Preparing the ocular surface for glaucoma filtration surgery: an unmet clinical need
Abstract
The mutual relationship among medical therapy, ocular surface (OS) and filtration surgery (FS) represents one of the most crucial issues in glaucoma management. As the long-term use of intraocular pressure-lowering medications significantly affect the OS health, patients with an uncontrolled disease frequently undergo glaucoma surgery in less-than-ideal conditions. As we known, OS changes strongly affect the post-operative bleb filtration capability. Therefore, improving the OS conditions before proceeding with FS is needed. Currently, given the rapid diffusion of new surgical procedures, this need is even more perceived. Nevertheless, despite surgeons retain the OS preparation of primary importance, and recognize the OS disease (OSD) as the only modifiable risk factor for filtration failure, there is no agreement on which strategies should be preferred to prepare patients. This is largely due to the lack of validated guidelines, which forces clinicians to adopt personal approaches based on evidence derived from low-quality studies. In this review, we provided an overview of risk factors involved in the FS failure, with particular attention to those depending on OS changes, and how OSD negatively affects the aqueous humor resorption after surgery. Moreover, we reported the most exploited measures to mitigate the OSD before surgery, the possible reasons underlying the absence of shared approaches, and the upcoming area of intervention to preserve the OS health during glaucoma management. Finally, based on the current evidence, we proposed a pre-operative outline reporting the main risk factors that should be considered before surgery, and the therapeutical options available to improve the OS.
Introduction
The term glaucoma refers to a multifactorial and progressive optic neuropathy, which includes a wide variety of clinical conditions, broadly classified in open-angle and angle-closure glaucoma. Currently, the only evidence-based strategy to slow the disease progression is to lower intraocular pressure (IOP) (Weinreb et al. 2014). Topical medications and selective laser trabeculoplasty are considered the first therapeutical options in the glaucoma treatment algorithm. The European Glaucoma Society (EGS) Guidelines recommend that medical treatment regimens should not exceed three daily drops and suggest to consider surgery in patients showing poor disease control under two drugs daily (European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition, 2020). Filtration surgery (FS), the most effective surgical procedure for glaucoma, is recommended, when the IOP is poorly controlled with medications or laser, there is evidence of damage progression, and in patients with low tolerance to medications and low adherence to medical therapy.
Overall, IOP lowering medications, ocular surface condition and the outcomes of FS show a close relationship, since medical therapy harms the ocular surface, which in turn is the ‘generator’ of the conjunctival filtration bleb (Yu et al. 2009). These mutual relationships were originally described in the milestone studies of Broadway et al. (1994a,b), who demonstrated that medical therapy induces profound alterations of the conjunctiva, and these modifications negatively affects the aqueous humor (AH) resorption after surgery.
This evidence indicated a strong clinical need to improve the ocular surface before proceeding with surgery. The first attempts to prepare the ocular surface date back to the last two decades of the 20th century, when Giangiacomo et al. (1986) reported the positive effects on post-operative IOP control of a subconjunctival injection of triamcinolone given before surgery. Several years later, other studies reported the beneficial effects of the pre-operative use of topical steroids on the FS outcome (Broadway et al. 1996; Breusegem et al. 2010). Overall, these pivotal studies provided positive data supporting that the pre-operative management of the ocular surface alterations is useful in minimizing the post-operative bleb-scarring.
Based on these assumptions, this review aims at providing an updated overview: (i) on the risk factors affecting FS outcome and (ii) on the most significant therapy-related ocular surface changes in glaucoma, with particular attention to those affecting the FS outcome; (iii) on the relationships between the pre-operative ocular surface status and the filtration bleb drainage function, and how the conjunctival alterations affect the AH resorption routes after surgery; (iv) to report the current management of the ocular surface adopted by clinicians before FS and (v) to identify the possible reasons underlying the absence of shared approaches, and the emerging area of interventions to preserve the OS during the management of the disease. Based on the current evidence, we proposed a pre-operative outline for glaucoma patients candidate to FS, and the measures available to improve the ocular surface.
Factors affecting the glaucoma filtration surgery outcome
The efficacy of FS depends on the achievement of an efficient filtration bleb. However, several elements were reported to affect the bleb formation and its drainage capability, thus acting as risk factors for FS outcome (Table 1).
| Demographic and systemic risk factors | Ocular surface - independent risk factors | Ocular surface - dependent risk factors |
|---|---|---|
| High pre-operative IOP | GTOSD Duration of medical therapy (years) Therapy regimen Cumulative dose of preservative |
|
| Young age | Advanced stage of disease (MD) | Non - GTOSD Previous cataract surgery Previous surgical violations of the conjunctiva Previous ocular trauma Concomitant inflammatory OSD |
| African ancestry | Impaired visual acuity | |
| Diabetes | Intraocular neovascularization | |
| Intraocular inflammation |
- GTOSD = glaucoma therapy-related ocular surface disease, IOP = intraocular pressure (mmHg), MD = mean defect (dB) at visual field.
Age
Younger age is considered one of the most important factors associated with a lower success rate of trabeculectomy (Fontana et al. 2006; Khaw et al. 2012). In a large retrospective study that considered 330 procedures over 20 years of follow-up, Landers et al. (2012) found that patients older than 80 years had a significantly lower failure rate than patients under 40 years. Similar results were found in the Advanced Glaucoma Intervention Study (AGIS), where a younger age was one of the four pre-operative risk factors associated with surgery failure (The AGIS Investigators 2002). This clinical evidence is likely supported by the fact that younger subjects have a more intense inflammatory reaction and a quicker healing response to surgery compared to older subjects (Sussman 1973). Moreover, since the Tenon's capsule is a reservoir of fibroblasts and is thicker in young, it could more intensively promote bleb fibrosis (Spencer & Zimmerman 1985).
Race
The AGIS Report (2001) found that black patients who underwent FS had a 79% greater risk of failure compared with white subjects. Broadway et al. (1994) reported a race-dependent difference in the conjunctival cell profiles, with a greater number of stromal macrophages in samples taken from non-white compared to white subjects. In addition, the thicker Tenon's capsule of patients with a black race has also been advocated as a risk factor for FS failure (McNair 1951). Although one cannot draw robust conclusions, it might be advisable to consider the African ancestry in the pre-operative assessment of the risk factors associated with FS outcome (Broadway et al. 1994).
Diabetes
To date, whether diabetes is a risk factor or not for FS failure is still a matter of debate. A prospective randomized controlled trial (RCT) found that patients with diabetes had a higher risk of FS failure (hazard ratio = 2.86) compared to other baseline factors (AGIS Investigators 2002). Several retrospective studies agreed with this finding, reporting that the survival rate of filtration procedures was significantly lower, and mean post-operative IOP was significantly higher, in diabetic patients compared to non-diabetic patients (Law et al. 2013; Schlenker et al. 2017). However, despite this evidence, the EGS Guidelines (Anon 2021), the American Academy of Ophthalmology recommendations (AAO PPP Glaucoma Committee 2020) and the Asia Pacific Glaucoma Society Guidelines (Asia Pacific Glaucoma Society 2016) did not mention diabetes among risk factors for FS failure. So far, definite conclusions cannot be drawn on the role of diabetes as a risk factor for FS failure. However, it seems prudent to consider with particular attention the presence of diabetes when a patient is undergoing glaucoma surgery, as it could negatively affect the FS outcome.
Ocular risk factors
High baseline IOP
A higher pre-operative IOP was reported as a risk factor for trabeculectomy failure in large RCTs. In the AGIS report, the risk of filtration failure increased by 4% for every mmHg increase in pre-operative IOP (AGIS Investigators 2002). In the Collaborative Initial Glaucoma Treatment Study (CIGTS) a higher baseline IOP was predictive of a higher post-operative IOP during the nine years of follow-up (Musch et al. 2008). These findings were confirmed in a large retrospective study in which the success rate of FS was found to decrease with the increase of baseline IOP, with a significantly better outcome in patients with a pre-operative IOP lower than 30 mm Hg (Landers et al. 2012). As patients with advanced glaucoma are likely to have a higher baseline IOP, high pre-operative IOP values can be considered as a marker of more severe or aggressive disease.
Stage of disease and visual impairment
Severe visual field (VF) loss and impaired visual acuity at the time of surgery were reported to increase the rate of trabeculectomy failure. Landers et al. (2012) found that the severity of VF damage, along with higher baseline IOP, previous ocular surgeries and the number of topical medications were risk factors significantly associated with poor surgical outcomes. Chiu et al. (2020) recently found that patients with visual acuity less than 6/60 and with a therapy regimen requiring a higher number of glaucoma medications had a higher risk of filtration failure.
Ocular comorbidity
Any condition associated with intraocular inflammation, such as uveitis, iris and angle neovascularization, or previous trauma, represent a significant risk factor for FS failure (Broadway & Chang 2001). The inflammatory cellular profiles of the conjunctiva described in these conditions, consisting of a higher density of fibroblasts, macrophages and lymphocytes, promote a more intense conjunctival inflammation in response to surgery and increase the risk of bleb fibrosis (Broadway et al. 1993; Broadway & Chang 2001).
Previous ocular surgery
Since cataract surgery induces a breakdown in the blood-aqueous barrier with an up-regulation of cytokines, the inflammation induced by this surgery was postulated to have an impact on the outcome of filtration procedures, promoting conjunctival fibrosis and bleb failure (Inoue et al. 2012; Khaw et al. 2012; European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition, 2020). Similar considerations were reported in case of aphakia, which is often the result of a complicated cataract surgery with release of inflammatory cytokines (Herschler 1981). Surgeries that require violation of the superior conjunctiva, such as vitreoretinal or strabismus surgery, or previous FS for glaucoma, represent additional risk factors for bleb dysfunction (Broadway & Chang 2001; Khaw et al. 2012). Conjunctival samples taken from patients who underwent previous incisional surgery contained more fibroblasts, macrophages and lymphocytes (Broadway et al. 1998).
Use of IOP lowering medications
The long-term use of IOP lowering medications and complex therapy regimens induce an iatrogenic form of ocular surface disease (OSD), which has been demonstrated to represent a strong risk factor for FS failure (Broadway et al. 1994b). Several studies showed that medical therapy for glaucoma profoundly modify the cellular profile of the ocular surface, inducing recruitment of inflammatory cells and a sub-clinical chronic inflammation (Broadway et al. 1994a,b, 1998; Baudouin et al. 1999, 2004, 2008, 2010; Baudouin 2008; Mastropasqua et al. 2013a; Villani et al. 2016). In presence of such sub-clinical inflammation, the conjunctiva intensely reacts to incisional surgery accelerating and intensifying the wound healing process, thus predisposing to bleb fibrosis.
Structure and drainage ability of the conjunctival bleb after glaucoma filtration surgery
A glimpse on the conjunctival filtration bleb anatomy
The anatomy of a functioning filtration bleb is very similar to that of normal, unmanipulated conjunctiva, from which it derives. Normal conjunctiva presents few layers (4–10) of epithelium, containing goblet cells (GCs) dispersed between the middle and superficial layers, and a stroma consisting of a superficial, lymphoid layer and a deeper fibrous layer, which hosts collagen fibres, lymphatics and blood vessels (Francis et al. 2005). The filtration bleb presents similar features, even though the surgical manipulation of the conjunctiva, along with the effects of mitomycin C (MMC), reduce the number of epithelial layers (≥ 2) and the density of GCs, making the bleb-epithelium wall thinner, irregular and partially dysfunctional. The bleb-wall stroma usually preserves the classical network of collagen fibres, but with reduced vascularity (Francis et al. 2005).
In the last two decades, the laser scanning in-vivo confocal microscopy (IVCM) improved the anatomical characterization of filtration blebs, expanding the histological information derived from excised abnormal leaking blebs (Hutchinson et al. 1994; Francis et al. 2005). IVCM is the only non-invasive diagnostic platform that provides tissue information at the cellular level, that permits evaluating the bleb function over time and recognizing earlier the loss of filtration (Labbé et al. 2005; Messmer et al. 2006; Amar et al. 2008; Ciancaglini et al. 2009; Carpineto et al. 2011; Mastropasqua et al. 2014).
The bleb-wall epithelium hosts the most specific confocal hallmarks for good AH filtration. These hallmarks are represented by fluid-filled, round and hypo-reflective structures, called as microcysts (10 to 300 μm in size). Microcysts can be found dispersed throughout the bleb-wall epithelium, especially in the site adjacent to the limbus, and are a sign of AH percolation through the bleb-wall. Very interestingly, the pivotal studies by Messmer et al. (2006) and Amar et al. (2008) observed that giant microcysts seen with IVCM corresponded to confluent GCs. Scattered, activated dendritic cells (DCs) can be also observed within the sub-basal epithelium, especially in MMC augmented trabeculectomies.
The bleb-wall stroma appears as a hypo-reflective and loosely arranged collagen network, sometimes containing stromal cysts, with rare rectilinear blood vessels. All these aspects are hallmarks of low density and fluid permeable connective tissue. Occasionally, adjacent to perfused blood vessels, IVCM depicts hypo-reflective compressed tubular structures, probably representing lymphatic vessels (Messmer et al. 2006).
Failed blebs show the opposite features, with rare or absent microcysts within the epithelium, and a hyper-reflective condensed stroma with tortuous vessels.
Anterior segment (AS-OCT) and OCT-angiography (OCT-A) further completed the anatomical characterization of filtration blebs, giving a macroscopic structural definition. At the AS-OCT assessment, functioning blebs present a thick bleb-wall, with numerous hypo-reflective cyst-like spaces and multiple parallel layers, which are signs of high hydraulic conductivity (Sacchi et al. 2020). At OCT-A, functioning filtering blebs show a reduced bleb-wall vessel density, along with the presence of non-flow and vessel displacement areas (Narita et al. 2018; Mastropasqua et al. 2020 b).
Routes involved in the aqueous humor resorption through the filtration bleb
Mechanisms involved in AH drainage after FS are different. As initially reported by Benedikt (1977), five potential drainage routes seem activated by filtration procedures: (I) the trans-conjunctival route; (II) diffuse resorption through degenerated veins; (III) bulk flow through lymphatic vessels; (IV) bulk flow through new developed atypical aqueous veins (trabeculectomy-veins); and (V) outflow through normal aqueous veins. Therefore, in a comprehensive view, one may distinguish a trans-conjunctival route (I) and a transvenous route (II-V), which occurs through blood and lymphatic vessels (Table 2).
| Routes of AH resorption | ||
|---|---|---|
| Trans-conjunctival | Trans-venous | |
| Moves the AH through the bleb-wall layers towards the ocular surface | Absorbs and moves the AH through vessels outside the bleb | |
| Anatomical components and functional interpretations | Epithelial Goblet Cells (intra-epithelial AH carriers) | |
| Epithelial Microcysts (Indicators of superficial AH flow) | Degenerated Veins (Peri-vascular AH movement) | |
| Basement Membrane (Resistance factor to the outer AH outflow) | Normal Aqueous Veins (Direct AH absorption) | |
| Stromal Microcysts (Indicators of deep AH flow) | Atypical Aqueous Veins (Newly developed post-surgical veins) | |
| Fibroblasts and Stromal Collagen (Most resistance factors to AH outflow) | Lymphatic Vessels (Direct AH absorption) | |
| Dendritic Cells (Stimulators of collagen deposition) | ||
- AH = aqueous humor.
In the trans-conjunctival route, the pioneer histological studies found that the contact of the AH with the bleb-wall induces stromal collagen degeneration, basement membrane collapse, and epithelial changes, which increases the hydraulic conductivity of bleb-wall layers (Teng et al. 1959; Benedikt 1977).
As reported above, the advent of IVCM allowed the detailed characterization of the AH outflow through the bleb-wall, clarifying several aspects of the trans-conjunctival route. The existence of this route was confirmed by the presence of intra-epithelial (and less frequently intra-stromal) microcysts in functioning filtration blebs (Labbé et al. 2005; Messmer et al. 2006). Amar et al. (2008) found that microcysts observed at the surface of functioning blebs corresponded to a confluence of GCs containing AH instead of mucins. Therefore, the transcellular pathway of the AH could be hypothesized to occur at the GC level towards the ocular surface.
The transvenous route occurs around blood capillaries of the conjunctiva and episclera, or after the formation of new drainage channels. This pathway is activated by the degeneration of the collagen surrounding vessels promoted by the mechanical pressure induced by AH, which leads to deep tissue remodelling (Teng et al. 1959). Messmer et al. (2006) confirmed the existence of this route by means of confocal microscopy, documenting blood and (presumedly) lymphatic vessels within the stroma of functioning filtration blebs.
Relations between the pre-operative conjunctival status and filtration bleb ability
The close relationship between the pre-operative status of the conjunctiva and the bleb filtration ability is supported by the fact that the conjunctiva hosts all the anatomical components that are post-operatively involved in the bleb formation and AH resorption.
Therefore, the development of an efficient filtration bleb depends on the pre-operative preservation of all conjunctival components that participate in the AH drainage. This assumption is supported by studies that investigated the relations between the structural characteristics of the pre-operative conjunctiva and the bleb filtration ability.
As stated above, the pioneer study by Broadway et al. (1994b) first reported the correlation between the cellular profile of the pre-operative conjunctiva and the surgical outcome. However, since ex-vivo sampling can be harmful for the filtration bleb function, this study did not investigate whether there was a correlation between the pre-operative conjunctival cell profile and the filtration bleb cellular profile after surgery. This was further investigated by Gwynn et al. (1993), who found that a higher post-operative GC density (GCD) was related to a better IOP control and a higher success rate of trabeculectomy. Their results were recently confirmed in a confocal and immunocytology study, in which patients with a successful FS and a good post-operative IOP control showed higher pre-operative GCD (and MUC5AC positivity) and preserved the same GCD after surgery compared to patients with filtration failure (Agnifili et al. 2016). In addition, both pre- and post-operative GCD presented a positive correlation with the IOP reduction and with the density and area of bleb-wall microcysts.
Similar correlations were found for other conjunctival components such as stromal collagen and DCs, indicators of tissue hydraulic resistivity and inflammation, respectively (Mastropasqua et al. 2017a,b). Interestingly, patients presenting a higher conjunctival stromal reflectivity (a confocal microscopic indicator of fibrosis) before surgery, presented lower density and area of bleb-wall microcysts after surgery, with a strong negative correlation between these parameters. These results indicate that a conjunctiva showing a high pre-operative hydraulic resistivity leads to the generation of a bleb-wall with low hydraulic conductivity, due to increased collagen content.
Hayek et al. (2019) found that a higher pre-operative conjunctival vessel density at the site of the future filtration bleb formation represents a significant risk factor for higher IOP, higher frequency of post-operative needling, and use of IOP lowering medications six months after FS. These findings can be explained by strong correlation between conjunctival vasculature and fibrotic processes.
All these aspects support the fact that when the conjunctival components involved in the AH resorption after surgery present unfavourable pre-operative conditions, the post-operative AH drainage through the bleb-wall is less-effective.
The ocular surface disease and its impact on glaucoma surgery outcome
Impact of OSD on glaucoma management
The definition of OSD includes a broad category of conditions characterized by an inadequate volume and a poor quality of tears, which lead to an unstable tear film and ocular surface breakdown (Moss et al. 2000; Brewitt & Sistani 2001).
Glaucoma and OSD frequently coexist in ophthalmic patients, with a strong mutual relationship (Anwar et al. 2013). Since the occurrence of OSD in glaucoma is related to the use of IOP lowering medications, Holló et al. (2018) introduced the term ‘glaucoma therapy-related OSD’ (GTOSD). They defined this condition as an imbalance of the ocular surface homeostasis caused by the toxic effect of medications, which leads to tear film instability, epithelial damage and inflammation.
The most used test for collecting GTOSD-related symptoms, is represented by the OSD index (OSDI) questionnaire (Schiffman et al. 2000; Skalicky et al. 2012; Mathews et al. 2013). Despite the OSDI score does not correlate well with the objective clinical measures of OSD, in certain subsets of patients this index correlates moderately with clinical signs (Schiffman et al. 2000). Therefore, the OSDI score may provide some indirect information about the ocular conditions.
GTOSD represents a common comorbidity of glaucoma, since 60% of patients shows a pathological OSDI score, and a third of them presents a severe form of GTOSD (Leung et al. 2008; Stewart et al. 2011; Skalicky et al. 2012; Figus et al. 2020).
Clinically, GTOSD induces important concerns on the management of glaucoma because it negatively affects the quality of life (QoL), therapy compliance and treatment outcomes, worsening the IOP control (Broadway et al. 1994b; Skalicky et al. 2012; Kaštelan et al. 2013; Batra et al. 2014; Dubrulle et al. 2018; Voicu & Salim 2021). Skalicky et al. (2012) found that patients with GTOSD had a significantly poorer QoL than those without OSD and that the OSDI score was related to the number of medications, the glaucoma severity, the cumulative daily dose of benzalkonium chloride (BAK) (>3 BAK-containing eye drops), and history of filtration surgery. Among the countless barriers to therapy compliance, those related to the presence of iatrogenic OSD are some of the most critical (Tsai 2009; Voicu & Salim 2021).
Patients with OSD complaining of more severe symptoms are less compliant with medical therapy and, thus, have a higher risk of worse IOP control and damage progression (Kaštelan et al. 2013). Because of this, modifications of glaucoma treatment can be required in many cases (more than 40% of patients) to mitigate the OSD and improve therapy compliance (Kaštelan et al. 2013). These modifications include switching to preservative-free (PF) formulations and fixed combinations, removal of selected active compounds, use of systemic medications to alternatively control IOP (oral acetazolamide), or performing laser trabeculoplasty. Dedicated medical approaches aimed at directly improving the ocular surface, such as the use of lubricants or short-term low-potency topical corticosteroids and lid hygiene are often recommended.
Glaucoma therapy-related ocular surface changes
The presence of preservatives, active compounds, the cumulative dose of preservative, the number of eye drops instilled per day, and the duration of therapy are the main determinants of GTOSD (Anwar et al. 2013; Mastropasqua et al. 2013a; Steven et al. 2018; Figus et al. 2020; Voicu & Salim 2021).
The determination of the relationship between preservatives (BAK) and the ocular surface alterations was the aim of countless studies. The majority of these studies stated that BAK is harmful to the entire ocular surface, and that the detrimental effects are related to the concentration, cumulative dose and duration of exposure (Baudouin 2008; Baudouin et al. 2010).
Direct cytotoxicity, apoptosis, inflammation and allergy, represent the most common mechanisms through which preservatives and active compounds induce ocular surface breakdown (Baudouin 2008; Baudouin et al. 2010; Stewart et al. 2011). GTOSD results from the combination of both preservatives and active compounds effects that jointly exacerbate their inflammatory effects (Baudouin et al. 2004; Mantelli et al. 2011; Mastropasqua et al. 2013a).
Overall, the entire ocular surface is damaged by the chronic use of anti-glaucoma medications (Martone et al. 2009; Agnifili et al. 2013, 2016; Mastropasqua et al., 2013a,b, 2015, 2016, 2017a,b, 2019) (Table 3). However, from a surgical perspective, the alterations of the conjunctiva represent the most critical factors since, as discussed, they affect the bleb function (Broadway et al. 1994b; Agnifili et al. 2016; Mastropasqua et al. 2017a).
| Ocular components | Modifications | References |
|---|---|---|
| Tear film | Mucus layer thinning, reduced mucin concentration, aqueous layer volume reduction, lipid layer thinning; hyper-osmolarity, instability | Mastropasqua et al. (2019); Figus et al. (2020) |
| Cornea | Dendritic cells increase and activation, stromal keratocyte activation, sub-basal plexus nerves density reduction, increased nerve tortuosity and beading, polymegathism and pleomorphism of endothelial cells | Baudouin et al. (2010); Martone et al. (2009); Mastropasqua et al. (2016) |
| Limbus | Irregularity and metaplasia of transitional epithelium, inflammation of Vogt's palisades, dendritic cells increase | Mastropasqua et al. (2015); Baudouin et al. (2010) |
| Conjunctiva | Goblet cells loss; epithelial metaplasia and desquamation; increased number of epithelial layers; mono-nuclear inflammatory cells infiltration within the epithelium and stroma; dendritic cells increase and activation; increased density of fibroblasts and collagen fibres deposition within the stroma; conjunctival thinning | Agnifili et al. (2016); Baudouin (2008); Baudouin et al. (1999, 2004, 2008, 2010); Broadway et al. (1994a,b); Mastropasqua et al. (2013a,b, 2017a,b, 2020,b) |
| Meibomian glands | Reduction of the acinar density and area, inhomogeneity of the acinar wall and interstice, increased secretion reflectivity and greater orifice area | Agnifili et al. (2013); Mastropasqua et al. (2014) |
| Calt | Lymphoid follicles and interfollicular crypts infiltrated by inflammatory cells; collagen deposition and increased reticular connective pattern within the follicular core | Baudouin et al. (2010); Mastropasqua et al. (2017a,b) |
- CALT = conjunctiva-associated lymphoid tissue.
The epithelial and stromal inflammation are the most common pathological features characterizing the conjunctiva of glaucomatous patients. In detail, the epithelium appears infiltrated by inflammatory cells such as macrophages, lymphocytes and mast cells, whereas the basal layer and the sub-epithelium host activated DCs (Broadway et al. 1994a; Baudouin et al. 1999; Baudouin 2008; Baudouin et al. 2008; Mastropasqua et al. 2013a, 2014; Mastropasqua et al. 2017a; Mastropasqua et al. 2020a,b). The higher density of DCs indicates a local immune activation, a common finding in other inflammatory ocular surface diseases (Steinman 1991).
The presence of a significant epithelial inflammation was widely supported by immune-histological studies that found increased tissue positivity for HLA-DR, IL-6, IL-8, IL-10, for lymphoid cells biomarkers (T-Helper 1 and 2) and for chemokine receptors (CCR4 and CCR5) in patients with medically controlled glaucoma (Baudouin et al. 2004, 2008).
Even though inflammation-related features can be also recognized within the stroma, the most critical alterations of this layer are represented by a higher density of fibroblasts and an abnormal deposition of collagen fibres, which lead to fibrosis and vessel tortuosity (Mastropasqua et al. 2017a). Inflammation and fibrosis of the conjunctiva are strongly correlated; in fact, in response to an inflamed microenvironment, transforming growth factor (TGF)-β levels increase and stromal myofibroblasts-induced collagen deposition is stimulated (Mietz et al. 1994; Baudouin et al. 2010). These pathophysiological concepts were investigated in a clinical study conducted on glaucomatous patient’s candidates for surgery, where it was found that the density of DCs positively correlated with stromal reflectivity (Mastropasqua et al. 2017a).
Besides the inflammation-related alterations, the epithelium presents disruption, metaplasia, accumulation of amorphous material, an increase of the number of cell layers, and GCs loss (Baudouin 2008; Mastropasqua et al. 2013a,b, 2014, 2017, 2020a,b; Agnifili et al. 2016).
Goblet cells (GCs) deserve particular attention in glaucomatous patients who are candidates to surgery since their function is of pivotal importance in the AH resorption after surgery. As observed in IVCM and immune-histological studies (Amar et al. 2008; Agnifili et al. 2016), GCs work as active carriers of AH through the bleb-wall epithelium, cooperating with other mechanisms in the final resorption of AH after FS. These findings underline the crucial importance of preserving this cell population in patients with glaucoma.
The bulk of literature demonstrated that preservatives, especially BAK, are highly toxic for GCs. Conversely, active compounds may have opposite effects on GCs. In fact, while the most part of medications induce GCs loss, prostaglandin analogues (PGAs) stimulate their proliferation and activity (Baudouin et al. 2010; Mastropasqua et al. 2013a,b).
Intraocular pressure (IOP) lowering drugs also induces macroscopic modifications, especially concerning the conjunctival thickness and vasculature. In general, patients present a thick epithelium and a thin stroma; however, since the stroma is anatomically more represented than the epithelium, the final effect is often a conjunctival thinning (Nuzzi et al. 1995; Terai et al. 2009; Mastropasqua et al. 2020a,b). Using angiographic imaging, it has been also shown that medical therapy increases the conjunctival vessel density, especially when the therapy regimen requires the use of PGAs (Akagi et al. 2019; Hayek et al. 2019).
Meibomian glands (MGs) play a crucial role in the ocular surface homeostasis since they synthesize the meibum, which forms the superficial layer of the tear film. Medical therapy for glaucoma deeply harms MGs, inducing an obstructive form of Meibomian gland dysfunction (MGD) (Agnifili et al. 2013). This MGD leads to an evaporative dry eye, suggesting that eyelid changes are relevant for patients with glaucoma, cooperating with the other ocular surface alterations in inducing the GTOSD (McCulley & Shine 2003; Mastropasqua et al. 2014).
To summarize, GTOSD has the aspect of a chronic inflammatory condition affecting the entire ocular surface, which shares many pathophysiological and clinical features with dry eye disease, and where the alterations of the conjunctiva are those that could have the highest impact on the management of glaucoma.
Impact of ocular surface disease on glaucoma surgery outcome
Glaucoma therapy-related OSD (GTOSD) is a major risk factor for glaucoma surgery failure. As stated above, the pioneering histological studies of Broadway et al. (1994a,b) observed that a pre-operative conjunctival cell profile characterized by a higher density of inflammatory cells (pale cells, macrophages and lymphocytes) within epithelium and stroma, and by an increased density of fibroblasts within the deep substantia propria were associated with FS failure. These cellular features, which correlated with the duration of therapy and the number of medications, were found to be, in part, reversible with the interruption of medications and treating the ocular surface with topical steroids (Broadway et al. 1996). Reversal of these histological changes led to an improvement of surgical outcomes.
Further evidence confirmed these initial findings. Leng et al. (2011) found that Tenon's capsule fibroblasts of patients with glaucoma present a higher proliferative activity and a higher expression of TGF-β1, TGF-β2 and TGF-β3. These aspects stimulate conjunctival scarring and predispose bleb to fail after surgery. In the PESO study, Boimer and Birt (2013) investigated the relationship between BAK exposure and surgical failure, observing that patients receiving higher pre-operative daily doses of BAK had a significantly shorter time to surgical failure than patients who had less BAK exposure. A recent study contributed to further clarify the relations between preservatives and glaucoma surgery, documenting a direct association between pre-operative exposure to BAK and the prevalence of glaucoma surgery (Chamard et al. 2020).
Ocular surface imaging techniques contributed to further characterize the relationship between the conjunctiva and glaucoma surgery (Agnifili et al. 2016; Mastropasqua et al. 2017a,b; Hayek et al. 2019). Thanks to the imaging capability of IVCM, AS-OCT and OCT-A, it was found that lower pre-operative GCs density, increased DCs density and reflectivity, reduced thickness and increased vessel density were predictors of higher post-operative IOP values, greater need for IOP lowering medications, and needling procedures, and a higher rate of FS failure.
In summary, over the last three decades, a consistent number of experimental and clinical evidence demonstrated that long-term medical therapy is harmful to the entire ocular surface, especially the conjunctiva. From this assumption, further lines of research progressively investigated the impact of ocular surface alterations on disease management, highlighting that GTOSD represents a major barrier for the control of the disease, and one of the most important risk factors for surgery failure.
Strategies to prepare the ocular surface for filtration surgery
Because of GTOSD and the common presence of concomitant risk factors, patients with uncontrolled glaucoma usually approach FS in less-than-ideal conditions. Among the numerous risk factors affecting FS outcome, those that are ocular surface-related are the only potentially modifiable. Therefore, measures aimed at mitigating the pre-operative OSD are crucial for increasing the chances of FS success.
Modulation of pre-operative IOP lowering therapy
The modulation of medical therapy represents the first important step for mitigating GTOSD before surgery. Some of the recommended measures include switching from preserved to unpreserved formulations and limiting the number of pre-operative medications for an adequate period before surgery (Boimer & Birt 2013; Tailor et al. 2016; Chamard et al. 2020). The rationale for this approach is to contain inflammation by reducing the cumulative daily dose of preservatives and active compounds. As demonstrated by Broadway et al. (1996), the interruption of a single medication one month before surgery may increase the success rate of FS by reducing the number of fibroblasts, macrophages, lymphocytes and pale cells and preserving GCs within the conjunctiva. However, it is to be noted that these results were obtained in patients who concomitantly received fluorometholone.
In a prospective randomized controlled study, Lorenz et al. (2017) compared two pre-operative approaches consisting in the interruption of all IOP lowering medications (‘drop holiday’) four weeks before surgery, and in the treatment with unpreserved dorzolamide/timolol fixed combination, or in the use of oral acetazolamide and topical dexamethasone. They found that the simplification of the pre-operative therapy regimen was safe and effective to increase the rate of success after surgery when using either approaches. However, in this study, patients had a contained mean pre-operative IOP (from 17.4 to 19.7 mmHg), which probably permitted suspending topical medications and using a potent steroid without fearing particular IOP spikes. Therefore, these approaches cannot be considered valid and optimal in all conditions.
A careful assessment of the pre-operative clinical conditions of patients is crucial because the decision of the number and type of active compounds to interrupt should be based on the stage of the disease, the baseline IOP levels and the stage of the OSD. In fact, it is well known that the majority of patients requiring surgery has high IOP values under maximal tolerated medical therapy and present a moderate to an advanced stage of the disease.
A national UK survey on the pre-operative management of the ocular surface reported that 95% of glaucoma specialists examine routinely the ocular surface and consider it useful or necessary to manage OSD (Tailor et al. 2016). 40.6% of specialists would prescribe or replace existing therapy with unpreserved formulations: 6.2% routinely and 34.4% in cases with conjunctival inflammation, intolerance, or allergy. A drop holiday (not specified in duration) was considered useful by 29.7% of specialists, with 1.6% of them implementing this approach routinely and 28% only in case of significant OSD.
However, a complete drop holiday is not always feasible, and some authors suggested stopping at least the PGA and replace preserved with unpreserved drugs (Rodríguez Uña et al. 2015; Dubrulle et al. 2018). Similar recommendations were suggested in case of minimally invasive FS, such as glaucoma gel stent implantation. It was proposed to replace preserved with unpreserved IOP lowering medications, and to discontinue the most offending drops (PGAs and/or brimonidine) in presence of conjunctival or lid inflammation, at least one month before surgery (Vera et al. 2018).
Management of the ocular surface
The direct management of OSD is the second crucial step to increase the success rate of surgery. It is mainly achieved by using topical anti-inflammatory agents.
Anti-inflammatory agents
Broadway et al. (1996) reported that the pre-operative use of fluorometholone 1% given four times daily one month before surgery was beneficial and efficient in reducing the density of inflammatory cells and fibroblasts within the conjunctiva, thus increasing the surgical success rate from 50% to 81%. In a randomized multicentre prospective study, Baudouin et al. (2002) compared the efficacy and safety of unpreserved indomethacin 0.1% vs preserved fluorometholone in patients undergoing trabeculectomy. The pre-operative treatment required the administration of the anti-inflammatory agents 4 times daily one month before surgery, without modifying the IOP lowering therapy. This strategy significantly reduced the conjunctival inflammation in both groups of treatment, as supported by the 30% reduction of the HLA-DR positivity at impression cytology. Six cases of superficial punctate keratitis were described in the fluorometholone group, probably related to the presence of preservatives. A prospective RCT conducted by Breusegem et al. (2010) investigated the effects of ketorolac and fluorometholone given four times daily one month before surgery on the outcomes of trabeculectomy. After a two-year follow-up, a similar success rate in both groups of treatment was observed, but with a significant reduction in the number of needling and the need for post-operative anti-glaucoma medications in the group treated with fluorometholone compared to the group treated with ketorolac (6% vs. 5% and 18% vs. 0%, respectively, vs. 41% and 24% of the placebo group).
Overall, this evidence suggests that anti-inflammatory treatments for an adequate period before surgery (at least one month), can reduce the ocular surface inflammation with beneficial effects, improving FS outcomes. Nevertheless, the use of steroids in patients with glaucoma, that often present an uncontrolled IOP before surgery, poses the risk of steroid-induced IOP increase. In the above discussed studies this complication was not reported, probably because they tested fluorometholone, which is safer than other steroids because of its limited ocular penetrance and low potency (Roberti et al. 2020).
To date, dexamethasone, the most potent steroid, was evaluated only in the study of Lorenz et al. (2017), where topical dexamethasone was administrated one week before surgery five times daily. The authors reported a significant IOP increase the day before surgery, with baseline values increasing by 40% (from 19.7 to 27.6 mmHg).
Therefore, the selection of the most appropriate steroid to administer, or whether to use non-steroid anti-inflammatory drugs (NSAIDs) should be made considering the baseline IOP levels, the stage of disease, the ocular surface-related signs and symptoms, and the presence of additional risk factors that may negatively affect surgical outcome. Besides these considerations, given the potential harmful effects of additional doses of BAK on GCs during pre-operative preparation, the anti-inflammatory therapy should be recommended without preservatives (Baudouin et al. 2002).
Immunosuppressive agents, such as cyclosporine A, could be considered as an additional class of drugs potentially useful for reducing ocular surface inflammation before FS. Because of its immunomodulatory activities (decrease of HLA-DR positivity, apoptosis markers and conjunctival T lymphocytes), topical cyclosporine A may have beneficial effects on the ocular surface increasing the conjunctival GCD and tear production, protecting human conjunctival epithelial cells, and reducing inflammation and fibrosis (Gao et al. 2013; Jones et al. 2017; Boboridis & Konstas 2018). Because of these properties, the recent approval of cyclosporine 0.1% in ophthalmic formulation represents a promising novel opportunity for the management of DED, meibomian gland dysfunction and different forms of inflammatory OSD, including GTOSD (Saini et al. 2015; Boboridis & Konstas 2018). On these bases, cyclosporine 0.1% may potentially reduce the negative consequences of the GTOSD on the FS outcomes, but this assumption has to be demonstrated in further prospective studies.
The use of topical anti-inflammatory drugs represents the most common strategy adopted by surgeons to prepare the ocular surface, as evident in the UK survey (Tailor et al. 2016). Glaucoma specialists recommend steroids in 50% of cases, especially in presence of concomitant risk factors for filtration failure such as OSD and lid margin inflammation, history of uveitis, or previously failed glaucoma surgery. Interestingly, despite the potential effects on IOP, about half of specialists prefer dexamethasone (in unpreserved formulation), with the remaining surgeons prescribing unpreserved prednisolone (30%) or fluorometholone (20%); usually, the dose regimen recommended is four times a day. A small portion of surgeons (8%) recommend the use of NSAIDs, mainly ketorolac or diclofenac.
Lubricants
The use of artificial tears represents another potential strategy to improve the ocular surface before surgery. The rationale for using lubricants is dual since they improve dry eye from one side, thus mitigating inflammation, while protecting and stimulating GCs from the other side, which is beneficial considering the role that these cells have in the AH resorption within the bleb-wall layers. It was demonstrated that the administration of topical sodium hyaluronate increases GCD after one month of therapy (Aragona et al. 2002; Yu et al. 2013). Moreover, in experimental studies, sodium hyaluronate was found to contrast fibroblast motility, proliferation and their metabolic activity, thus potentially decreasing the collagen deposition after glaucoma surgery (Balazs & Darzynkiewicz 1973; Alpar 1986). Lubricants are prescribed 4–8 times a day by 42% of glaucoma specialists, under the form of carmellose sodium (with or without glycerol), sodium hyaluronate, or polyethylene and propylene glycol (Tailor et al. 2016).
Because of the toxic effects on GCs (Mastropasqua et al. 2013a,b), as suggested by the EGS guidelines (European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition, 2020) and the Tear Film and Ocular Surface Society (TFOS) International Dry Eye Workshop II (DEWS II) (Jones et al. 2017), lubricants should be prescribed without preservative in the presence of any form of OSD, especially in patients requiring frequent eye drops instillation.
Systemic tetracyclines
Systemic tetracyclines (especially doxycycline) were demonstrated to work as inhibitors of matrix metalloproteinases (MMPs), being therefore useful in ocular diseases that induce scarring, such as trachoma (Li et al. 2013). Because doxycycline inhibits MMPs and downregulates MMP-induced proinflammatory cytokines, it can dampen inflammation and prevent matrix remodelling, which lead to tissue contraction and fibrosis. Other than in trachoma, these results were also reported in glaucoma where doxycycline, even if applied topically, proved effective in inhibiting the scarring processes following FS (Sen et al. 2010). Therefore, the oral administration of doxycycline (50–100 mg/day; 2–8 weeks before surgery) in patients undergoing FS may contribute to reduce the ocular surface inflammation and contrast the fibrotic modifications of the conjunctival stroma (Tailor et al. 2016). Of note, it was previously reported that systemic doxycycline is adsorbed and concentrated in conjunctival GCs (Dilly & Mackie 1985). Thus, it seems that to exert this favourable action, doxycycline requires the preservation of this cell population.
Management of the eyelids
As reported in previous studies, medical therapy for glaucoma induces an obstructive form of MGD (Agnifili et al. 2013). Therefore, treating MGD represents a crucial step for the comprehensive management of the ocular surface since lid hygiene concurs in mitigating OSD and optimizing IOP control (Batra et al. 2014; Dubrulle et al. 2018).
On these bases, the evaluation of the lid margin should be routinely performed in patients undergoing FS (Baudouin 2013; Vera et al. 2018). Lid hygiene is recommended by more than a third of UK glaucoma specialists before proceeding to FS, and usually requires digital massage, application of hot compresses (twice daily) and the administration of oral doxycycline (50–100 mg per day, from 4 weeks pre-op to 12 weeks post-op) (Batra et al. 2014; Tailor et al. 2016; Dubrulle et al. 2018).
To summarize, the most adopted measures by clinicians to improve the OS are performed one month before surgery and are characterized by the interruption of at least one of the most harmful medications to the OS (PGAs or brimonidine (Dubrulle et al. 2018)), the substitution of preserved with unpreserved IOP lowering medications, the administration of low potency (unpreserved) steroids four times per day, and the use of adjunctive treatments consisting in (unpreserved) lubricants, systemic doxycycline (50–100 mg per day) and lid hygiene for several weeks.
Fig. 1 shows a useful guide for clinicians that summarizes the most important risk factors for FS failure that should be pre-operatively considered, and the currently available measures to improve the ocular surface in patients undergoing FS.
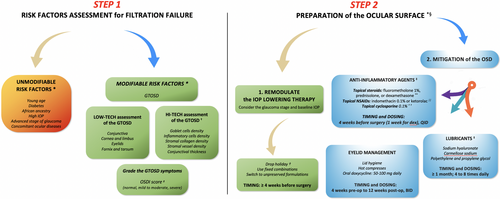
Major gaps in knowledge and possible solutions
Overall, the implementation of measures to optimize the ocular surface before surgery is considered beneficial by the vast majority of glaucoma specialists (86%), but only half of them (48.4%) consider it necessary. Of note, glaucoma surgeons (84%) would modify the routine management of glaucoma in presence of OSD, but only one-third of them (33%) would apply specific strategies to reduce the ocular surface inflammation. Surprisingly, only 11% of surgeons that treat the ocular surface would combine two or more treatments (Tailor et al. 2016). These data indicate that there is a consistent gap between what clinicians consider beneficial for patients undergoing FS, and what they really do.
Different reasons may account for these discrepancies. The first one is related to the absence of a significant number of RCTs that analysed this topic. To date, very few randomized prospective studies evaluated the real effects of the ocular surface preparation on surgery outcomes, the most appropriate measures to adopt, how to combine measures, and the correct timing for each measure. At this stage, clinicians adopt measures according to their personal experience or basing their decisions on studies with a low level of evidence. This appears evident in the study of Tailor et al. (2016), in which the authors recommend strategies depending on the OSD severity, according to their routine practice: (i) interruption of PGAs (and/or brimonidine) and administration of steroids one month before surgery in all cases; (ii) lubricants up to 6 times per day, lid hygiene 2 times per day, doxycycline 50 mg for three months and a switch to PF IOP lowering medications in presence of mild to moderate OSD; and (iii) same therapy and referral to cornea specialists and immunologists in presence of severe OSD.
The second reason, which derives from the former, is that even though there is a consensus for ocular surface optimization before surgery, the lack of guidelines that regulate the correct management in terms of measures to undertake, regimen and duration of pharmacological and physical treatments, and timing, hinders the broad diffusion of this practice. These two reasons account for the discrepancy between what clinicians consider useful and what they really do in clinical practice.
A good approach would be to adopt strategies that maximize the preservation of the ocular surface health during the entire management of the disease, thus lowering the need to prepare the ocular surface before surgery or to adopt milder or easier measures. The availability of a wide spectrum of fixed combinations, unpreserved formulations for all available anti-glaucoma eye drops, as well as for lubricants, NSAIDs, and steroids are crucial to maintaining the OSD and pre-operatively optimize the ocular surface in a gentler manner. Moreover, alternative delivery systems for IOP lowering medications, which do not require the daily eye drops administration, will permit the reduction of the cumulative burden of the medical therapy on the ocular surface. Based on this need, slow-release devices designed to be introduced in the lacrimal punctum, or into the fornix, the subconjunctival space, or inside the anterior chamber are being developed (Figus et al. 2020; Sartini et al. 2021).
Finally, a crucial point is represented by the timing of surgery, which is frequently delayed. This is a major issue since the correct timing may limit the GTOSD, which is at least in part induced by an unnecessarily prolonged duration of the medical therapy. Because of this, when the therapy regimen consists of three eye drops per day and the disease and/or the IOP is uncontrolled, surgery is recommended (European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition, 2020).
In conclusion, because of the strong mutual relationship between the condition of the ocular surface and glaucoma management outcomes, the need to improve the ocular surface before proceeding with FS represents a primary clinical need that should be soon fulfilled. However, the lack of studies that address this topic and the low level of evidence of the currently existing studies, have not resulted in clear guidelines. Prospective RCTs and new ocular surface-sparing medical approaches are mandatory to fill the numerous existing gaps, to define the most useful measures for preparing the ocular surface, and the correct timing for each measure.
In the meantime, considering that the available evidence indicates a potential benefit when ocular surface is correctly treated and prepared, we recommend always to consider the ocular surface status before proceeding with surgery, adopting measures in a timely manner, and offering patients the most personalized strategy based on their risk profile.




