Viewing the choroid: where we stand, challenges and contradictions in diabetic retinopathy and diabetic macular oedema
Abstract
Diabetic macular oedema (DMO) is the leading cause of vision loss in the working-age population. Blood–retinal barrier (BRB) dysfunction in diabetic retinopathy (DR), mainly at the level of the retinal vessels, has long been related with leakage and fluid accumulation, leading to macular oedema. However, the nourishment of the macula is provided by the choroid and a diabetic choroidopathy has been described. Therefore, there has been a growing interest in studying the role of the choroid in the pathophysiology of DR and DMO, mainly by optical coherence tomography (OCT). Nevertheless, there are conflicting results in the different studies. We summarize the results from the available studies, describe the limitations and confounding factors and discuss future procedures to avoid bias.
Introduction
Diabetes mellitus (DM) has become one of the most dramatic challenges worldwide (Wild et al. 2004). Sedentary life, lack of exercise and overweight are risk factors for diabetes and its complications, including diabetic retinopathy (DR). In developed countries, DR is the leading cause of blindness in the active population (Williams et al. 2004) and it became a burden on healthcare facilities (Javitt & Aiello 1996; Brown et al. 1999). Progression of DR causes microvascular damage leading to increased permeability, retinal ischaemia, macular oedema and neovascularization (Klein et al. 1994; Engerman & Kern 1995). Retinal neuropathy with evidence of neural apoptosis associated with DM was reported (Barber et al. 1998; Antonetti et al. 2012). As DR may lead to loss of vision, it is extremely important to evaluate the stage of DR in order to establish an adequate follow-up and therapy (Klein et al. 1984a).
The breakdown of the inner blood–retinal barrier (BRB) is believed to be the initial event in the development of DR (Cunha-Vaz et al. 1975). However, experimental studies demonstrated that the BRB breakdown occurs at both the inner and outer BRB (Bento et al. 2010). Inner and outer BRB disruption leads to the accumulation of fluid, exudation and haemorrhages, thickening of the macular region, resulting in diabetic macular oedema (DMO) (Cunha-Vaz et al. 1975).
Diabetic macular oedema (DMO) is the leading cause of visual loss in patients with DR (Moss et al. 1994). The prevalence of DMO increases from 0% to 3% in individuals recently diagnosed up to 28–29% in those with diabetes duration of over 20 years (Klein et al. 1984b). In a UK diabetic population, an overall prevalence of DMO has been estimated as 13.9% (Keenan et al. 2013).
The current treatment of DMO, wherein anti-vascular endothelial growth factor (VEGF) agents and steroids are increasingly being used, demands optical coherence tomography (OCT) as an essential tool for the follow-up (Diabetic Retinopathy Clinical Research et al. 2012). This shift in treatment led to a classification of DMO as centre-involving (CI), treated with intravitreal injections of drugs, and non-centre-involving DMO, treated with laser (Keenan et al. 2013). Currently, based on OCT, centre-involving diabetic macular oedema (CI-DMO) is defined as a 300-μm thickness at the central macula. This definition avoids the interobserver variability in funduscopy and is very useful because fluorescein angiography has been replaced by OCT as the mainstay for the follow-up (Diabetic Retinopathy Clinical Research et al. 2010). Despite the alterations in the BRB are believed to be mainly responsible for the development of DR and DMO, several studies indicate the choroid as an important player in the pathophysiology of DR and DMO (Hidayat & Fine 1985; Nickla & Wallman 2010; Hua et al. 2013). However, the role of the choroid in DR and DMO is still under discussion. The authors briefly review the anatomy and physiology of the choroid, the application of OCT to the study of the choroid and compare the published data regarding the variations of the choroidal thickness (CT) in DR and DMO.
Anatomy and Physiology of the Choroid
The choroid is a highly vascularized and pigmented structure localized between the lamina fusca of the sclera and the retinal pigment epithelium (RPE), extending anteriorly from the ora serrata to the optic nerve posteriorly. The choroid is composed of the choriocapillaris, the basal membrane of which forms the outer part of the 5-laminar structure of Bruch's membrane, the middle layer of medium-sized vessels (Sattler's layer), the outer layer of large vessels (Haller's layer) and the suprachoroid, limited externally by the lamina fusca (Fig. 1).
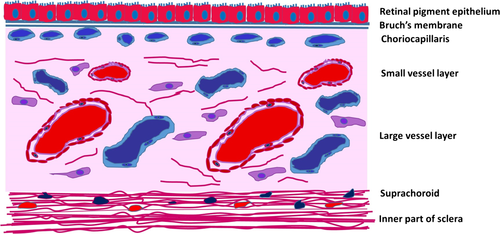
The choriocapillaris is a network of capillaries about 10 μm thick at the fovea, thinning to about 7 μm at the periphery. The medium-sized arteries of the Sattler's layer give rise to a hexagonal or lobe-shaped structure of a single layer of fenestrated capillaries originating the patchlike structure of the choriocapillaris (Figs 2 and 3) (Hayreh 1975). The pores of those capillaries are mainly facing the RPE and are permeable to proteins, originating a high oncotic pressure in the stroma, directing the movement of fluids out of the retina into the choroid (McLeod et al. 2009).

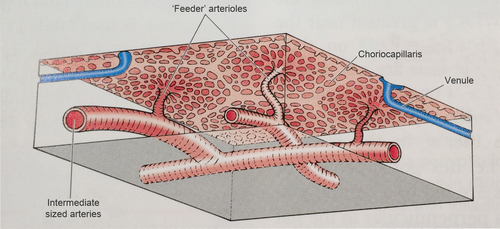
From the Sattler's layer, at the level of the outer choriocapillaris, there are columns of collagen fibres running between the capillaries and attaching to the outer fibrous layer of the Bruch's membrane probably supporting the capillaries network (Krebs & Krebs 1988). The Bruch's membrane is a five-layered structure comprehending the basement membrane of the choriocapillaris, an outer collagenous zone, an elastic layer, an inner collagenous zone and the basement membrane of the RPE (Nickla & Wallman 2010). Between the vessels of Sattler's and Haller's layers, there are melanocytes, a ganglion cell plexus whose cells express nicotinamide adenine dinucleotide phosphate diaphorase and nitric oxide synthase (NOS), connective tissue and other cellular elements (Flugel et al. 1994). The suprachoroid lies between the choroid and the sclera, containing fibroblasts, collagen fibres and melanocytes. The suprachoroid has large endothelial-lined spaces receiving fluid via the uveoscleral route and from the remaining choroid due to an oncotic gradient and emptying into veins (Alm & Nilsson 2009). The 30-μm-thick outmost layer of the suprachoroid is the lamina fusca, consisting of several layers of melanocytes and fibroblast-like cells disposed in plates, with bundles of myelinated axons (Nickla & Wallman 2010). The choroid receives more than 70% of the blood supply from the ophthalmic artery, which is the highest rate per unit weight in any tissue, but due to the large area of the choriocapillaris, the speed of the flow is decreased to 77% of the flow speed in the capillaries of the retina (Wajer et al. 2000).
The main physiologic function of the choroid is to provide oxygen and nutrients to the highly metabolic outer retinal layers, namely the central avascular fovea and the prelaminar portion of the optic nerve (Hayreh 2001). The RPE is so intimately related with the choroid and choriocapillaris under homoeostasis and disease that photoreceptors, RPE, Bruch's membrane and choriocapillaris might be considered as the tapetoretinal unit (Dzhemileva et al. 2008). The choriocapillaris originates from the mesenchyme, but it needs to be in contact with the developing RPE, derived from the neural crest, in order to differentiate (Mrejen & Spaide 2013). Homoeostasis and nourishment of the RPE is intimately under choroidal mediation as demonstrated by the age-related choroidal atrophy that goes along with age-related macular degeneration (AMD) (Spaide 2009). In addition, the choroid is of outmost importance in temperature regulation by conveying heat (Nickla & Wallman 2010), accumulated due to the focused light onto the macula and due to the high metabolism of the tapetoretinal unit (Parver et al. 1980; Wangsa-Wirawan & Linsenmeier 2003). Despite choroidal blood flow in the fovea compensates better for an increase in arterial blood pressure than for an increase in intraocular pressure (Polska et al. 2007), a fundamental role of the choroid in angle-closure glaucoma mediated by choroidal expansion has been demonstrated (Quigley 2009). The choroid also plays a role in the drainage of the aqueous humour via the uveoscleral pathway. This drainage is about 35% of the total aqueous drainage and is enhanced by atropine and epinephrine but blocked by pilocarpine. Additionally, non-vascular smooth muscle cells in the lamellae of the suprachoroid are responsive to neurogenic stimulation and may account for variations in the CT as well as for the stabilization of the position of the fovea during accommodation. As there is no evidence hitherto of classic lymphatic vessels in the adult human choroid, clearance of toxins and debris of metabolism might go through the suprachoroid to the episcleral veins and to the vortex vein system (Schroedl et al. 2014).
The choroid provides most of the blood supply the retina needs. Photoreceptors use about 90% of oxygen delivered to the retina, mainly under mesopic and scotopic environments. To bypass the Bruch's membrane and the RPE, specific adaptations were needed: a blood flow in the choroid 10-fold higher than in the brain, a high oxygen tension in the choroid (arterial/venous difference of about 3% compared with 38% in the retinal circulation) and the choriocapillaris pores disposed mainly on the Bruch's membrane side (Linsenmeier & Braun 1992; McLeod & Lutty 1994; Yu & Cringle 2001). Furthermore, a higher haemoglobin oxygen saturation level in the vessels of the choroid, when compared with the vessels of the retina, and further rise in that difference with inhalation of 100% oxygen, was demonstrated in vivo in human subjects using a non-invasive spectrophotometric oximeter (Kristjansdottir et al. 2013). Unlike the retinal circulation, the choroidal circulation consists of fenestrated blood vessels due to the continuous secretion of VEGF by the RPE cells (Blaauwgeers et al. 1999). Fenestration dependent on VEGF allows prompt delivery of oxygen and nutrients to the outer retina and macula. The positive oncotic pressure at the level of the Bruch's membrane, created by the extravascular accumulation of large molecules, allows fluid to flow out of the retina into the choroidal stroma and suprachoroid (Marmor et al. 1980; Saint-Geniez et al. 2009). Thus, one possible mechanism accounting for the CT changes is the expansion of the lacunae in the suprachoroid. The lacunae expansion is mediated by the synthesis of large proteoglycans (Wallman et al. 1995), by the modulation of the size and number of fenestrations in the choriocapillaris (Pendrak et al. 2000), by changes in the flux of the uveoscleral pathway (Alm & Nilsson 2009), by altered transport from the retina across the RPE (Liang et al. 2004) and by changes in the tonus of the non-vascular smooth muscle of the suprachoroid (Nickla & Wallman 2010). Nitric oxide (NO) synthase-positive axon terminals are found in the non-vascular smooth muscle cells of the choroid, suggesting a role of NO in the regulation of the CT by reducing the degree of contraction of these cells (Nickla & Wallman 2010).
Currently, it is considered that the choroid may contribute to the pathogenesis of several retinal diseases. Choroidal atrophy seems to be associated with high myopic retinal degeneration (Wang et al. 2015) and with atrophic AMD (Spaide 2009). Several inflammatory conditions of the retina are in fact choroiditis (Jampol et al. 1979; Campos et al. 2014). It was also reported a general disturbance in the choroidal blood flow of both eyes in central serous choroidopathy (CSC), with increased CT and serous macular detachment (SMD), probably dependent on a mineralocorticoid receptor mediation (Scheider et al. 1993; Imamura et al. 2009; Zhao et al. 2012). In diabetes, the choroid behaves as a pro-inflammatory environment. In fact, inflammation, glial cell activation and cell migration from the retina to the choroid are involved in the pathogenesis of diabetic retinopathy (Omri et al. 2011; Grigsby et al. 2014). Hitherto, the study of the choroid in vivo has been limited by the lack of an adequate technique of visualization and evaluation.
Optical Coherence Tomography
Optical coherence tomography (OCT) was introduced as a non-invasive modality for imaging transparent and translucent samples and tissues with a resolution of a few micrometres (Huang et al. 1991). As the eye is essentially transparent, it provides easy optical access to the retina and therefore OCT was first investigated in ophthalmology (Huang et al. 1991; Fercher et al. 1993).
The introduction of the spectral domain (SD) principle changed the paradigm in the OCT technology. Its improved sensitivity enabled to operate at 800 nm with a higher acquisition speed, with light suffering minimal optical attenuation and scattering (Mrejen & Spaide 2013). The resolution of about 5 μm provides high-quality images of the retina, from the inner limiting membrane down to the RPE (Geitzenauer et al. 2011). This wavelength is suitable to resolve all main intraretinal layers, but its penetration depth is limited by absorption and scattering at the RPE level. As the absorption of light by melanin is strongly wavelength-dependent, the use of longer wavelengths may improve the penetration depth into the choroid (Drexler 2004). Nevertheless, when going towards longer wavelengths water absorption of light increases. The advantage of a better tissue penetration, using longer wavelength devices, is overshadowed by a poorer resolution in an organ consisting mostly of water (Mrejen & Spaide 2013). The signal double passing through the ocular media to the retina is significantly attenuated (up to 50%) (Unterhuber et al. 2005). The enhanced deep imaging (EDI) is the approach developed to overcome these difficulties. The 800 nm SD-OCT device is positioned closer to the eye in order to change the focal point backwards to the choroid. This avoids the loss of signal caused by the RPE (Spaide et al. 2008; Margolis & Spaide 2009). Moreover, a correlation has been found between data collected from the choroid with the longer wavelength devices and the EDI procedure with the 800 nm SD-SPECTRALIS® (Heidelberg Engineering GmbH, Heidelberg, Germany) device (Ikuno et al. 2011; Tan et al. 2015).
OCT and the Choroid
Choroidal thickness (CT) was first evaluated in a focal fashion from the posterior edge of the RPE to the choroid/sclera junction at 500 μm intervals up to 2500 μm temporal and nasal to the fovea (Margolis & Spaide 2009). The choroid is thicker in the subfoveal area and CT decreases from the subfoveal area to the nasal and temporal choroid (Ikuno et al. 2011; Regatieri et al. 2012), with the nasal CT being usually thinner (Margolis & Spaide 2009; Ouyang et al. 2011; Noori et al. 2012). Margolis & Spaide (2009) reported a subfoveal choroidal thickness (SFCT) of 287 ± 76 μm, using a 870-nm device in the EDI mode and others reported a SFCT up to 10–20 μm thicker, using a longer wavelength device, and found reproducibility between these two devices (Esmaeelpour et al. 2011; Ikuno et al. 2011). There is great variability in the CT according to age (Spaide 2009), refraction and even the time of day (Margolis & Spaide 2009). Previous studies, using both OCT and histologic findings, have found statistically significant negative correlations between CT and age (decreasing CT with increasing age) (Ramrattan et al. 1994; Margolis & Spaide 2009; Ikuno et al. 2011). It was reported that SFCT decreased 1.56–1.95 μm for each additional year of age (Margolis & Spaide 2009; Ouyang et al. 2011), or 15.6 μm for each decade of life (Margolis & Spaide 2009). Based on histologic evaluation, a decrease in thickness of 1.1 μm per year of age was found, which represents a rough estimation of the actual in vivo CT (Ramrattan et al. 1994). Subfoveal choroidal thickness (SFCT) also decreases with increasing axial length (AL), 31.96 μm for each 1-mm increase in AL (Ouyang et al. 2011). In addition, a person with a normal choroid may manifest differences in thickness at intervals of a few hours or days. Unlike the retinal thickness, it has been reported that CT shows a diurnal variation of about 30 μm (thinnest at 6 p.m. and thickest at 3 a.m.), decreasing roughly 8% from 9 a.m. to 5 p.m. (Tan et al. 2012; Usui et al. 2012). In addition, there is interindividual variability in CT, independently of age, AL or time of the day, which must be taken into account when including both eyes of the same patient (Fig. 4). The layers of the choroid do not behave as the layers of the retina. Unlike the retina, where layers are stacked as regular sheets one underneath the other almost like sheets of paper, in the choroid the large vessels from underneath (Haller's layer) press upwards the superior layers (Sattler and choriocapillaris layers), leaving thicker areas in-between the large vessels in the unpressed sites (Fig. 5). This results in a generalized intertwined network of ‘hills and valleys’. This fingerprint-like structure of the choroid layers and the presence of the suprachoroid make the actual evaluation of the sublayers of the choroid and the correct identification of the choroid–scleral border occasionally difficult by OCT.
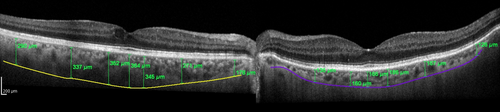
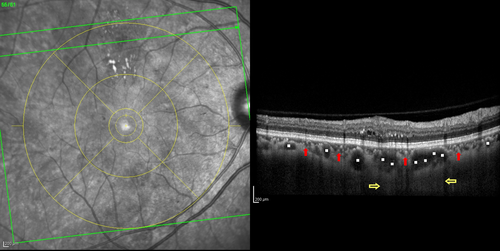
Choroid and Diabetes
Clinical and experimental findings suggest that a choroidal vasculopathy in diabetes may play a role in the pathogenesis of DR. Diabetic choroidopathy (DC) was defined in DM using indocyanine green angiography. Late-phase choroidal hypoperfusion along with an inverted inflow phenomenon has been related to DR severity, and choroidal vascular resistance has been related to retinal ischaemia (Hua et al. 2013). Large hyperfluorescent spots were associated with high glycosylated haemoglobin levels and might be an indicator for choroidal microangiopathy (Shiragami et al. 2002). Histopathological studies of eyes in type 2 diabetes reported decreased alkaline phosphatase activity in the choriocapillaris, loss of viable endothelial cells, degeneration of the choriocapillaris, obstruction and choroidal aneurysms, Bruch's membrane degenerative changes and choroidal neovascularization (Fukushima et al. 1997). Lutty & McLeod (2005) demonstrated that the decrease in the alkaline phosphatase enzyme activity is related to choriocapillaris loss in DC. Nitric oxide synthase expression is increased in the retina even in early-onset type 1 (do Carmo et al. 1998) or type 2 DM (Carmo et al. 2000). The neuronal NO release in the parasympathetic perivascular nerve fibres of the choroid may also result in a diabetes-induced neuronal damage. Therefore, DC may encompass a microangiopathy along with a diabetic neuropathy (Sakurai et al. 2002). In fact, a previous study hypothesized that the unexplained loss of visual acuity in diabetic patients, regardless of the inexistence of retinopathy, might be due to DC (Cao et al. 1998). Choroidal inflammation and ischaemia may disturb the outer BRB, leading to the accumulation of subretinal fluid (Kaur et al. 2008). Indeed, there is growing evidence indicating that the choroid is implicated in the onset of SMD (Hua et al. 2013). As the nourishment of the macula depends on the choroid, DMO with SMD may be associated with macular ischaemia in some cases (Koleva-Georgieva & Sivkova 2009). Furthermore, SMD is a main feature of CSC, a disease located primarily in the choroid (Gemenetzi et al. 2010). Once disturbed the outer BRB in diabetes, the inner BRB would be further unbalanced as there is a crosstalk between the two components of the BRB (Bento et al. 2010). Despite the alterations reported above, the crosstalk between the choriocapillaris and the BRB is not fully understood yet.
CT in DM without DR
When comparing CT between diabetic eyes without DR and controls, the results are contradictory. Some studies reported that there is no difference in CT between diabetic eyes without retinopathy and controls (Vujosevic et al. 2012; Adhi et al. 2013b; Lee et al. 2013; Unsal et al. 2014), while other studies reported either a significant increase (Xu et al. 2013) or, more commonly, a significant decrease (Esmaeelpour et al. 2011, 2012; Querques et al. 2012; Kim et al. 2013).
In the Beijing Eye Study, no correlation was found between DM and SFCT. However, the range of CT reported was too wide (from 8 to 854 μm), which does introduce a bias towards axial length (AL)-dependent parameters (Wei et al. 2003). A report issued later, using the same sample corrected for AL and age, found diabetes to be related with choroidal thickening, but added no additional risk to the DR stage. However, diabetics were only 12% of the total number of subjects enrolled, which reduced the strength of the analysis (246 in 2041 eyes). More importantly, only 23 diabetic eyes had DR (0.7%) with scarce numbers in the different subgroups of DR, not allowing any consistent statement as to whether CT relates to DR or the grade of DR (Xu et al. 2013).
Therefore, CT seems to decrease or remain unchanged in diabetic eyes without DR, but a consensus still has to come from future studies. A recent study correlated microalbuminuria with a thinner choroid, in diabetic patients whose eyes had no DR or mild DR. Hence, microalbuminuria and CT would be prognostic markers for disease progression in eyes with early-stage DR. Unfortunately, the study enrolled a small sample size, included both eyes, and considered a small cut-off as significantly different for CT (15 μm). Considering small differences as significant, the sensibility is overweighted at the expense of specificity. Small differences in CT may not be independent from other variables, such as individual variability in CT, duration of diabetes and hour of day for the collection of the OCT data (Farias et al. 2014).
CT during DR progression
A decrease in CT in diabetic eyes unrelated to the DR stage was reported in some studies (Esmaeelpour et al. 2011, 2012; Querques et al. 2012) correlating diabetes with a smaller CT, while others found a decrease in CT related to the stage of DR, but no difference between diabetic eyes without retinopathy and controls, correlating the severity of DR but not diabetes with choroid thinning (Table 1) (Vujosevic et al. 2012; Adhi et al. 2013b; Lee et al. 2013; Unsal et al. 2014). Regatieri et al. (2012) found no difference in CT in eyes with non-proliferative diabetic retinopathy (NPDR) when compared with controls, but found a CT decrease in advanced disease stages, either DMO or proliferative diabetic retinopathy (PDR). By opposition, Kim et al. (2013) related DR progression not to a decrease but to an increase in CT. This report had important drawbacks; therefore, the evidence seems to indicate that the choroid thins in diabetic eyes, but the correlation with the grade of DR is still elusive.
| Author | N diabetic eyes/controls | Type of study | Device | CT and DR staging | CT and DMO | Strength | Drawbacks |
|---|---|---|---|---|---|---|---|
| Kim et al. (2013) |
235 of 36 5 subgroups |
Retrospective transversal approach | SD-spectralis | ↑ | ↑ |
|
|
| Esmaeelpour et al. (2011) |
63 of 16 eyes 4 subgroups |
Prospective transversal approach | 1060 nm OCT | No, only ↓ in all diabetic eyes | No change |
|
|
| Esmaeelpour et al. (2012) |
33 of 20 2 subgroups |
Prospective transversal approach | 1060 nm OCT | No, only ↓ in all diabetic eyes | Not searched |
|
|
| Querques et al. (2012) |
63 of 21 3 subgroups |
Prospective transversal approach | SD-Spectralis | No, only ↓ in all diabetic eyes | ↓ |
|
|
| Vujosevic et al. (2012) |
102 of 48 3 subgroups |
Observational case series Transversal approach |
SD-Nidek | ↓ | No change |
|
|
| Regatieri et al. (2012) |
49 of 24 3 subgroups |
Retrospective transversal approach | Cirrus-HD | ↓ | ↓ |
|
|
| Xu et al. (2013) |
246 of 2041 3 subgroups |
Epidemiological study | SD-Spectralis | No change | Not searched |
|
|
| Adhi et al. (2013b) |
33 of 24 3 subgroups |
Retrospective transversal approach | Cirrus-HD | ↓ | ↓ |
|
|
| Lee et al. (2013) |
203 of 48 4 subgroups |
Prospective, institutional, transversal approach | SD-Spectralis | ↓ | No change |
|
|
| Unsal et al. (2014) |
151 of 40 3 subgroups |
Retrospective transversal approach | Optovue RT Vue 100-2 | ↓ | ↓ |
|
|
- CT = choroidal thickness; D = dioptres; DMO = diabetic macular oedema; DR = diabetic retinopathy; EDI = enhanced deep imaging; NPDR = non-proliferative diabetic retinopathy; OCT = optical coherence tomography, PDR = proliferative diabetic retinopathy; PRP = panretinal photocoagulation; SRD = serous retinal detachment; CMT = central macular thickness.
Choroidal thickness in diabetic macular oedema
Most authors relate DMO with decreased CT or decreased choroidal circulation (Table 1) (Nagaoka et al. 2004; Querques et al. 2012; Regatieri et al. 2012; Adhi et al. 2013b; Unsal et al. 2014).
Others do not confirm this finding, reporting no independent association between DMO and CT (Esmaeelpour et al. 2011; Vujosevic et al. 2012; Lee et al. 2013). At least in the study of Lee et al. (2013), reporting no change in CT with DMO, age was a confounding factor, as the authors were dealing with an aged sample. Moreover, there was a wide range of variation in age (32 – 78 years old) and the type of diabetes was not stated. Vujosevic et al. (2012) also found no correlation between CT and DMO but did not employ the EDI procedure which is more prone to disclose the choroidoscleral border. In addition, type 1 and type 2 diabetic eyes were included. In the report of Esmaeelpour et al. (2011), both eyes were enrolled and the sample in each subgroup was small.
By opposition to most authors, Hua et al. (2013) and Kim et al. (2013) found that CT increases in eyes with DMO and with the severity of DR (highest SFCT in PDR eyes). However, in the study of Hua et al. (2013) there was no control group. Surprisingly, Kim et al. (2013) found that the SFCT decreases in eyes with no DR and in eyes with NPDR. Ischaemia of the choriocapillaris in early diabetic choroidopathy would be responsible for such decrease. Nevertheless, in the more advanced stages of DR, where the authors found an increase in SFCT, ischaemia of the choriocapillaris is likely to be present as well. The authors theorized that an increased secretion of VEGF would account for the increased SFCT in the more advanced stages of DR. However, such increased secretion of VEGF is likely to occur in the early stages of ischaemia where SFCT is reduced. Perhaps more consistent is the possibility that the increase in the SFCT in the more advanced stages of DR was due to the presence of SMD. Serous macular detachment (SMD) might be related with increased CT, increased choriocapillaris permeability and outer BRB dysfunction. These alterations occur more frequently in naïve eyes with recent DMO, where SMD is more likely to occur as well. Therefore, SMD may be related to, or result from, an increased thickness of the choroid in early-onset DMO. Furthermore, SMD is present in CSC where CT is increased and patients are younger (Gemenetzi et al. 2010). Unfortunately, the work of Kim et al. (2013) has important drawbacks: no correction for age, no separation between type 1 and type 2 diabetes, inclusion of both eyes, no follow-up and small numbers in each group (20 to 45 eyes). Moreover, the heterogeneity of eyes enrolled is clearly expressed in the wide range of variation in CT in all groups. The differences between CT within each cohort (±58 to ±108 μm) are greater than the differences in the mean CT between cohorts (14 to 73 μm), except for the panretinal photocoagulation (PRP) group (124 μm). Interestingly, a reduction in CT in PRP-treated eyes was reported as in other studies (Regatieri et al. 2012; Lee et al. 2014; Unsal et al. 2014).
The role of prior focal laser photocoagulation should be considered as a confounding factor when comparing the effect on CT caused by DMO or by DMO treatment. Naïve eyes ought to be in a different cohort from eyes with history of treatment for DMO. One study discards focal laser as having effect on CT. However, there are serious limitations in that study: small cohort, no EDI procedure, scans only within 500 μm from the fovea where laser burns would unlikely be present, short follow-up and set of data from focal scans only, without determination of an area of CT (Adhi et al. 2013a).
In conclusion, most of the evidence hitherto available correlates DMO with a CT decrease.
The Influence of Treatment of DR on CT
Panretinal photocoagulation
Panretinal photocoagulation alters choroidal blood flow in patients with PDR and, in one study, it was reported to increase SFCT (Cho et al. 2013), while in most studies PRP was associated with a decrease in SFCT (Table 2) (Regatieri et al. 2012; Kim et al. 2013; Lee et al. 2014; Unsal et al. 2014; Zhang et al. 2015).
| Author/Treatments and drawbacks | Takahashi et al. (2008) | Regatieri et al. (2012) | Adhi et al. (2013a,b) | Kim et al. (2013) | Cho et al. (2013) | Hwang et al. (2014) | Zhang et al. (2015) | Lains et al. (2014) | Lee et al. (2014) | Unsal et al. (2014) | Yiu et al. (2014) | Sonoda et al. (2014) | Rayess et al. (2015) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRP | Increase in choroidal blood flow and volume | ↓ | ↓ | ↓ | ↑ | 0 | ↓ | ↓ | ↓ | ||||
| Anti-VEGF | ↓ | 0 | ↓ | ↓ | ↓ | ↓ | ↓ | 0 | ↓ | ||||
| Steroids | ↓ | ||||||||||||
| Drawbacks |
|
|
|
|
|
|
|
|
|
|
|
|
|
- D = dioptres; DMO = diabetic macular oedema; PDR = proliferative diabetic retinopathy; PRP = panretinal photocoagulation; VEGF = vascular endothelial growth factor. ↓ indicates decrease in choroidal thickness, 0 indicates no change in choroidal thickness.
A study evaluating choroidal blood flow in the foveal area one month after PRP, using a laser Doppler flowmetry technique, showed that PRP induces an increase in both choroidal blood flow and choroidal blood volume shortly after PRP (Takahashi et al. 2008). An increase in SFCT can be interpreted as either an increase in choroidal blood flow due to vasodilation or choroidal effusion induced by choroidal vascular obstruction from laser photocoagulation. Another study found SFCT thickening after PRP, but OCT measurements were made only one week after PRP and may reflect the effect of choroidal effusion rather than a real thickening of the choroid (Cho et al. 2013).
Evidence for a decrease in CT long after PRP, unbiased by choroidal effusion is more consistent. It was found a significant SFCT thinning 1 month after either PRP or anti-VEGF treatment (Lee et al. 2013). These data concerning choroidal thinning after PRP were confirmed by other studies (Regatieri et al. 2012; Kim et al. 2013; Unsal et al. 2014). A plausible explanation for this contradiction may be the time of measuring SFCT after PRP. Studies where SFCT is found to be thinner after PRP had treatment completed 3 months before the study, while studies where SFCT was found to be increased collected the measurements just 1 week to 1 month after the PRP treatment. Thus, increased blood flow, vasodilation and effusion in the retina and choroid might account for such increase. A possible confounding factor is that PDR may reduce CT along with PRP, thus contributing to SFCT reduction attributed to PRP (Adhi et al. 2013b; Unsal et al. 2014). However, PRP often follows shortly after the diagnosis of PDR; thus, it is unlikely that PDR masks the effect of PRP on CT. Long-term follow-up of PRP-treated eyes is crucial to evaluate the effect of PRP on CT. Indeed, a longitudinal study showed the pattern described by other studies: an increase in CT one week after PRP with a decrease in CT beneath the baseline at 12 weeks (Zhang et al. 2015).
Unchanged CT after PRP was also reported, but there were some possible confounding factors in the study: small sample, the eyes were previously treated for DMO, and the period of follow-up after PRP was very short (one month) (Hwang et al. 2014). As the first end-point to evaluate whether neovascularization regresses in PDR eyes treated with PRP is 3–4 months (Mohamed et al. 2007; Bressler et al. 2011), it would be more appropriate to check for a possible alteration in the choroid after PRP at least after a similar end-point.
Overall, most studies indicate that PRP decreases CT in the long term (after 3 months of treatment).
Intravitreal therapy
Most studies correlate the use of anti-VEGF agents to treat DMO with a decrease in CT (Table 2) (Regatieri et al. 2012; Kim et al. 2013; Lains et al. 2014; Lee et al. 2014; Unsal et al. 2005; Yiu et al. 2012; Rayess et al. 2015). Nevertheless, there are studies contradicting this finding.
One study found that anti-VEGF therapy does not affect SFCT. However, the number of eyes enrolled was small, the EDI protocol was not used and the choroidoscleral interface was not clearly identified in 36% of the eyes with DMO (Adhi et al. 2013b). Another study did not correlate CT with the treatment of DMO, either with focal laser or with anti-VEGF agents, but only with age. As this study found no correlation between the severity of DMO and the changes in CT, the authors concluded that the CT is not an important factor in the pathophysiology of DMO. However, this study was retrospective, lacked a control group, included both eyes and enrolled small numbers in each subgroup (Manjunath et al. 2013). The finding of no significant change in CT with anti-VEGF treatment of DMO eyes was also described in an uncontrolled, prospective, longitudinal study, within a 12-month follow-up period. Unfortunately, the number of eyes enrolled was rather small (n = 23) and there was no available data on disease duration or previous treatments (Giorno et al. 2010). A prospective study with a follow-up of only 3 months correlated a decrease in CT while treating DMO with steroids but not with bevacizumab. However, only one injection of either drug was used, the sample was small (n = 25 in the triamcinolone group and n = 26 in the bevacizumab group) and the study was short lasting (Sonoda et al. 2014).
Lains et al. (2014) found that anti-VEGF agents reduce CT either in PDR eyes with DMO or in NPDR/DMO eyes. Eyes were compared with the fellow non-treated eyes. Yiu et al. (2014) found that the choroid thins with anti-VEGF agents although with no cumulative effect of the number of injections given. Despite this study had a longitudinal profile, with a follow-up of six months, it had some drawbacks. Eyes were included until -6D without further correction for AL, and eyes were treated until three months with different treatments, including PRP, focal laser and intravitreal steroids. Additionally, it had a non-uniform treatment regimen with an average of 2.73 (range 1–6) anti-VEGF injections over the 6 months of follow-up and a small number of eyes treated (n = 33) (Yiu et al. 2014). The study of Rayess et al. (2015) was important due to its longitudinal profile, and by the cut-off chosen to consider a significant CT decrease (≥ 50 μm). The choroid is a vascular dynamic layer, and therefore, the thickness cut-off for the choroid has to be higher than that used for the retina. The study involved naïve eyes therefore excluding a previous effect on the CT of other therapies. The main drawbacks were the use of Snellen charts that do not consistently evaluate changes in the best-corrected visual acuity (BCVA) and a short follow-up (3 months).
In summary, there seems to be plenty of agreement that the use of anti-VEGF agents to treat DMO decreases CT (Lains et al. 2014; Lee et al. 2013; Yiu et al. 2012; Rayess et al. 2015), but there is some inconsistency with respect to whether CT may be taken as a prognostic factor for the treatment response.
The Role of CT as a Biomarker for Disease Progression or Treatment Response
There are arguments claiming that the thinning of the choroid may play a role in the development of DMO and that CT may be taken as a biomarker for DR progression or treatment response. Studies have been performed trying to correlate either the BCVA or the treatment with CT. Yiu et al. (2014) pointed that the decrease in CT does not seem to be correlated with the number of anti-VEGF injections or with the anatomic outcome in the retina. Hence, the decrease in CT does not seem to modulate or to be a good marker of DMO, seeming to be a secondary effect of treatment instead (Yiu et al. 2014).
A study by Lee et al. (2013) showed a decrease in CT with anti-VEGF therapy for DMO or DMO+PDR, but no correlation between CT changes and anatomic central macular thickness (CMT) or functional (BCVA) outcome or the number of injections given. However, a correlation was found between the decrease in CT and the first intravitreal injection of anti-VEGF, with no further reduction in CT after additional injections. This was different from the floor effect after three injections reported in AMD (Yamazaki et al. 2013) or after four injections in DMO (Yiu et al. 2014). As CMT increased and CT decreased after PRP, while CMT and CT decreased after anti-VEGF therapy, the authors concluded that CT was not a good biomarker of response to treatment. However, DMO worsening after PRP has been reported long ago (McDonald & Schatz 2013) and is known to be related with acute inflammation and release of cytokines (Nonaka et al. 2010). Central macular thickness (CMT) changes rapidly after PRP, probably due to an inflammation-driven process, while CT changes more gradually, probably due to an atrophic effect of PRP on the choroid. In fact, a longitudinal study involving PRP-treated eyes, with a follow-up of 3 months, confirmed that CT was not a good biomarker of anatomic or functional outcome shortly after PRP. Central macular thickness (CMT) increased very rapidly after PRP while CT increased at one week, but took up to 12 weeks to decrease beneath the baseline level (Zhang et al. 2001). The study of Lee et al. (2013) had important drawbacks, including a non-uniform treatment regimen (1–3 injections), a small number of eyes in the anti-VEGF-treated group (n = 31), short follow-up (3 months) and a low cut-off value for a significant CT decrease (5–14%).
The study from Yiu et al. (2014) compared DMO eyes treated with anti-VEGF versus DMO non-treated eyes. The study revealed that baseline DMO was more severe in treated than non-treated eyes (higher CMT and lower BCVA in treated eyes) while the CT was similar. Therefore, CT could not be taken as a biomarker for the severity of DMO. The choroidal thinning resulting from the anti-VEGF treatment neither correlated with the number of anti-VEGF injections nor with the improvement in BCVA or CMT. Hence, the authors concluded that CT was not a good marker for response to treatment either. The thinning of the choroid after the anti-VEGF therapy might just be a side-effect rather than a modulation of DMO. Moreover, eyes with DMO left untreated did not progress in severity for six months, which probably was related to a less severe disease at baseline, despite no differences in CT. More pronounced DMO with higher retinal thickness was an obvious choice to treat, but there was no difference in the baseline CT related to DMO severity (Yiu et al. 2014). There were, however, confounding factors involved: none of the eyes had history of previous anti-VEGF treatment, but several of them had a history of previous steroid treatment, focal laser, PRP, or a combination, which is related to long-standing DMO. Additionally, the fact that cumulative injections did not cause further choroid thinning seems to confirm the presence of long-standing previously treated DMO (Brown et al. 2013).
In the study of Rayess et al. (2015) enrolling naïve eyes with DMO, a higher CT prior to treatment was predictive of good response to the anti-VEGF treatment. Also, an enhanced decrease in SFCT was correlated with better anatomic (decrease in CMT ≥ 50 μm) and functional outcome (increase in BCVA of 1 line or more). Comparing both studies (Yiu et al. 2014; Rayess et al. 2015), the study of Rayess et al. (2015) was more consistent, as it involved a larger number of eyes and a uniform and defined schedule of treatment. Overall, the greater consistency of the study lies on involving naïve DMO eyes, without factors that could biased the conclusions, namely chronic DMO associated with PDR, PRP or previous steroid treatment. As aforementioned, the drawbacks of this study were the use of Snellen charts for assessing BCVA, a relatively wide range in age, the mixture of eyes treated with ranibizumab or bevacizumab and a short follow-up (Rayess et al. 2015). Evaluating a dynamic vascular structure would require the validation of those important findings throughout a longer period of time. However, the study enhanced an important issue: DMO is not a homogeneous entity as revealed by the RISE and RIDE and the RESTORE studies. Eyes with chronical DMO (≥2 years) failed to reach the same gain achieved by DMO eyes treated with anti-VEGF agents right from the start (Brown et al. 2013). In addition, a delay of 1 year in treatment took two additional years of treatment in order that DMO eyes achieved the same functional level of the DMO eyes promptly treated from baseline (Schmidt-Erfurth et al. 1993). Henceforth, the duration of DMO will be of crucial importance in considering outcomes and a thicker choroid may be related to early-onset DMO and thereafter better response to treatment and better prognosis.
In conclusion, a thicker choroid before treatment of DMO with anti-VEGF agents seems to be a good biomarker of treatment response and correlates better with a naïve or short-standing DMO. However, evidence hitherto available does not seem to correlate the variation of CT with treatment or with the anatomic or functional response of the retina.
Discussion
There has been an increasing interest and research on the choroid lately. The evaluation of the choroid by OCT concerns mainly its structure and thickness. Therefore, it must be clarified whether the thickness and structure of the choroid have importance for the evolution of metabolic and vascular diseases such as diabetes or DR. Pathology has established a clear DC. Hence, does the OCT technique have a capability similar to pathology of describing cut-off thicknesses for a DC? Moreover, do the alterations in CT have diagnostic and prognostic values, and might they be reliable biomarkers of the disease? In fact, there are conflicting results. Thus, an effort should be made to identify reasons for such discrepancy, so that henceforth a more consistent set of data may be obtained. The evaluation of CT leads to conflicting results because there is a poor control of variables, a wide range of approaches, different methods of collecting data, use of different devices, resulting in an inacceptable variability between studies. Several publications do not mention what kind of diabetic eyes they are dealing with nor do not take age into account, which is the most important variable capable of misleading data. It is of outmost importance to report whether type 1 or type 2 diabetic patients are enrolled. Choroidal thickness (CT) is known to be strongly dependent on age and type 1 diabetic patients are commonly younger than type 2, sometimes with more than two decades of difference, which means a difference in CT of about 32 μm (Margolis & Spaide 2009). Some studies do not correct data for age (Cho et al. 2013; Hua et al. 2013; Lee et al. 2013; Sim et al. 2002; Wei et al. 2003; Hwang et al. 1991; Lains et al. 2014; Rayess et al. 2015), other studies use mean age for comparison between groups (Vujosevic et al. 2012; Adhi et al. 2013b; Kim et al. 2013; Lee et al. 2013; Yiu et al. 2014) and others use age-matched groups (Esmaeelpour et al. 2011, 2012; Querques et al. 2012; Regatieri et al. 2012; Unsal et al. 2014). Age-matching often compares samples of different sizes, and median age may include eyes of very different age range. The age range may tell us more about age heterogeneity than the mean age. Moreover, as the control sample is usually smaller than the diseased sample, a wider variation related to age within the larger group might exist. This sort of transversal approach ignores interindividual variability of the choroid. Additionally, it is important to report whether measurements were taken in the morning or during the afternoon, as CT decreases about 8% from 9 a.m. to 5 p.m.
Focal or multifocal measurements have been widely used to access the CT profile, but choroidal area (CA) was also found to be a quantitative index for DC (Hua et al. 2013). Thus, focal measurements should be complemented with data from area measurements (Gupta et al. 2014). There are studies where SFCT was the only parameter evaluated (Adhi et al. 2013b; Cho et al. 2013; Lee et al. 2013; Xu et al. 2013; Rayess et al. 2015) while others used multifocal or area measurements (Esmaeelpour et al. 2011, 2012; Querques et al. 2012; Regatieri et al. 2012; Vujosevic et al. 2012; Hua et al. 2013; Kim et al. 2013; Hwang et al. 1991; Lains et al. 2014; Lee et al. 2013; Yiu et al. 2014). One study mismarked the boundaries to be measured, from the RPE to the choroid–scleral junction (Unsal et al. 2014). Some studies used the EDI procedure to evaluate the choroid–scleral junction (Querques et al. 2012; Hua et al. 2013; Kim et al. 2013; Lee et al. 2013, 2013; Xu et al. 2013; Hwang et al. 1991; Lains et al. 2014; Yiu et al. 2014; Rayess et al. 2015) while others did not (Esmaeelpour et al. 2011, 2012; Regatieri et al. 2012; Vujosevic et al. 2012; Adhi et al. 2013b; Unsal et al. 2014).
The devices used were of different types: Cirrus™ HD-OCT (Carl Zeiss Meditec AG, Dublin, CA, USA) (Regatieri et al. 2012; Adhi et al. 2013b), SD-NIDEK (Gamagori, Japan) (Vujosevic et al. 2012), swept-source OCT (SS-OCT, Topcon, Japan) (Esmaeelpour et al. 2011, 2012), RTV® (Optovue, Freemont, CA, USA) (Unsal et al. 2014) and SD-SPECTRALIS® (Heidelberg Engineering GmbH, Heidelberg, Germany) (Querques et al. 2012; Adhi et al. 2013b; Cho et al. 2013; Hua et al. 2013; Kim et al. 2013; Lee et al. 2013, 2013; Xu et al. 2013; Hwang et al. 1991; Lains et al. 2014; Yiu et al. 2014; Rayess et al. 2015), which have different characteristics, as follows: SD-Spectralis visualizes the choroid by the EDI procedure, RTV Optovue 100-2 using the deep choroidal imaging (DCI) mode, SD-Nidek RS-3000 by averaging 50 B-scans in a single raster line and using the ‘toggle switch’ option, and SS-OCT using a longer wavelength. All these devices have a similar depth resolution of about 5 μm. The measurements with the different devices seem to be strongly correlated (Branchini et al. 2012). Nevertheless, the Cirrus™ HD-OCT 500 lacks eye tracking, and hence, the need of averaging the images with this system makes it difficult to improve the signal-to-noise ratio, and therefore, the visualization of the choroid–scleral border is worse, mainly when there is an increase in CMT (Manjunath et al. 2005). These difficulties were largely overcome by the newer version, the Cirrus™ HD-OCT 5000 (Hardin et al. 2014). SS-OCT seems to bring an enhanced visualization of the choroid, but it has been reported to be as accurate as the SD-OCT EDI procedure (Waldstein et al. 2000). However, another study reported that the CT measured with a SS-OCT was thicker than that measured with a SD-OCT. Despite good reproducibility, CT measurements should not be compared between the two instruments (Matsuo et al. 1980).
Another issue was related to HbA1c levels. HbA1c was clearly stated in some studies (Esmaeelpour et al. 2012; Cho et al. 2013; Kim et al. 2013; Lee et al. 2013, 2013; Hwang et al. 1991; Lains et al. 2014; Unsal et al. 2014; Yiu et al. 2014), while absent in others (Esmaeelpour et al. 2011; Regatieri et al. 2012; Adhi et al. 2013b; Hua et al. 2013; Rayess et al. 2015) or not clearly stated to each group under study (Querques et al. 2012; Vujosevic et al. 2012).
Taking into account all the listed confounding factors, and as the choroid keeps a steady morphology in the same eye throughout time, it would be advisable to compare differences dependent on disease, treatment and time within the same eye, avoiding the individual variability in CT (Yiu et al. 2014).
There is evidence that the choroid thins with DMO. As aforementioned, there are several drawbacks in the studies that are not in accordance with this finding. Nevertheless, due to the individual variability of the choroid, a longitudinal approach is mandatory to have a more decisive conclusion. Transversal approaches do not avoid individual variability. Another important point to be considered is the influence of treatment. There is a large body of evidence indicating that the choroid thins after PRP (Regatieri et al. 2012; Adhi et al. 2013b; Kim et al. 2013; Lee et al. 2013; Unsal et al. 2014). There is also large evidence showing that the choroid thins with anti-VEGF therapy (Lains et al. 2014; Lee et al. 2014; Yiu et al. 2014; Rayess et al. 2015). Concerning CT as biomarker for outcome, the study of Rayess et al. was the first one proving a relationship between baseline SFCT and outcome (Rayess et al. 2015), being in opposition to previous studies (Lee et al. 2014; Yiu et al. 2014). The strength of the study lies in dealing with naïve eyes and in its longitudinal profile. This results need to be confirmed by future longitudinal studies with naïve eyes or, at least, with naïve eyes and already treated eyes in separate cohorts. Data from the RISE and RIDE trials (Brown et al. 2013) report that long-term DMO has a worse anatomical and functional response to anti-angiogenic agents. Hence, as CT tends to decrease with the duration of DMO, a thicker choroid prior to treatment would probably be a marker of a short-standing DMO and consequently a marker of a better response. Therefore, the time of onset of DMO should be stated in future studies.
In the future, transversal matching should be replaced whenever possible by longitudinal analysis. That requires a longer time of follow-up, but it will tell more about the evolution of CT with DR or DMO and will avoid age-related changes as well as individual variability. Moreover, the cut-off for CT ought to be higher than that for CMT. The choroid is not a neurosensorial continuous tissue; it is rather a vascular bed with a wider range of physiological differences. Hitherto, differences in CT taken as significant have been approximately 10%, the value taken as significant for the retina (Polito et al. 2005), or at times even less (Farias et al. 2014). Perhaps only a bigger difference ought to be considered as significant.
Conclusion
In conclusion, in order to get reliable data on CT, future studies ought to accomplish certain requirements, such as: (1) to get a clear identification of the choroid–scleral junction, (2) to collect multifocal scans in the horizontal and vertical lines encompassing the fovea supplemented with data from area measurements, (3) to include age and refractive correction of CT, (4) to mention the history of the evolution of the disease, (5) to state HbA1c values and microalbuminuria whenever possible, (6) to include only one eye per patient in transversal analysis to discard individual variability or when including both eyes correcting data with multilevel mixed models (7) to make a longitudinal approach, wherein both eyes may be included and (8) to use longer follow-up intervals.
Methods of Literature Search
Studies were identified by systematically search of the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) and Association for Research in Vision and Ophthalmology (ARVO) Meeting Abstracts website http://arvojournals.org/solr/searchresults.aspx?q=choroid%20thickness%20diabetic%20macular%20oedema&restypeid=1. Key words used were as follows: Diabetic macular edema; optical coherence tomography; choroidal thickness; subfoveal choroidal thickness; diabetic retinopathy. All matching study abstracts were reviewed, and appropriate studies were obtained in full for complete review and inclusion. Additional articles were identified from bibliographies of the references. The search used the English language in PubMed and ARVO databases only. Additional search was made using the Spanish and French languages in the MedlinePlus database but yielded no relevant articles.




