Corneal wavefront aberrations and subjective quality of vision after small incision lenticule extraction
Abstract
Purpose
To analyse in depth the associations between objectively measured corneal higher-order aberrations (HOAs) and subjectively perceived visual quality after small incision lenticule extraction (SMILE) as quantified with the standardized and clinically validated quality of vision (QOV) questionnaire.
Methods
This cross-sectional study included patients after bilateral simultaneous SMILE for the treatment of myopia and/or myopic astigmatism with plano target refraction. Scheimpflug imaging (Pentacam HR; Oculus Optikgeräte GmbH, Wetzlar, Germany) was used to objectively quantify corneal HOAs. The standardized and validated QOV questionnaire was employed to gauge patients’ subjectively perceived visual quality regarding frequency, severity and bothering effect of visual disturbances.
Results
A total of 394 eyes of 197 patients with a mean age of 32.4 ± 7.7 years and a mean postoperative follow-up of 24.3 ± 14.1 months were included. SMILE induced a statistically significant (p < 0.001) increase in spherical aberration (0.074 ± 0.131 µm), coma (0.142 ± 0.179 µm), trefoil (0.018 ± 0.067 µm) as well as in total HOAs (0.191 ± 0.176 µm). Surgically induced and postoperative levels of HOA showed no correlation with the three QOV scores representative of overall visual symptom frequency, severity and bothering effect (all R2 values ≤ 0.016). In addition, the associations between specific visual symptoms (e.g. starburst) and singular HOA terms (e.g. haloes) were very weak (all Rho values ≤ 0.164).
Conclusions
Small incision lenticule extraction induced significant amounts of corneal HOAs that, however, showed no clear relationships to patient-reported QOV or specific long-term visual symptoms.
Introduction
The all-femtosecond laser-based small incision lenticule extraction (SMILE) technique has become a commonly accepted alternative to excimer-laser-based techniques [i.e. femtosecond laser in situ keratomileusis (fs-LASIK) and photorefractive keratectomy (PRK)] for the surgical correction of myopia and myopic astigmatism (Zhang et al. 2016). As compared with SMILE, one potential advantage of excimer-laser-based photoablation is the ability to deliberately modulate corneal higher-order aberrations (HOAs). In keratorefractive surgery, an excess of iatrogenically induced HOAs over a certain threshold is well known to convey an increased risk for bothersome visual symptoms such as haloes, starburst and glare (Chalita et al. 2004; McCormick et al. 2005; Reinstein et al. 2005; Zhang et al. 2016) Hence, controlled modulation of corneal HOAs has been realized by excimer-laser ablation profiles that are ‘customized’ to the preoperative corneal or ocular wavefront in an effort to address pre-existing HOAs and prevent new HOAs. On the one hand, topography-guided excimer laser systems link the patient’s individual corneal topography data to the ablation profile, thereby specifically targeting surface elevations. On the other hand, wavefront-guided platforms incorporate the patient’s individual ocular wavefront into a customized ablation profile (Kligman et al. 2016).
Despite these apparent technological advantages, current data are highly contradictory as to whether customized ablation treatments actually translate into lower postoperative levels of HOAs as compared with the purely spherocylindrical SMILE technique. For instance, some comparative studies between wavefront-guided excimer-ablation techniques and SMILE found significantly lower levels of total HOAs (Xia et al. 2018) and spherical aberration (Lee et al. 2018; Xia et al. 2018) for the latter technique, one study detected less HOA induction for wavefront-guided LASIK (Khalifa et al. 2017) while others suggested equivalent induction of total HOAs (Ye et al. 2016; Lee et al. 2018; Shetty et al. 2018) and spherical aberration (Ye et al. 2016; Shetty et al. 2018) between both techniques. In contrast, the body of literature seems more unequivocal regarding the surgical induction of coma with multiple studies pointing towards increased postoperative coma after SMILE as compared with wavefront-guided excimer-ablation techniques (Ye et al. 2016; Khalifa et al. 2017; Lee et al. 2018; Xia et al. 2018). This is related to the second potential advantage of excimer-based treatment platforms over SMILE: automated centration control of the optical zone. In contrast to eye tracker-based excimer ablation, centration of the SMILE treatment zone is reliant upon the patient’s fixation and, hence, potentially subject to increased variability. Since it is well known that optical zone decentration entails increased coma induction (Moreno-Barriuso et al. 2001; Li et al. 2014), some authors suggest that special measures should be employed to ensure optimal treatment centration in SMILE in an effort to minimize postoperative HOAs (Kang et al. 2018).
Interestingly, no evidence exists as to whether these differences in HOA induction between customized excimer-ablative techniques and SMILE are in fact clinically relevant for patients’ subjective quality of vision (QOV). Moreover, our knowledge and understanding of the actual relationships between objectively measured HOAs and subjectively perceived QOV after SMILE are very basic. A recent study by Gyldenkerne et al. (2019) was the first to investigate the influence of corneal HOAs on patients’ visual satisfaction after SMILE. Surprisingly, no clear relationships between surgically induced or postoperative corneal HOAs and subjectively perceived visual symptoms were identifiable. However, the small sample size of 51 eyes as well as the monocular use of a self-developed and not clinically validated questionnaire on visual quality might have compromised the power of their study (Gyldenkerne et al. 2019).
Our group recently characterized the subjective QOV and the incidence of long-term visual symptoms after SMILE for treatment of myopia and myopic astigmatism (Schmelter et al. 2019). The purpose of the present study was to analyse in depth the relationship between objectively measured corneal HOAs and subjectively perceived visual disturbances after SMILE as quantified with the standardized and clinically validated QOV questionnaire (McAlinden et al. 2010) in a large sample of SMILE patients.
Materials and Method
Patient selection
This cross-sectional study included patients after bilateral simultaneous SMILE for the treatment of myopia and/or myopic astigmatism. In all cases, target refraction was plano for both eyes. The minimum postoperative follow-up was 3 months. Mean follow-up was 24.3 ± 14.1 months (range 3–39 months). As the questionnaire was used in its original (English) form, patients with insufficient knowledge of the English language according to the investigators’ judgement were ineligible for participation in the study. Institutional review board approval was obtained for all aspects of this study; consent to use their data for analysis and publication was obtained from all subjects and all study-related procedures adhered to the tenets outlined in the Declaration of Helsinki.
Small incision lenticule extraction surgery
All SMILE procedures were performed by one of two highly experienced corneal surgeons (M.D., S.G.P.) using the VisuMax 500-kHz femtosecond laser system (Carl Zeiss Meditec AG, Jena, Germany). The technical principles of the SMILE procedure have been outlined in detail previously (Reinstein et al. 2014). In all cases, an optical zone of 6.5 mm was created. The intended cap diameter was 7.8–7.9 mm, and the intended cap thickness ranged between 120 and 140 μm. For manual extraction of the refractive lenticule, a 4.00-mm incision was created by the femtosecond laser centred at the 135° position in right eyes and at the 45° position in left eyes (Luft et al. 2018).
Postoperatively, patients were prescribed dexamethasone 0.1% and tobramycin 0.3% eyedrops six times daily for 1 week. Thereafter, corticosteroid eyedrops were tapered over the course of 1 month starting with a four times daily regimen. Additionally, patients were encouraged to use preservative-free lubricating eye drops as often as individually required (Luft et al. 2018).
Patient-reported quality of vision
A total of 197 consecutive patients were presented with the original English version of the QOV questionnaire developed by McAlinden et al. (2010) at a regular postoperative visit a minimum of 3 months after surgery. The questionnaire represents a validated, standardized instrument, which has been used in several other studies for the assessment of QOV, for example after LASIK and intraocular lens implantation (Luger et al. 2015; Maurino et al. 2015).
The questionnaire encompasses 10 different items of visual disturbance: glare, haloes, starbursts, hazy vision, blurred vision, distortion, double or multiple images, fluctuation in vision, focusing difficulty and difficulty judging distance/depth perception. All but the latter three symptoms are illustrated by standardized colour images (the so-called ‘QoV Pictures’ printed in standardized size (163 × 232 mm, 300 × 300 DPI). The questionnaire evaluates QOV in three dimensions: patients are encouraged to report how often [never (0), occasionally (1), quite often (2), very often (3)] and how severe [not at all (0), mild (1), moderate (2), severe (3)] they experienced the respective symptoms and, finally, how much they are bothered by them [not at all (0), a little (1), quite (2), very (3)]. After entering all data into a spread sheet, three separate QOV scores are calculated for the respective dimensions of visual disturbance (frequency, severity, bothersome) as established by the inventor. Incorporating all 10 tested visual symptoms, the proprietary QOV scores represent linear-scaled measures of the three dimensions of visual disturbance in general and can range from 0 (=no disturbance) to 100 (=maximum disturbance). In addition, the six most commonly reported long-term visual symptoms after SMILE (fluctuations in vision, glare, starburst, focusing difficulties, haloes and blurred vision; all with a prevalence of ≥37% (Schmelter et al. 2019) were analysed for potential associations with corneal HOAs as described below.
Corneal tomography and higher-order aberrations
Preoperative and postoperative corneal tomography scans were obtained using a high-resolution rotating Scheimpflug camera system (Pentacam HR; Oculus Optikgeräte GmbH, Wetzlar, Germany). All measurements were obtained under standard scotopic ambience light conditions and subjects had to refrain from using eye drops 1 hr prior to scanning. For the present analysis, total (i.e. anterior + posterior) corneal HOAs were calculated for the central 6.00 mm zone using the Optical Society of America (OSA) notation (Thibos et al. 2002). Root mean square (RMS) values were automatically calculated by the system’s onboard software for spherical aberration (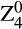 ), total coma (
), total coma (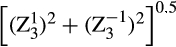 ), and total trefoil (
), and total trefoil (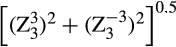 ) and the total amount of HOAs (Thibos et al. 2002).
) and the total amount of HOAs (Thibos et al. 2002).
Data analysis
All statistical analysis was performed using spss 25.0.1 for Windows (IBM Corp.; Armonk, NY, USA). Unless indicated otherwise, all data are reported as mean ± standard deviation. Normality of QOV score data was confirmed by histogram frequency analysis and the Shapiro–Wilk test. Paired-samples t-tests were employed to compare the levels of preoperative and postoperative HOAs. For all correlation analyses, mean values of HOAs from paired eyes were taken into account. Pearson’s correlation coefficients were calculated to quantify the relationships between corneal HOAs and the three linear-scaled QOV scores (frequency, severity, bothersome). In addition, Spearman’s correlation coefficients were computed to analyse the associations between the frequency, severity and bothering effect of specific visual symptoms (ordinal-scaled) and specific corneal HOAs (e.g. spherical aberration).
Results
A total of 394 eyes of 197 patients (117 females; 59.4%) with a mean age of 32.4 ± 7.7 years were included in this study. Mean preoperative manifest refraction was −4.43 ± 1.94 dioptres (range −0.86 to −9.86) of spherical equivalent.
Induction of higher-order aberrations
As depicted in Fig. 1, SMILE induced a statistically significant increase in spherical aberration, coma, trefoil as well as in total HOAs (p < 0.001 for all comparisons; paired-samples t-tests). A detailed summary of preoperative, postoperative and surgically induced HOAs is given in Table 1. With a mean delta of 0.142 µm, coma was the predominantly induced HOA, followed by spherical aberration (0.074 µm) and trefoil (0.018 µm).
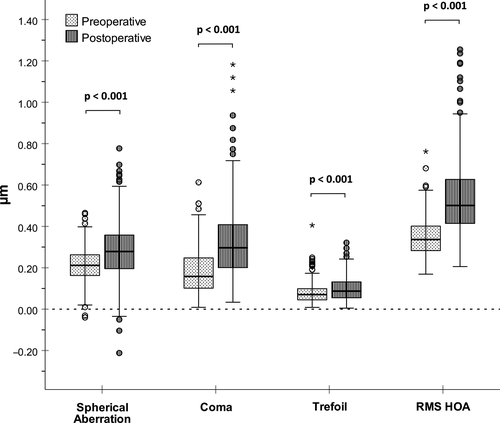
| RMS values | Mean (µm) | SD (µm) | Minimum (µm) | Maximum (µm) |
|---|---|---|---|---|
| Spherical aberration | ||||
| Preoperative | 0.213 | 0.077 | −0.040 | 0.465 |
| Postoperative | 0.287 | 0.139 | −0.212 | 0.777 |
| Induced | 0.074 | 0.131 | −0.305 | 0.443 |
| Coma | ||||
| Preoperative | 0.177 | 0.104 | 0.009 | 0.613 |
| Postoperative | 0.318 | 0.171 | 0.034 | 1.182 |
| Induced | 0.142 | 0.179 | −0.267 | 0.965 |
| Trefoil | ||||
| Preoperative | 0.077 | 0.047 | 0.008 | 0.405 |
| Postoperative | 0.096 | 0.054 | 0.005 | 0.321 |
| Induced | 0.018 | 0.067 | −0.347 | 0.250 |
| Total HOAs | ||||
| Preoperative | 0.346 | 0.087 | 0.169 | 0.762 |
| Postoperative | 0.536 | 0.174 | 0.206 | 1.255 |
| Induced | 0.191 | 0.176 | −0.177 | 0.958 |
- HOAs = higher-order aberrations, RMS = root mean square, SD = standard deviation.
Quality of vision scorings
The mean QOV scores for visual symptom frequency, severity and bothering effect amounted to 34.63 ± 13.69 (range 0–93), 29.60 ± 12.38 (range 0–80) and 24.56 ± 16.00 (range 0–75), respectively. The minimum score of 0 indicates no visual disturbance and the maximum of 100 indicates maximum visual disturbance. The most commonly reported visual symptoms were fluctuation in vision and glare: a cumulative of 73.3% and 65.5% of patients reported these symptoms either ‘occasionally’, ‘quite often’ or ‘very often’. Fluctuation in vision and glare was also concordantly perceived as the most severe and the most bothersome postoperative visual disturbances (Schmelter et al. 2019).
Corneal higher-order aberrations and quality of vision scores
Table 2 summarizes the produced Pearson coefficients (Pearson’s R2) for the linear correlations between surgically induced/postoperative HOAs and the three linear-scaled QOV scores: symptom frequency, severity and bothering effect (Table 2). No statistically significant correlations were detected with all calculated R2 values amounting ≤0.016, indicating that 1.6% or less of the variance in the respective QOV scores could be explained by variation in the examined surgically induced and postoperative HOA values, respectively.
| QOV scores | Postoperative | Induced | ||||||
|---|---|---|---|---|---|---|---|---|
| Spherical aberration | Coma | Trefoil | Total HOAs | Spherical aberration | Coma | Trefoil | Total HOAs | |
| Frequency | ||||||||
| R | −0.012 | −0.107 | 0.021 | −0.036 | −0.005 | −0.021 | −0.070 | −0.018 |
| R 2 | 0.000 | 0.011 | 0.000 | 0.001 | 0.000 | 0.000 | 0.005 | 0.000 |
| p | 0.868 | 0.138 | 0.138 | 0.613 | 0.950 | 0.769 | 0.337 | 0.803 |
| Severity | ||||||||
| R | −0.040 | −0.126 | 0.028 | −0.063 | −0.023 | −0.057 | −0.064 | −0.05 |
| R 2 | 0.002 | 0.016 | 0.001 | 0.004 | 0.001 | 0.003 | 0.004 | 0.003 |
| p | 0.576 | 0.079 | 0.693 | 0.378 | 0.754 | 0.434 | 0.381 | 0.490 |
| Bothering effect | ||||||||
| R | −0.001 | −0.129 | 0.031 | −0.067 | −0.001 | −0.054 | −0.080 | −0.063 |
| R 2 | 0.000 | 0.011 | 0.000 | 0.005 | 0.000 | 0.003 | 0.006 | 0.004 |
| p | 0.988 | 0.073 | 0.662 | 0.353 | 0.987 | 0.454 | 0.273 | 0.384 |
- HOAs = higher-order aberrations, QOV = quality of vision.
Corneal higher-order aberrations and specific visual symptoms
The associations between surgically induced/postoperative HOAs and the frequency, severity and bothering effect of specific visual symptoms as quantified by Spearman’s correlation analysis are presented in detail in Table 3. All Rho values lay between −0.180 and 0.164. The predefined level of statistically significance of p < 0.05 was reached for the following five positive correlations: higher postoperative (Rho = 0.160, p = 0.025) and surgically induced spherical aberration (Rho = 0.156, p = 0.032) as well as higher surgically induced total HOAs (Rho = 0.164, p = 0.024) were statistically significantly positively correlated with the frequency of starburst perception. Moreover, higher postoperative spherical aberration (Rho = 0.148, p = 0.039) and higher surgically induced HOAs (Rho = 0.147, p = 0.043) were positively correlated with the bothering effect of the visual symptom starburst.
| Specific visual symptoms | Postoperative | Induced | ||||||
|---|---|---|---|---|---|---|---|---|
| SA | Coma | Trefoil | Total HOAs | SA | Coma | Trefoil | Total HOAs | |
| Glare | ||||||||
| Frequency | ||||||||
| Spearman’s Rho | 0.008 | −0.105 | −0.086 | −0.044 | 0.034 | −0.016 | −0.103 | −0.020 |
| p-Value | 0.913 | 0.144 | 0.234 | 0.540 | 0.640 | 0.822 | 0.155 | 0.781 |
| Severity | ||||||||
| Spearman’s Rho | −0.033 | −0.122 | −0.054 | −0.071 | 0.005 | −0.035 | −0.040 | −0.029 |
| p-Value | 0.643 | 0.089 | 0.453 | 0.324 | 0.951 | 0.626 | 0.583 | 0.695 |
| Bothering effect | ||||||||
| Spearman’s Rho | −0.022 | −0.130 | −0.128 | −0.070 | −0.005 | 0.015 | −0.122 | −0.008 |
| p-Value | 0.763 | 0.070 | 0.076 | 0.331 | 0.942 | 0.840 | 0.093 | 0.916 |
| Haloes | ||||||||
| Frequency | ||||||||
| Spearman’s Rho | −0.047 | −0.022 | 0.111 | −0.010 | −0.018 | 0.047 | 0.035 | 0.040 |
| p-Value | 0.514 | 0.764 | 0.122 | 0.886 | 0.803 | 0.520 | 0.627 | 0.581 |
| Severity | ||||||||
| Spearman's Rho | −0.054 | −0.047 | 0.136 | −0.018 | −0.030 | 0.011 | 0.044 | 0.021 |
| p-Value | 0.450 | 0.514 | 0.058 | 0.800 | 0.683 | 0.885 | 0.544 | 0.773 |
| Bothering effect | ||||||||
| Spearman’s Rho | −0.084 | −0.095 | 0.132 | −0.086 | −0.039 | −0.041 | 0.079 | −0.043 |
| p-Value | 0.242 | 0.185 | 0.065 | 0.233 | 0.594 | 0.568 | 0.276 | 0.559 |
| Starburst | ||||||||
| Frequency | ||||||||
| Spearman’s Rho | 0.160 | 0.026 | 0.008 | 0.133 | 0.156 | 0.121 | −0.074 | 0.164 |
| p-Value | 0.025 | 0.723 | 0.915 | 0.064 | 0.032 | 0.094 | 0.308 | 0.024 |
| Severity | ||||||||
| Spearman’s Rho | 0.123 | 0.003 | 0.082 | 0.112 | 0.108 | 0.081 | −0.011 | 0.127 |
| p-Value | 0.088 | 0.968 | 0.256 | 0.120 | 0.138 | 0.267 | 0.884 | 0.081 |
| Bothering effect | ||||||||
| Spearman’s Rho | 0.148 | −0.015 | 0.060 | 0.082 | 0.140 | 0.134 | 0.002 | 0.147 |
| p-Value | 0.039 | 0.834 | 0.402 | 0.256 | 0.054 | 0.064 | 0.983 | 0.043 |
| Blurred vision | ||||||||
| Frequency | ||||||||
| Spearman’s Rho | −0.033 | −0.096 | −0.018 | −0.084 | −0.057 | −0.110 | 0.036 | −0.137 |
| p-Value | 0.652 | 0.181 | 0.806 | 0.239 | 0.433 | 0.128 | 0.620 | 0.059 |
| Severity | ||||||||
| Spearman’s Rho | −0.020 | −0.109 | −0.036 | −0.086 | −0.042 | −0.120 | 0.019 | −0.139 |
| p-Value | 0.779 | 0.128 | 0.622 | 0.233 | 0.565 | 0.097 | 0.792 | 0.056 |
| Bothering effect | ||||||||
| Spearman’s Rho | −0.021 | −0.116 | −0.012 | −0.098 | −0.049 | −0.152 | 0.054 | −0.175 |
| p-Value | 0.769 | 0.105 | 0.863 | 0.170 | 0.499 | 0.036 | 0.456 | 0.016 |
| Fluctuation in vision | ||||||||
| Frequency | ||||||||
| Spearman's Rho | 0.052 | −0.005 | 0.002 | 0.055 | 0.039 | 0.015 | −0.022 | 0.005 |
| p-Value | 0.472 | 0.940 | 0.975 | 0.447 | 0.592 | 0.842 | 0.760 | 0.941 |
| Severity | ||||||||
| Spearman’s Rho | 0.053 | 0.024 | 0.003 | 0.054 | 0.057 | 0.053 | −0.023 | 0.027 |
| p-Value | 0.466 | 0.739 | 0.970 | 0.455 | 0.431 | 0.463 | 0.748 | 0.710 |
| Bothering effect | ||||||||
| Spearman's Rho | 0.087 | 0.055 | 0.027 | 0.120 | 0.062 | 0.037 | −0.025 | 0.050 |
| p-Value | 0.226 | 0.447 | 0.704 | 0.094 | 0.395 | 0.614 | 0.728 | 0.497 |
| Focusing difficulties | ||||||||
| Frequency | ||||||||
| Spearman's Rho | −0.180 | −0.052 | −0.071 | −0.097 | −0.142 | −0.036 | −0.048 | −0.109 |
| p-Value | 0.012 | 0.471 | 0.327 | 0.178 | 0.050 | 0.623 | 0.510 | 0.133 |
| Severity | ||||||||
| Spearman’s Rho | −0.167 | −0.041 | −0.087 | −0.093 | −0.134 | −0.039 | −0.045 | −0.124 |
| p-Value | 0.020 | 0.566 | 0.227 | 0.198 | 0.064 | 0.588 | 0.538 | 0.087 |
| Bothering effect | ||||||||
| Spearman's Rho | −0.096 | −0.063 | −0.027 | −0.095 | −0.070 | −0.047 | −0.036 | −0.135 |
| p-Value | 0.184 | 0.386 | 0.713 | 0.186 | 0.337 | 0.520 | 0.620 | 0.064 |
- Bold numbers indicate a significance level of p < 0.05.
- HOAs = higher-order aberrations, SA = spherical aberration.
Moreover, four statistically significant inverse correlations were detected: higher amounts of induced coma (Rho = −0.152, p = 0.036) and total HOAs (Rho = −0.175, p = 0.016) were negatively correlated with the bothering effect of fluctuations in vision. In addition, higher postoperative spherical aberration was negatively associated with the frequency (Rho = −0.180, p = 0.012) and severity (Rho = −0.167, p = 0.020) of focusing difficulties. For the symptoms halo, glare and fluctuations in vision, no statistically significant associations with postoperative or surgically induced HOAs were detectable.
Discussion
The purpose of this study was to unravel the relationships between objectively quantified HOA of the corneal wavefront and subjectively perceived visual disturbances after SMILE. The present work is the first to address this question in a large cohort of post-SMILE patients and to utilize a standardized and validated patient-reported outcome (PRO) instrument, the QOV questionnaire.
The mean QOV scores for visual symptom frequency (34.6), severity (29.6) and bothering effect (24.6) of the present study ranged within previous QOV scorings reported for postkeratorefractive surgery populations. For example, Luger et al. reported slightly worse QOV scores of 47.3, 41.2 and 41.6, respectively, for a cohort of 32 patients 1 year after femtosecond LASIK (Luger et al. 2015). In contrast, Wang Yin et al. (2016) reported better visual quality (QOV scores of 20.7, 18.0 and 23.7, respectively) for a sample of 69 hyperopic patients that had undergone presbyopic fs-LASIK with micro-monovision.
In accordance with several previous reports, SMILE induced statistically significant amounts of corneal HOAs, while the level of the respective induced HOAs lay within the ranges reported in past studies (Gyldenkerne et al. 2015; Miao et al. 2015; Pedersen et al. 2015). Of all HOAs, coma was the predominantly induced HOA, which is a well-known phenomenon in SMILE (Miao et al. 2015) that has been shown to be related to treatment decentration (Li et al. 2014). As compared with excimer-based keratorefractive techniques, its lack of eye tracking technology for automated optical zone centration as well as its technical incapability to perform treatments customizable to the individual patient’s preoperative topography or wavefront may be regarded as technical shortcomings of SMILE, potentially entailing suboptimal aberrometric outcomes. Nevertheless, the current literature comparing HOA induction between SMILE and topography- or wavefront-guided photoablative techniques is highly controversial (Ye et al. 2016; Khalifa et al. 2017; Lee et al. 2018; Shetty et al. 2018; Xia et al. 2018). In addition, it is entirely unclear whether these (apparently subtle) differences in HOA induction between techniques actually manifest in our patients’ subjective QOV.
Interestingly, the present study did not reveal any relevant coherence between SMILE-induced HOAs with subjectively reported visual quality as assessed with the QOV questionnaire, a validated PRO instrument gauging: overall visual symptom frequency, severity and bothering effect (McAlinden et al. 2010). In contrast, statistically significance was found for associations between corneal HOAs and three of the six most commonly reported (Schmelter et al. 2019). Visual symptoms after SMILE. For instance, increased spherical aberration and total HOA induction were associated with more frequent and bothersome starburst perception. However, also inverse correlations between HOAs (e.g. coma) and specific visual symptoms (e.g. blurred vision) surpassed the predefined level of statistical significance suggesting a protective effect of increased coma induction on postoperative visual quality. This is a paradox finding, as coma is well known to cause a ‘smearing’ effect or comet like tails on a perceived image and is regarded as one of the HOAs most detrimental to perceived image quality (Kligman et al. 2016). Surprisingly, the commonly purported association between spherical aberration and halo perception (Kligman et al. 2016) was not confirmed in the present study. In light of these counterintuitive findings, the fact that all calculated correlation coefficients were very weak (ranging between −0.180 and 0.164) and, moreover, a plethora of correlation analyses was conducted (thereby significantly increasing the risk for type I errors) we hypothesize that these statistically significant associations may be regarded as clinically negligible.
Our findings resonate well with Gyldenkerne et al. (2019), who very recently reported no clear relationship between corneal HOAs and visual symptoms in patients that underwent SMILE for treatment of myopia or myopic astigmatism. In contrast to the present work, however, their study design was limited by a relatively small sample size and the monocular use of a self-developed and not clinically validated questionnaire. In the recent Patient-Reported Outcomes With Laser In Situ Keratomileusis (PROWL-1, PROWL-2) studies that included 262 military personnel and 312 civilians undergoing LASIK, respectively, 43–46% of patients reported new visual symptoms postoperatively with haloes and starburst being the most common visual disturbances. In accordance with our findings, no clear correlations between visual symptom scores and optical aberrations could be established. In contrast, moderately strong associations between visual disturbances and dry eye symptoms (as quantified with the ocular surface disease index) were detected at 6 months postoperatively (Eydelman et al. 2017).
In general, visual symptoms intractable to treatment with spectacles are well-established phenomena attributable to corneal irregularities caused by certain complications of keratorefractive surgery. For instance, decentred ablation zones (Mrochen et al. 2002), small optical zones in presence of larger pupil diameter (Rajan et al. 2006) and so-called central islands (McCormick et al. 2005) leading to elevated levels of HOAs give rise to bothersome visual disturbances. After uneventful keratorefractive surgery with apparently ‘regular’ postoperative corneal topography, however, the associations between HOAs and visual symptoms appear to not have been fully unravelled. Several factors other than corneal HOAs can influence subjectively perceived QOV such as tear film quality (Eydelman et al. 2017), pupil size (McCormick et al. 2005) and interindividual neuroadaptation to surgically induced HOAs (Artal et al. 2004), and hence, more research seems warranted to further elucidate these apparently complex interactions.
Limitations to the present study may be found. Firstly, the original (English) version of the QOV questionnaire was used in a German native population. However, patients with insufficient English language knowledge were excluded from participation, the questionnaire items were accompanied by standardized colour photographs that give clear illustrations of the respective visual symptoms and one of the investigators was always available in case of questions in order to avoid language-related misunderstandings. A second limitation of the study is the lack of preoperative baseline QOV scorings, as also spectacle wearers and contact lens users are known to have significant visual symptoms (Luger et al. 2015; Kang et al. 2018). Moreover, potential cofounding factors influencing QOV such as dry eye symptoms were not assessed.
To conclude, this large-scaled study utilizing a standardized and validated patient-reported outcome instrument found only very weak and probably clinically irrelevant associations between corneal HOAs and subjectively perceived visual quality after SMILE. It seems reasonable to assume that more important determinants of subjective QOV other than corneal HOAs exist, and further research is warranted to address this question.




