Ocular findings and growth in 5-year-old preterm children born to mothers with preeclampsia
Abstract
Purpose
To evaluate growth, blood pressure and ophthalmological status in preschool children born preterm to mothers with preeclampsia.
Methods
In a prospective cohort study, 78 children (34 girls) born preterm without retinopathy of prematurity were examined regarding length/height, weight, head circumference and insulin-like growth factor I (IGF-I) at birth and at 5 years of age. At 5 years, IGF-binding protein 3 and blood pressure were also measured. A detailed ophthalmological examination including ocular dimensions, fundus morphology, visual fields, visual evoked potentials and perceptual visual dysfunction was performed. Children born to preeclamptic mothers (n = 24) were compared to children with non-preeclamptic mothers (n = 54).
Results
Children exposed to preeclampsia had lower weight (p = 0.0002, mean difference −1.46, 95% CI −2.09; −0.83), length (p = 0.013, −1.10, 95% CI −1.92; −0.23) and IGF-I levels (p = 0.0002, −26.0, 95% CI −36.0; −16.1) at birth compared to non-exposed children. At 5 years of age, the preeclamptic group had larger optic cup areas (p = 0.0006, 0.32, 95% CI 0.15; 0.46, in right eye, p = 0.049, 0.18, 95% CI 0.001; 0.35, in left eye). There was no significant difference between the groups regarding other ophthalmological findings or blood pressure. Children with reduced eye motility had lower neonatal IGF-I levels (p = 0.033, 15.5, 95% CI 1.1; 30.3).
Conclusion
Preeclampsia was shown to affect growth and IGF-I levels, confirming previous studies. Children exposed to preeclampsia were shown to have larger optic cup areas. Furthermore, lower neonatal IGF-I levels were seen in preterm children with reduced eye motility at 5 years of age.
Introduction
Hypertensive disorders in pregnancy are classified by the International Society for the Study of Hypertension in Pregnancy (ISSHP) into gestational hypertension, preeclampsia, chronic hypertension and white coat hypertension. Gestational hypertension is defined as de novo hypertension developing after 20 weeks of gestation. The definition of preeclampsia is hypertension arising de novo after 20 weeks of gestation, in the presence of one or more of the following: proteinuria, other maternal organ dysfunction and/or uteroplacental dysfunction (Tranquilli et al. 2014; Brown et al. 2018). Hypertensive disorders affect 10% of all pregnancies (World Health Organization 2011) and are among the most common causes of maternal mortality (Clark et al. 2008), where preeclampsia globally causes over 70 000 maternal deaths each year (Brown et al. 2018). Furthermore, preeclamptic women have a higher risk of developing cardiovascular and cerebrovascular diseases (McDonald et al. 2008).
Infants of hypertensive pregnancies have a higher mortality rate compared to infants born to normotensive mothers (Ananth & Basso 2010) with 500 000 offspring deaths each year (Brown et al. 2018). Children exposed to preeclampsia have a higher risk of being hospitalized and developing infectious, endocrine, nutritional, metabolic, haematological and respiratory diseases in childhood and young adulthood (Wu et al. 2009). Moreover, these children have an increased risk of developing long-term outcomes such as elevated blood pressure (Davis et al. 2015). Because of the risks of a prolonged pregnancy, most children exposed to preeclampsia are born preterm by iatrogenic delivery (Wu et al. 2009; Geelhoed et al. 2010).
Women suffering from preeclampsia are known to have an elevated risk of ophthalmological complications (Abu Samra 2013). However, there is a lack of evidence on the impact of preeclampsia on ocular and visual development in children born to preeclamptic mothers. The aim of the study was to evaluate growth, blood pressure and ophthalmological status in preschool children born preterm to mothers with preeclampsia.
Methods
Study participants
The present study is a part of an ongoing population-based and prospective cohort study including children born moderate-to-late preterm (gestational age (GA) 32–36 weeks) in Gothenburg, Sweden. Children born with chromosomal abnormalities, syndromes or severe malformations, or born to mothers with severe chronic diseases were not included (Raffa 2016). At 5 years of age, 127 children were invited to the present study through their guardians. There were 30 rejections and 16 non-responses, and three children were not included since their guardians chose to provide only the medical record. Out of the remaining 78 children, 24 (10 girls) were born to preeclamptic mothers and 54 (24 girls) were born to non-preeclamptic mothers. Preeclampsia was defined as blood pressure >140/90 mmHg, coexisting with proteinuria (≥+2, equals ≥1 g/l) after gestational week 20. There was no significant difference between the preeclamptic and non-preeclamptic groups regarding GA, age at assessment, gender, twin/singleton birth, in vitro fertilization, maternal diabetes mellitus or maternal age, as shown in Table 1. In Sweden, only children with GA <31 weeks are screened for retinopathy of prematurity (ROP). However, participants of the present study were ophthalmologically examined at birth (Allvin et al. 2014) and all medical records were scrutinized. According to this, the participating children did not show any history of ROP.
| Characteristics |
PE group n = 24* |
Non-PE group n = 54* |
p-value | Mean difference with 95% CI |
|---|---|---|---|---|
| Girls, n (%) | 10 (42%) | 24 (44%) | >0.99 | −2.8 (−29.6; 24.0) |
| Boys, n (%) | 14 (58%) | 30 (56%) | >0.99 | 2.8 (−24.0; 29.6) |
| Twins, n (%) | 6 (25%) | 14 (26%) | >0.99 | −0.9 (−24.8; 23.0) |
| In vitro fertilization, n (%) | 0 (0%) | 8 (15%) | 0.089 | 14.8 (2.3; 27.3) |
| Maternal diabetes mellitus, n (%) | 0 (0%) | 2 (4%) | 0.95 | 3.7 (−4.3; 11.7) |
|
Maternal age, years mean (SD), median (range) |
32.7 (4.3) 33.4 (23.9; 42.8) |
30.9 (4.8) 30.2 (22.7; 40.0) |
0.12 | 1.78 (−0.45;4.02) |
| Variables at birth | ||||
|
Gestational age, weeks mean (SD), median (range) |
35.1 (1.53) 35.6 (32.0; 36.9) |
35.1 (1.46) 35.6 (32.0; 36.9) |
0.85 | 0.078 (−0.63; 0.81) |
|
Weight, g mean (SD), median (range) |
2041 (503) 2160 (945; 2270) |
2418 (486) 2445 (1035; 3420) |
0.002 | −376 (−616; −131) |
|
Weight, SDS mean (SD), median (range) |
−2.10 (1.41) −1.58 (−5.17; −0.02) |
−0.64 (1.21) −0.55 (−4.55; 1.57) |
0.0002 | −1.46 (−2.09; −0.83) |
|
Length, cm mean (SD), median (range) |
44.1 (3.68) 45.0 (33.0; 50.0) |
45.6 (3.06) 46.5 (35.0; 51.0) |
0.087 | −1.44 (−3.03; 0.19) |
|
Length, SDS mean (SD), median (range) |
−1.83 (1.74) −1.49 (−7.35; 0.90) |
−0.73 (1.69) −0.56 (−5.91; 2.85) |
0.013 | −1.10 (−1.92; −0.23) |
|
BMI, kg/m2 mean (SD), median (range) |
10.3 (1.36) 10.0 (8.0; 12.5) |
11.5 (1.33) 11.7 (8.4; 14.2) |
0.0006 | −1.21 (−1.86; −0.56) |
|
HCF, mm mean (SD), median (range) |
316 (18.7) 318 (280; 350) |
320 (19.8) 320 (258; 350) |
0.37 | −4.39 (−13.9; 5.20) |
|
HCF, SDS mean (SD), median (range) |
−0.69 (0.95) −0.65 (−1.95; 1.11) |
−0.29 (0.99) −0.27 (−3.64; 1.87) |
0.11 | −0.39 (−0.87; 0.08) |
|
SGA, n (%) |
12 (50%) | 9 (17%) | 0.006 | 33.3 (8.0; 58.7) |
| Variables at assessment | ||||
|
Age, years mean (SD), median (range) |
5.8 (0.25) 5.7 (5.4; 6.3) |
5.7 (0.22) 5.6 (5.0; 6.3) |
0.065 | 0.10 (−0.01; 0.21) |
|
Weight, kg mean (SD), median (range) |
19.7 (2.92) 19.4 (13.8; 25.8) n = 22 |
20.4 (3.23) 19.7 (15.1; 33.8) n = 53 |
0.37 | −0.71 (−2.45; 0.80) |
|
Weight, SDS mean (SD), median (range) |
−0.73 (1.09) −0.83 (−2.92; 1.72) n = 22 |
−0.31 (1.16) −0.51 (−2.54; 4.12) n = 53 |
0.13 | −0.42 (−1.04; 0.13) |
|
Height, cm mean (SD), median (range) |
115 (6.82) 115 (104; 130) n = 22 |
115 (4.78) 115 (106; 128) n = 53 |
0.93 | 0.17 (−2.80; 2.92) |
|
Height, SDS mean (SD), median (range) |
−0.21 (1.41) −0.74 (−2.01; 2.91) n = 22 |
−0.04 (0.99) −0.23 (−1.92; 2.91) n = 53 |
0.55 | −0.16 (−0.78; 0.39) |
|
BMI, kg/m2 mean (SD), median (range) |
14.7 (1.53) 14.5 (12.7; 18.5) n = 22 |
15.3 (1.53) 15.1 (13.0; 20.5) n = 53 |
0.15 | −0.55 (−1.36; 0.19) |
|
HCF, cm mean (SD), median (range) |
52.0 (1.75) 52.1 (49.3; 55.2) n = 20 |
51.6 (1.76) 51.8 (47.5; 55.2) n = 53 |
0.46 | 0.35 (−0.60; 1.27) |
|
IGFBP-3, mg/l mean (SD), median (range) |
3141 (708) 3345 (2213; 4754) n = 17 |
3776 (4210) 3119 (1971; 30636) n = 44 |
0.70 | −635 (−2762; 377) |
| Blood pressure readings | ||||
|
Systolic blood pressure, mmHg mean (SD), median (range) |
102.4 (8.39) 100.7 (92.0; 123.0) n = 18 |
103.3 (6.59) 103.3 (92.0; 117.7) n = 51 |
0.63 | −0.95 (−4.97; 3.00) |
|
Diastolic blood pressure, mmHg mean (SD), median (range) |
65.0 (5.77) 63.7 (56.3; 76.7) n = 18 |
64.6 (4.97) 65.0 (51.3; 76.3) n = 51 |
0.82 | 0.34 (−2.46; 3.21) |
|
Median arterial pressure, mmHg mean (SD), median (range) |
77.4 (6.43) 76.0 (68.6; 91.2) n = 18 |
77.5 (5.16) 77.7 (65.2; 87.3) n = 51 |
0.96 | −0.09 (−3.14; 2.97) |
- BMI = body mass index, CI = confidence interval, HCF = head circumference, IGFBP-3 = insulin-like growth factor-binding protein 3, PE = preeclampsia, SD = standard deviation, SDS = standard deviation score, SGA = small for gestational age.
- The bold values indicate significant results.
- * Where numbers differ from the number of children in the group, this is given separately for each category.
Paediatric data
Length/height, weight and head circumference (HCF) were measured at birth and at 5 years of age. Body mass index (BMI) was calculated as weight (kg) divided by length squared (m2). Standard deviation scores (SDS) were calculated using Swedish reference values (Albertsson-Wikland et al. 2002; Niklasson & Albertsson-Wikland 2008). The number of children born small for gestational age (SGA), defined as birth length and/or weight below −2 SDS (Karlberg & Albertsson-Wikland 1995), or appropriate for gestational age (AGA) was noted. Preeclampsia, high blood pressure, diabetes mellitus before pregnancy, as well as any impaired glucose tolerance (plasma glucose >10.0 mmol/l 2 h after an oral glucose tolerance test) during pregnancy were recorded.
At birth, umbilical cord concentrations of insulin-like growth factor I (IGF-I) were measured using specific radioimmunoassay (RIA) (Mediagnost GmbH, Tübingen, Germany) (Blum & Breier 1994). At 5 years of age, serum levels of IGF-I and IGF-binding protein 3 (IGFBP-3) were analyzed with an immunoassay (IDS-iSYS, Immunodiagnostic Systems, London, UK). As the two methods have good overall agreement (r = 0.98) (Bidlingmaier et al. 2014), the change in IGF-I levels from birth to 5 years of age (ΔIGF-I) could be calculated. At 5 years of age, blood pressure was obtained as a mean of three measurements taken in an upright position using blood pressure cuffs (Welch Allyn, Welch Allyn Inc., Skaneateles Falls, NY, USA).
Ophthalmological data
Visual acuity (VA) was measured monocularly at 3.0 m for distance vision and binocularly at 0.33 m for near vision, using a linear KM-Boks chart or an HOTV chart (Hedin et al. 1980; Moutakis et al. 2004). Values from the charts were measured in Snellen decimal and later converted to logarithm of minimum angle of resolution (logMAR) for calculation. Subnormal VA was defined as Snellen decimal <0.63 (equals >0.2 logMAR). Refraction was evaluated using an autorefractor (Topcon A6300, Topcon Corporation, Tokyo, Japan) after instilment of dilating eye drops consisting of cyclopentolate (0.85%) and phenylephrine (1.5%).
Ocular motility was tested with a penlight in nine directions of gaze. Reduced motility was defined as any under- or overreaction (Vivian & Morris 1993). Heterotropia and heterophoria were tested using a cover test with fixation at near (0.33 m) and distance (3 m) (Aring et al. 2005). Near point of convergence (NPC) and near point of accommodation (NPA) were measured using a Royal Air Force (RAF) ruler. The near limit of convergence was 6 cm (Von Norden & Campos 2002). The cut-off limit for NPA was set to 20 D, and a push-up method was used (Duane 1922). Stereoacuity was tested using the TNO (de Nederlandse Organísatie voor toegepast-natuurwetenschappelijk onderzoek TNO) random dot test, where subnormal stereoacuity was defined as >60 sec of arc (Von Norden & Campos 2002).
Visual fields (VFs) were evaluated in the right eye using computerized rarebit perimetry (Frisen 2002; Martin 2005; McKendrick 2005). The participants were asked to fixate a fixation mark that moved across the screen. At the same time, small bright dots (<0.5 minimum angle resolution) appeared on the screen for 200 ms, one or two at a time. Stimuli were presented in 24 separate test areas within the 20 × 30-degree VF. The participants responded with a single mouse click for one dot and with double clicks for two dots. A percentage mean hit rate (MHR) was calculated. If there were no gaps between the receptive fields, the participant would have near 100% hit rate. If there was a loss in receptive fields, the participant would have a lower hit rate.
Ocular fundus photographs were taken using a Topcon 50-VT fundus camera (Topcon Corporation, Tokyo, Japan). The optic disc area, the optic cup area and the neuroretinal rim area were analyzed using the RetinaSizeTool (RST) program (Bartling et al. 2008). Furthermore, the ocular fundus photographs were analyzed subjectively by one examiner (L.R.) (blinded and according to a standardized study protocol) to evaluate increased or decreased retinal vessel tortuosity. The palpebral fissure length (PFL) and the inner intercanthal distance (ICD) were measured in mm using a ruler (Hall et al. 1989).
Pattern-reversal visual evoked potentials (VEPs) were performed using Keypoint (SynMed, Medicinteknik AB, Stockholm, Sweden) according to the International Society for Clinical Electrophysiology of Vision standards (ISCEV) (Odom et al. 2010). Electrodes were placed according to the international 10–20 electrode electroencephalograph system. Recordings were performed at a stimulus rate of 2 Hz at a distance of 1 m. The electrode resistance was set below 5 kΩ; however, for less accommodative children, the resistance was adjusted up to 10 kΩ. Moreover, the amplifier bandpass filter was set at 1–100 Hz and the artifact reference filter was set at an amplitude of ±100 μV. The recording window was 300 ms, with the recording pausing if fixation of the screen was lost. Two trials were performed, first binocularly followed by two trials on each eye separately. A minimum of 30 reversals was recorded for each trial, or lower if the child had difficulties cooperating.
A structured history was taken from the children, together with their guardians, regarding perceptual visual dysfunction (PVD) in the following areas: recognition, orientation, perception of depth, perception of motion and simultaneous perception (Dutton et al. 1996; Mitry et al. 2016). The PVD questionnaire is used to evaluate deficiencies in visuocognitive and visual processing (Mitry et al. 2016).
Statistical analysis
Means, standard deviation (SD), median, range and confidence interval (CI) were calculated for descriptive purposes. For comparison between two groups, the Fisher’s exact test was used for dichotomous variables, and the Fisher’s non-parametric permutation test was used for continuous variables. The 95% confidence interval (CI) for the mean difference between groups was based on Fisher’s non-parametric permutation test. Mean difference in proportions was given with 95% CI. The levels of IGF-I and IGFBP-3 were analyzed for any correlation with length/height, weight, HCF and BMI at birth and at assessment, as well as with GA, SGA/AGA, gender, twin/singleton birth, PFL and ICD. Correlations were analyzed using Spearman’s rank correlation coefficient analysis. The p-values <0.05 were considered statistically significant and all tests were two-tailed. All statistical analyses were performed by Senior Biostatisticians with SPSS Statistics (IBM Corporation, Armonk, NY, USA) and SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
The study was approved by the Research Ethical Review Board in Gothenburg and all examinations were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from the guardians of the participating children.
Results
Paediatric data
Paediatric data of the examined children, subdivided into children exposed and not exposed to preeclampsia, are shown in Table 1. As reported in Fig. 1, IGF-I levels at birth were significantly lower in the preeclamptic group compared to the non-preeclamptic group; however, there was no significant difference at 5 years of age. In the non-preeclamptic group, girls had significantly higher IGF-I levels (117 ng/ml versus 89.6 ng/ml, p = 0.022, mean difference −27.4, 95% CI −50.5; −4.0, Fisher’s non-parametric permutation test) and IGFBP-3 levels (4746 ng/ml versus 2890 ng/ml, p = 0.006, mean difference −1856, 95% CI −3835; −197) at 5 years of age compared to boys. In all preterm children, neonatal IGF-I levels correlated with weight (p < 0.0001, r = 0.67, Spearman’s rank correlation coefficient analysis), length (p < 0.0001, r = 0.50) and HCF (p < 0.0001, r = 0.45) expressed as SDS at birth. When using a general linear model, the correlation between neonatal IGF-I levels and birth weight in the preeclamptic group (p = 0.0002, r = 0.70) was not significantly larger than the correlation in the non-preeclamptic group (p = 0.0003, r = 0.47).
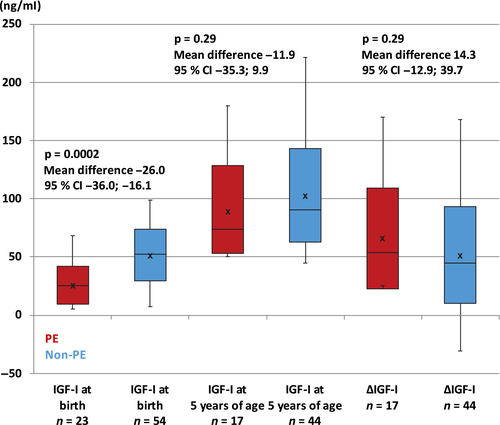
Ophthalmological data
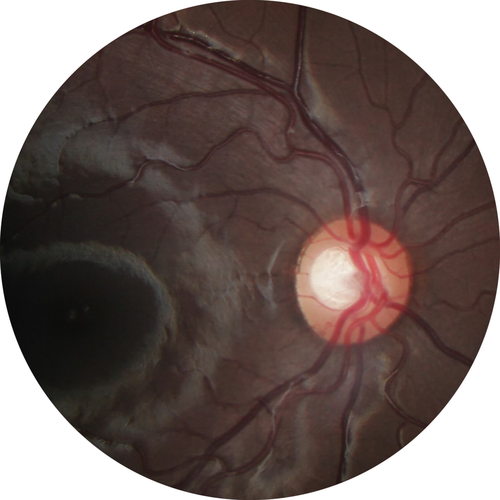
At 5 years of age, the preeclamptic group had larger optic cup areas compared to the non-preeclamptic group, as shown in Table 2. An ocular fundus photograph of a child exposed to preeclampsia with a large optic cup area is shown in Fig. 2. There was no significant difference between the groups regarding other ophthalmological findings, including PVD, presented in Table 2. Three out of the 24 children (13%) with preeclamptic mothers showed signs of PVD in one area (two children) or in two areas (one child), whereas two out of the 54 children (4%) with normotensive mothers showed signs of PVD, both in two areas. All of these five children reported recognition problems. The preterm children who showed reduced eye motility at 5 years of age had significantly lower neonatal IGF-I levels (30.6 ng/ml versus 46.1 ng/ml, p = 0.033, mean difference 15.5, 95% CI 1.1; 30.3) compared to children with normal eye motility. However, the association was not seen in either the preeclamptic or the non-preeclamptic group. Levels of IGF-I were not found to be related to PFL or ICD in either the preeclamptic or the non-preeclamptic group.
| Characteristics |
PE group n = 24* |
Non-PE group n = 54* |
p-value | Mean difference with 95% CI |
|---|---|---|---|---|
| Visual acuity | ||||
|
RE, logMAR mean (SD), median (range) |
0.09 (0.10) 0.10 (−0.10; 0.30) |
0.12 (0.12) 0.10 (0.00; 0.50) |
0.41 | −0.03 (−0.09; 0.03) |
|
LE, logMAR mean (SD), median (range) |
0.08 (0.09) 0.10 (−0.10; 0.30) |
0.13 (0.15) 0.10 (0.00; 0.70) |
0.14 | −0.05 (−0.12; 0.01) |
| Refraction | ||||
|
RE, SE D mean (SD), median (range) |
1.96 (1.46) 1.50 (0.75; 7.20) |
1.75 (1.24) 1.50 (0.50; 7.50) |
0.54 | 0.20 (−0.48; 0.79) |
|
LE, SE D mean (SD), median (range) |
1.93 (1.34) 1.63 (0.75; 7.25) |
1.72 (1.34) 1.25 (−0.50; 7.00) |
0.54 | 0.21 (−0.48; 0.82) |
| Eye motility and strabismus | ||||
| Reduced eye motility, n (%) | 6 (25%) | 6 (11%) | 0.22 | 13.9 (−8.4; 36.1) |
| Heterotropia†, n (%) | 1 (4%) | 2 (4%) | >0.99 | −0.5 (−12.9; 12.0) |
| Heterophoria†, n (%) | 7 (29%) | 19 (36%) | 0.80 | 6.0 (−19.2; 31.2) |
| NPC >6 cm, n (%) | 2 (8%) | 1 (2%) | 0.45 | 6.5 (−8.2; 21.1) |
| NPA <20 D, n (%) | 4 (17%) | 9 (17%) | >0.99 | 0.0 (−20.9; 20.9) |
| Stereoacuity | ||||
| Stereoacuity >60 sec arc, n (%) | 2 (8%) | 11 (20%) | 0.32 | 12.0 (−6.4; 30.5) |
| Ocular fundus morphology | ||||
| Increased tortuosity of arteries, n (%) | 3 (13%) | 10 (19%) | 0.76 | −6.0 (−25.8; 13.8) |
| Increased tortuosity of veins, n (%) | 1 (4%) | 8 (15%) | 0.33 | −10.6 (−26.1; 4.8) |
|
Optic disc area, RE, mm2 mean (SD), median (range) |
2.59 (0.37) 2.54 (1.99; 3.31) n = 14 |
2.40 (0.43) 2.36 (1.57; 3.60) n = 29 |
0.17 | 0.20 (−0.08; 0.47) |
|
Optic disc area, LE, mm2 mean (SD), median (range) |
2.56 (0.47) 2.52 (2.05; 3.74) n = 14 |
2.53 (0.50) 2.57 (1.64; 3.80) n = 29 |
0.85 | 0.03 (−0.30; 0.35) |
|
Optic cup area, RE, mm2 mean (SD), median (range) |
0.76 (0.21) 0.74 (0.43; 1.29) n = 14 |
0.47 (0.25) 0.40 (0.12; 1.07) n = 29 |
0.0018 | 0.29 (0.12; 0.44) |
|
Optic cup area, LE, mm2 mean (SD), median (range) |
0.72 (0.28) 0.69 (0.39; 1.33) n = 14 |
0.55 (0.26) 0.49 (0.19; 1.25) n = 29 |
0.049 | 0.18 (0.001; 0.35) |
|
Neuroretinal rim area, RE, mm2 mean (SD), median (range) |
1.84 (0.31) 1.76 (1.51; 2.65) n = 14 |
1.93 (0.42) 1.85 (1.33; 2.72) n = 29 |
0.48 | −0.09 (−0.35; 0.16) |
|
Neuroretinal rim area, LE, mm2 mean (SD), median (range) |
1.84 (0.34) 1.82 (1.24; 2.65) n = 14 |
1.99 (0.42) 1.91 (1.29; 3.03) n = 29 |
0.26 | −0.15 (−0.42; 0.11) |
| Ocular dimensions | ||||
|
PFL, RE, mm mean (SD), median (range) |
25.6 (2.02) 26.0 (21.0; 30.0) |
26.1 (1.63) 26.0 (22.0; 30.0) |
0.31 | −0.47 (−1.33; 0.39) |
|
PFL, LE, mm mean (SD), median (range) |
25.6 (2.02) 26.0 (21.0; 30.0) |
26.1 (1.63) 26.0 (22.0; 30.0) |
0.36 | −0.43 (−1.29; 0.43) |
|
ICD, mm mean (SD), median (range) |
29.1 (2.23) 30.0 (25.0; 35.0) |
29.1 (1.78) 29.0 (26.0; 34.0) |
0.94 | 0.07 (−0.88; 1.00) |
| Visual field | ||||
|
Visual field MHR, % mean (SD), median (range) |
65.2 (21.5) 68.0 (32.0; 99.0) n = 20 |
69.7 (20.2) 80.0 (36.0; 99.0) n = 34 |
0.46 | −4.45 (−16.2; 7.45) |
| Visually evoked potentials | ||||
|
VEP, binocular, ms mean (SD), median (range) |
101.9 (5.24) 100.7 (92.5; 111.5) n = 22 |
100.8 (5.65) 100.7 (87.0; 113.2) n = 49 |
0.46 | 1.04 (−1.78; 3.85) |
|
VEP, RE, ms mean (SD), median (range) |
102.3 (4.25) 101.5 (93.8; 111.3) n = 17 |
101.4 (4.58) 102.0 (89.5; 116.5) n = 40 |
0.51 | 0.90 (−1.80; 3.44) |
|
VEP, LE, ms mean (SD), median (range) |
101.3 (3.45) 101.5 (94.0; 106.8) n = 16 |
101.6 (3.90) 101.5 (90.3; 112.0) n = 39 |
0.75 | −0.33 (−2.49; 1.96) |
- D = dioptre, ICD = intercanthal distance, LE = left eye, logMAR = logarithm of minimal angle of resolution, MHR = mean hit rate, NPA = near point of accommodation, NPC = near point of convergence, PFL = palpebral fissure length, PE = preeclampsia, RE = right eye, SD = standard deviation, SE = spherical equivalent, VA = visual acuity, VEP = visual evoked potential.
- The bold values indicate significant results.
- * Where numbers differ from the number of children in the group, this is given separately for each category.
- † At near and/or distance.
Discussion
In the present study, children born to preeclamptic mothers had significantly lower birth size and were more likely to be born SGA compared to children born to non-preeclamptic mothers, confirming previous studies (Odegard et al. 2000; Wu et al. 2009; Geelhoed et al. 2010). Children born with intrauterine growth restriction (IUGR) (i.e. recorded deviated growth with several ultrasound measures), including most SGA children, will have a postnatal catch-up growth (Prader et al. 1963) resulting in a higher risk of hypertension, diabetes and metabolic syndrome (Class et al. 2014; Kelishadi et al. 2015). In the present study, a majority of children exposed to preeclampsia were seen to have a catch-up growth, which is in agreement with a study by Rätsep et al. (2016b).
Reduced neonatal IGF-I levels are seen in children born to preeclamptic mothers (Diaz et al. 2002; Verhaeghe et al. 2003), as well as in children born preterm or with IUGR (Cance-Rouzaud et al. 1998; Verhaeghe et al. 2003; Cutfield et al. 2004; Hansen-Pupp et al. 2007; Allvin et al. 2014; Lind et al. 2018). Since most children exposed to preeclampsia are born preterm and with IUGR (Odegard et al. 2000; Wu et al. 2009; Geelhoed et al. 2010), it is difficult to determine the individual aetiology of the reduced IGF-I levels. In the present study, where all children were born preterm enabling the possibility to better determine the impact of preeclampsia, lower neonatal IGF-I levels were seen in the preeclamptic group compared to the non-preeclamptic group.
The present study found an association between neonatal IGF-I levels and weight, length and HCF at birth. These correlations were seen in all preterm children, confirming previous studies (Verhaeghe et al. 2003), as well as in both the preeclamptic group and the non-preeclamptic group. The correlation between IGF-I levels and birth weight was not significantly larger in the preeclamptic group than in the non-preeclamptic group, suggesting that preeclampsia may not have the greatest impact on this association. Regarding gender, in the present study, girls had significantly higher IGF-I and IGFBP-3 levels at 5 years of age compared to boys, in the non-preeclamptic group. Further research is needed to evaluate the individual effects of preeclampsia, prematurity, IUGR and gender on IGF-I levels.
In a study of 9-year-old children, preeclampsia was associated with elevated blood pressure. However, after adjustment for confounders, the association was no longer apparent (Geelhoed et al. 2010). In a follow-up study for 20 years, participants born to hypertensive pregnancies showed higher blood pressure compared to participants born to normotensive mothers at each measuring point (Davis et al. 2015). In the present study, there was no difference in blood pressure at 5 years of age between participants exposed and not exposed to preeclampsia, which may be due to their early age as the difference in blood pressure tends to increase in older ages (Law et al. 1993).
As reviewed by Abu Samra, preeclampsia has an impact on the visual system in women affected, where 25% of women with severe preeclampsia develop ophthalmological complications, such as visual loss due to pathological processes in the optic nerve or the occipital cortex, or due to serous retinal detachment. Furthermore, hypertension is leading to abnormal retinal vascularization including arteriolar narrowing, which is seen in women with hypertensive pregnancies (Abu Samra 2013). Also, it has been speculating whether preeclampsia may have an impact on the retinal vascularization and the development of ROP in the offspring, due to oxidative stress and altered angiogenic factors (Özkan et al. 2011).
Reduced placental growth factor, associated with preeclampsia, has been found to be related to narrower retinal arteriolar calibre in children exposed to preeclampsia (Gishti et al. 2015). Moreover, a study of very preterm neonates (GA ≤32) found an increased prevalence of ROP in the preeclamptic group and preeclampsia was shown to correlate significantly with ROP (Özkan et al. 2011). However, a study of preterm infants (GA ≤36) with very low birth weight found no elevated risk of ROP in children exposed to preeclampsia when adjusting for SGA birth (Huang et al. 2015). In the present study of moderate-to-late preterm children with no history of ROP, there was no significant difference in the occurrence of increased retinal tortuosity in the preeclamptic group compared to the non-preeclamptic group. Further research is needed to evaluate the impact of preeclampsia on the visual and ocular development, including retinal vascularization, in children exposed.
Nevertheless, it is well known that prematurity is related to ophthalmological complications, such as refractive errors and abnormal ocular fundus morphology (Fazzi et al. 2004; Wikstrand et al. 2010; Allvin et al. 2014; Holmstrom et al. 2014; Raffa et al. 2016; Lind et al. 2018). In the present study, preterm children presenting reduced eye motility had significantly lower neonatal IGF-I levels, compared to children with normal eye motility. Accordingly, low neonatal IGF-I levels may have an impact on the development of eye moment impairments in preterm individuals. However, the association was not seen in either the preeclamptic or the non-preeclamptic group, suggesting that preeclampsia may not have a great impact on this association.
A magnetic resonance imaging study by Rätsep et al. showed increased volumes in the cerebellum, amygdala, temporal lobe and brain stem, as well as reduced vessel radii in the parietal and occipital lobes at 7–10 years of age in children exposed to preeclampsia (Rätsep et al. 2016b). In addition, Rätsep et al. have found eye motility impairments in both prosaccade and antisaccade tasks, as well as impairments in working memory in children born to preeclamptic mothers (Rätsep et al. 2016a). In the present study of preterm children, there was no significant difference between children exposed and not exposed to preeclampsia regarding VA, refraction, strabismus, reduced eye motility (defined as any under- or overreaction), stereoacuity, retinal vascularization, ocular dimensions, VFs, VEP or PVD.
However, in the present study, the preeclamptic group showed significantly larger optic cup areas at 5 years of age compared to the non-preeclamptic group. A previous study found a correlation between a large optic cup-to-disc ratio and poorer cognitive function (Vajaranant et al. 2019). Furthermore, large optic cup areas within normal-sized optic discs have been found in individuals with brain lesions, such as periventricular leukomalacia (PVL) (Jacobson et al. 1997; Groth et al. 2019) and cerebral palsy (CP) (Wikstrand et al. 2010), both associated with cerebral visual impairment (CVI) (Fazzi et al. 2004; Mitry et al. 2016) including PVD and deficiencies in VA, VFs and eye motility (Fazzi et al. 2004). Children born to preeclamptic mothers have a higher risk of CP (Wu et al. 2009), and it would be of great interest to further investigate the effects of preeclampsia on the children’s optic nerve, brain development and the risk of CVI.
The small sample size and the relatively high number of participant dropouts are limitations of the study. The study design, as a prospective and population-based cohort study, is a major strength. Furthermore, the study cohort included only preterm children with no history of ROP, enabling us to better determine the impact of preeclampsia on growth, blood pressure and ophthalmological status. To our knowledge, no previous study has evaluated all the investigated ophthalmological variables in preschool children exposed to preeclampsia.
In conclusion, the present study of children born preterm showed that preeclampsia has an impact on birth size and IGF-I levels, confirming previous studies. Additionally, children with reduced eye motility at 5 years of age showed lower neonatal IGI-I levels compared to children with normal eye motility. Children born to mothers with preeclampsia had larger optic cup areas at 5 years of age compared to children born to normotensive mothers. However, there was no significant difference regarding other ophthalmological variables, or regarding blood pressure. To be able to offer children born to preeclamptic mothers the best health care possible, further research is needed to evaluate the effects of preeclampsia on growth, cardiovascular status, brain development, as well as on ocular development and visual function.




