reach a statistically significant difference (p = 0.07). Furthermore, there was no statistically significant difference between the nCSC and the controls when the A-SO2, V-SO2 and A-V SO2 values were considered. (p > 0.33, Table 2).
Comparison of retinal vessel diameters in CSC subgroups against controls
When compared with controls, eyes suffering from aCSC or nCSC did not show significant differences when the D-A and D-V were taken into account (p > 0.38).
The effect of quadrant on RO values
In regard to the peripapillary quadrant (IN = inferonasal, IT = inferotemporal, SN = superonasal, ST = superotemporal) distribution, the aCSC group showed in general a tendancy for increased A-SO2 and V-SO2 values, when compared to controls and the nCSC group.
Quadrant analyses in controls showed the highest A-SO2 values in the SN (100.12%) and the lowest A-SO2 values in the IT peripapillary retina (93.74%; Table 3; Fig. 2). The same also held true for the nCSC and the aCSC patients with values 100.25% and 93.91% and, respectively, 101.18% and 95.11% (p ≤ 0.007; Fig. 2). Compared to the entire CSC group but also in subgroups, the A-SO2 values in the ST quadrant was significantly increased in the CSC compared to that of controls (p ≤ 0.03; Table 3). Also, in the IN, the A-SO2 was significantly increased in the aCSC when compared to that in the nCSC. Both results thus influenced the overall group effect for the A-SO2 (p = 0.06; Table 3).
| Variables | Adjusted mean values, based on mixed-effects models [SE] | Mean difference Controls versus CSC in subgroups (p-values) |
Mean difference aCSC versus nCSC (p-values) |
p-values | ||
|---|---|---|---|---|---|---|
| Values: average/in quadrants | Controls | Controls-CSC | Controls-aCSC | Controls-nCSC | aCSC-nCSC | Group effect |
| A-SO2, % | ||||||
| SN | 100.12 [0.84] | −0.74 (0.50) | −1.02 (0.37) | −0.08 (0.95) | 0.93 (0.34) | 0.06 |
| IN | 98.92 [0.88] | 0.93 (0.42) | 0.36 (0.76) | 2.27 (0.10) | 1.91 (0.08) | |
| IT | 93.74 [0.84] | −0.61 (0.59) | −1.02 (0.39) | 0.28 (0.84) | 1.30 (0.22) | |
| ST | 94.35 [0.81] | −2.51 (0.01) | −2.75 (0.02) | −2.75 (0.02) | −0.36 (0.70) | |
| V-SO2, % | ||||||
| SN | 67.08 [1.37] | −3.54 (0.03) | −3.64 (0.03) | −3.32 (0.07) | −0.32 (0.82) | 0.06 |
| IN | 66.48 [1.46] | −0.24 (0.89) | −0.18 (0.92) | −0.39 (0.83) | −0.21 (0.89) | |
| IT | 64.10 [1.26] | −1.14 (0.95) | −1.07 (0.64) | 2.04 (0.44) | 3.10 (0.13) | |
| ST | 68.10 [0.24] | −2.54 (0.27) | −2.93 (0.08) | −1.68 (0.38) | 1.25 (0.40) | |
- aCSC = active CSC, CSC = central serous chorioretinopathy, nCSC = nonactive CSC, SN = superonasal, IN = inferonasal, IT = inferotemporal, ST = superotemporal.
- Statistical sifnificance was defined as p-values <0.05 in bold) and statistical trend as p-values between 0.05 and 0.10 (in italic).
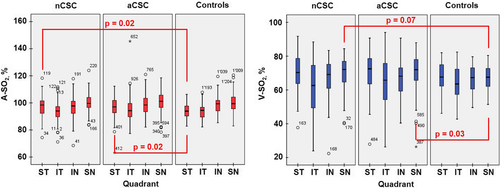
In regard to the quadrant analyses of the V-SO2 values, in controls, the highest values were found in the ST (68.10%) and the lowest V-SO2 values in the IT peripapillary retina (64.10%; p = 0.03; Table 3; Fig. 2). This quadrant distribution in the V-SO2 also held true for the aCSC patients with corresponding values for the ST of 71.03% and for the IT peripapillary retina of 65.16% (p < 0.001). In contrast, in the nCSC group, quadrant V-SO2 values were highest in the SN peripapillary area (70.41%) and lowest in the IT peripapillary retina (62.06%; p < 0.001; Table 3; Fig. 2). Compared to controls, the V-SO2 values in the SN quadrant were significantly increased in the aCSC but also in the nCSC (p ≤ 0.07; Table 3).
Quadrant analyses for the A-V SO2, D-A and D-V showed no statistically significant difference between all examined groups (p > 0.005). Nevertheless, the D-A of the entire CSC group showed a trend to be more dilated than that of controls (Table 2). Therefore, and since the diameter of the retinal vessels is known to influence the SO2 values (Beach et al. 1999; Vandewalle et al. 2013), we looked also for interractions between the SO2 and the diameter values within subgroups (p = 0.028). Here, statistically significant values were reached for the A-SO2 in the ST quadrant, where not only aCSC, but also nCSC could be differentiated from controls with increased A-SO2 values (p = 0.010 and p = 0.026 respectively). For the V-SO2, the same held true in the ST and SN quadrants, where aCSC patients could clearly be differentiented from controls by this sign (p = 0.031 and p = 0.036).
Correlations between the RO and the distribution of the lesion
Within the aCSC patients, increased A-SO2 and V-SO2 values correlated positively with the foveal involvement (p = 0.019, R = 0.089; p = 0.040, R = 0.119 respectively). Furthermore, the A-SO2 values correlated positively with the subretinal fluid in the SN peripapillary area (0.046, R = 0.076) and the V-SO2 values with the subretinal fluid in the IT peripapillary area (0.040, R = 0.086).
In regard to the A-V SO2, within the aCSC group, positive correlations were found with the presence of a focal leak in the ST quadrant (p < 0.001, R = 0.162), as well as with the subretinal fluid in the ST and SN quadrants (p = 0.034, R = 692; p = 0.041, R = 692 respectively).
Discussion
Central serous chorioretinopathy (CSC) is characterized by increased choroidal thickness, hyperpermeability and congestion of choroidal vessels.
Theories related to infections (Djabri & Diallinas 1964; Szreter 2014; Burucoa & Axon 2017) and stress (Yannuzzi 1986) in patients with CSC have been replaced by those paying attention to alterations in the choroidal blood flow measured on laser Doppler flowgraphy (LDF). These disturbances of choroidal blood flow were mainly found to be the result of altered vascular resistance (Tittl et al. 2005; Saito et al. 2018). Also, active CSC has been found to be associated with increased fundus pulsation amplitudes and an abnormal local variability of fundus pulsation amplitudes, indicative of increased pulsatile choroidal blood flow (Tittl et al. 2003). Furthermore, it has been shown that patients with active CSC have lower choroidal blood flow on LDF than healthy control subjects (Kitaya et al. 2003).
The recently emerged pachychoroid concept has changed the understanding of CSC. Novel CSC susceptible hyperpermeability loci, expressed in the choroid, which is the main focus of CSC, have been identified (Spaide & Ryan 2015). Central serous chorioretinopathy (CSC) as part of the pachychoroid spectrum disease have a thickened choroid with attenuation of the choriocapillaris which is thought to be due to venous overload choriopathy (Kaye et al. 2020). Thus, and since alterations in the ocular and choroidal blood flow, choroidal vascular hyperpermeability and excessive leakage have been reported in patients with CSC, metabolic demand would also be expected to occur in CSC and may possibly reflect the activity of the disease.
Retinal vessel oximetry (RO) has recently been introduced as a marker of metabolic disease activity in a wide variety of retinal and systemic diseases (Stefánsson et al. 2019). A recent study found increased retinal vascular oxygen saturation in arterioles and venules in acute CSC (aCSC) compared to that in the nonactive fellow eye (Li et al. 2017).
Increased retinal vessels oxygen saturation in CSC
Consistent with the study cited above, we again found an increased A-SO2 and increased V-SO2 within the CSC group. However, in the present study, we were also able to distinguish between the active the nonactive stage of the disease.
Within the aCSC group, we found that the metabolic alterations were significantly more profound in the aCSC group compared to that in controls and also in the nCSC group. The results were consistent both in the evaluation of the average SO2 values and in the evaluation of the SO2 values in the quadrants. A possible explanation for this finding could be the fact that when the CSC is active as part of pachychoroidal disease, there is decreased oxygen consumption, which is reflected in generally increased arteriolar and venous SO2 levels (Spaide et al. 2021a). Ma et al. (2012) have shown that retinal and choroidal blood flow is inversely related to oxygen tension in the extracellular space. Impaired metabolic function therefore can also be attributed to the increased A-SO2 levels, as a consequence of reduced metabolic demand and a destroyed blood-retinal barrier. Consistent with this, we found higher mean A-SO2 levels in the aCSC group than in the control group or in patients with nCSC.
A possible hypothesis for the increased V-SO2 in the aCSC subgroup could be based on hyperoxygenation of the extracellular space due to reduced metabolic demand and altered ocular blood flow.
In the nCSC group, the destroyed blood-retinal barrier, following tissue remodelling and apoptosis due to the chronic stage of the disease could be accounted for the increased V-SO2. Of particular interest is the fact that the greater portion of oxygen, supplying the outer retina, comes from the choriocapillaris (Yu & Cringle 2001; Cringle & Yu 2002; Cringle et al. 2002). When the function of the blood-retinal barrier is compromised in nCSC, as for instance as part of the pachychoroidal disease with a secondary retinal pigment epithelium apoptosis, an increased oxygenation of the extracellular space would occur (Spaide et al. 2021b). In agreement with the above statements, the level of the V-SO2 in nCSC was significantly higher.
The question of why the arterial oxygen saturation should be increased although the peripapillary arterial blood has not yet participated in the ocular metabolism might on the one hand be explained by the above-mentioned increased extracellular oxygen pressure. On the other hand, the reduced oxygen exchange between the central artery and vein in the optic nerve might contribute to this. Since the oxygen saturation in the venules is increased as is to be expected because of the reduced metabolism due to the CSC, there is a reduced counter current oxygen exchange and a correspondingly higher A-SO2 (Eliasdottir 2018). The role of extraocular, systemic oxygenation in patients with CSC remains unclear.
Further possible explanations for measured metabolic alterations found in our CSC patients could be the excessive hydrostatic pressure discussed nowadays, which is due to choroidal vascular hyperpermeability and excessive leakage (Pietruschka 1965). All of the discussed probably lead to increased oxygen gradient toward inner retina, where the oximetry measurements on retinal vessels are performed.
A set of findings occur in the choroid following venous outflow abnormalities, including venous dilatation and choroidal hyperpermeability, which are now referred to as clinical hallmarks of CSC (Agrawal et al. 2016). Consistently, Indocyanine green (ICG) angiography and enhanced depth imaging OCT (EDI-OCT) reports confirmed enlarged choroidal vessels and thickened choroid, as well as an increase in the diameter of choroidal vessels, particularly of the larger veins in Haller's layer (Prünte 1995, Prünte & Flammer 1996a, Prünte & Flammer 1996b). Thus, increased oxygenation of the retinal vessels seems to be part of the pachychoroidal alterations in CSC now confirmed in the present study.
Quadrant oxygen distribution in CSC as compared to controls
It is usually thought that the macula is primarily affected in CSC (Miyake et al. 1988; Marmor & Tan 1999). According to the vascular anatomy, the choroidal veins within an individual vortex system do not show the common branching structure found in other vessel systems, such as the retina. Instead, there are many vessels that independently course toward the ampule. This reflects the anatomical hallmarks on wide-field ICG angiography of the choroid and its relation to each one of the vortex vein per quadrant (Spaide et al. 2021b). Therefore, contrary to the group of Li et al. (2017), we performed our quadrant analyses taking the optic disc-centred image into account (Fig. 1).
Interestingly, when compared to the entire CSC group but also in subgroups, the A-SO2 values in the ST quadrant was significantly increased in the aCSC compared to that in controls (p ≤ 0.03). Also, in the IN quadrant, the A-SO2 showed a trend to increase in the aCSC when compared to the nCSC (p = 0.008). Furthermore, the A-SO2 values correlated positively with the subretinal fluid in the SN peripapillary area (0.046; R = 0.076) and the V-SO2 values with the subretinal fluid in the IT peripapillary area (0.040; R = 0.086).
One might speculate that these results are related to the predominantly anastomotic distribution of the vessels between the IT and ST vortex vein systems observed in CSC patients (Matsumoto et al. 2020). In a study of wide-field ICG angiography of patients with CSC, peripapillary pachychoroid syndrome, and macular neovascularization including polypoidal choroidal vasculopathy associated with CSC, intervortex venous anastomoses have been observed uniformly presented among the SN, ST and IT vortex vein systems, whereas the IN vortex vein was less often involved (Spaide et al. 2021b).
Oxygen use in CSC
Another measure, which is an indirect indicator for oxygen use in the retina, the A-V SO2 difference, was in the present study not affected in the CSC patients. This was the case also when compared in subgroups and against controls (p ≥ 0.651). The result could well be attributed to the simultaneous increase of both parameters A-SO2 and V-SO2.
Nevertheless, within the aCSC group, positive correlations were found with the presence of a focal leak in the ST quadrant (p < 0.001), as well as with the subretinal fluid in the ST and SN quadrants (p = 0.034, R = 692; p = 0.041, R = 692 respectively), consistent with a metabolic demand in the affected areas.
Retinal vessels and CSC
Consistent with the activity of the pachychoroidal disease occurring in aCSC patients, dilatation of the retinal arteriolar vessels is expected to occur. Our aCSC showed a slight trend to be distinguished from controls, as well as from nCSC by this sign. To rule out the effect of retinal vessel diameter on oxygen saturation measurements (Beach et al. 1999; Vandewalle et al. 2013), even having a software incorporated in the Oxymap system, we evaluated the relationship between the A-SO2 and the diameter of arterioles, as well as between the V-SO2 and the diameter of venules. Results of our study showed a significant influence of retinal vessel diameters on vessel SO2 measurements in quadrants for the arterial as well as for the venous measurements in subgroups (linear mixed-effects model). This was the case for the A-SO2 in the ST quadrant, where not only aCSC, but also nCSC could be differentiated from controls with increased A-SO2 values (p = 0.010 and p = 0.026 respectively), but also for the V-SO2 in the ST and SN quadrants, where aCSC patients could clearly be differentiented from controls by this sign (p = 0.031 and p = 0.036). This means that D-A and D-V, even though to be almost equally dilated as part of the activity of the disease, influence oxygen saturation values, again being more affected in patients with aCSC.
Summary
In summary, our preliminary data on 72 eyes of patients suffering from CSC and on 21 eyes of healthy controls suggest the oxygen metabolism to be affected in CSC patients by means of increase in the arterial A-SO2 and venous V-SO2 saturation, where the activity of the disease seems to contribute even more the retinal metabolic demand. Thus, retinal vessel oximetry constitutes a useful tool for differentiating aCSC patients from nCSC, and even more from controls.
Further studies need to evaluate the metabolic choroidal alterations in patients with pachychoroidal disease.




