Changes in choroidal thickness after vitrectomy for epiretinal membrane combined with vitreomacular traction
Abstract
Purpose
To compare choroidal thickness after vitrectomy between epiretinal membrane (ERM) with and without vitreomacular traction (VMT).
Methods
In this retrospective study, 228 consecutive participants with ERM who underwent vitrectomy were categorized into two groups according to the presence of VMT on spectral domain–optical coherence tomography: VMT group (ERM with VMT, n = 21) and non-VMT group (ERM without VMT, n = 207). The primary outcome was the mean subfoveal choroidal thickness (SFCT) at baseline, and at 3 and 6 months postsurgery.
Results
At baseline, the prevalence of VMT in eyes with ERM was 9.6% (21/228), and mean SFCT was greater in the VMT than in the non-VMT group (270.3 ± 93.4 vs. 223.7 ± 82.1 μm; p = 0.015). After surgery, mean SFCT decreased in the VMT group (241.7 ± 92.3 μm at 3 months and 228.8 ± 86.4 μm at 6 months; p < 0.001), but remained unchanged in the non-VMT group (223.6 ± 78.9 μm at 3 months and 223.3 ± 82.6 μm at 6 months; p = 0.696). There were no differences in mean SFCT between the groups at 3 and 6 months after surgery (p = 0.339 and p = 0.772, respectively).
Conclusion
Choroidal thickness was greater in ERM eyes with than without VMT possibly due to direct anteroposterior traction on the retina and choroid, increased vascular endothelial growth factor associated with stress on retinal pigment epithelial cells and inflammation. After vitrectomy, mean SFCT reduced in the eyes with VMT, but not in those without VMT.
Introduction
Epiretinal membrane (ERM) is characterized by fibrocellular proliferation on the inner retinal surface (Bu et al. 2014; Scheerlinck et al. 2015). Epidemiologic studies demonstrate a marked discrepancy in the prevalence of ERM from 1.06% to 28.9% among different ethnic groups (You et al. 2008; Ng et al. 2011; Yiu et al. 2013). Although the pathogenesis of ERM is not completely understood, posterior vitreous detachment (PVD) is regarded as one of the most important pathogenic factors, because previous research revealed that ERM was associated with complete PVD at the time of diagnosis in 70% of patients (Kishi & Shimizu 1994). Various hypotheses have been proposed regarding the generation of ERM. One is that microscopic disruptions in the internal limiting membrane (ILM) during the development of PVD might induce migration and proliferation of retinal glial cells (Foos 1974). However, transmission electron microscopic study showed that defects in the ILM rarely occurred (Gandorfer et al. 2011). A subsequent hypothesis is that hyalocytes in cortical vitreous remnants produced during PVD might be a factor in the generation of ERM (Sebag 2004).
Another theory regarding the generation of ERM involves vitreoretinal traction (Kampik 2012; Zhao et al. 2013; Bu et al. 2014). Anteroposterior (AP) traction can promote the expression of growth factors including basic fibroblast growth factor and vascular endothelial growth factor (VEGF) in Müller cells and retinal pigment epithelium (RPE) cells (Seko et al. 1999; Lindqvist et al. 2010). These growth factors may induce the proliferation of hyalocytes and extracellular matrix production by them. Hence, vitreomacular traction (VMT), which may occur with ERM, may be a factor associated with the pathogenesis or progression of ERM (Kampik 2012; Zhao et al. 2013; Pierro et al. 2014). However, the role of VMT in ERM is not fully understood, and the characteristics and surgical outcomes of ERM combined with VMT have not been compared with those of ERM without VMT in previous studies.
Previous studies demonstrate differences in choroidal thickness in various diseases including pathologic myopia, diabetic retinopathy, neovascular age-related macular degeneration (AMD), polypoidal choroidal vasculopathy (PCV) and central serous chorioretinopathy (CSCR) (Chung et al. 2011; Kuroda et al. 2013; Gupta et al. 2015; Lee et al. 2015). Alterations can be observed after treatments such as anti-VEGF treatment for neovascular AMD and PCV, photodynamic therapy for CSCR and panretinal photocoagulation for proliferative diabetic retinopathy, and this may help in the estimation of treatment response (Kim et al. 2013; Alkin et al. 2014; Kang et al. 2014).
This study aimed to investigate changes in subfoveal choroidal thickness (SFCT) for ERM without VMT and ERM with VMT from the baseline to 6 months after surgery. Additionally, we compared the baseline characteristics and surgical outcomes including visual acuity (VA), intraocular pressure (IOP) and mean central subfield thickness (CSFT) between the two groups.
Patients and Methods
This retrospective study included 228 consecutive patients who underwent phacovitrectomy for ERM and cataract at the Vitreoretinal Service Clinic of Yonsei University Medical Center from January 2011 through October 2014. Patients who were followed up for at least 6 months after the surgery were included in this study. Patients who had VMT syndrome without ERM, CSCR, AMD, diabetes mellitus, uveitis, glaucoma, or retinal vessel occlusion, or who had undergone laser photocoagulation or previous intraocular surgery, were excluded from this study. This study was approved by the Institutional Review Board of Yonsei University College of Medicine and was conducted in accordance with the tenets of the Declaration of Helsinki.
Patients who had ERM with VMT on spectral domain–optical coherence tomography (SD-OCT) were categorized as the VMT group and patients who had ERM without VMT on SD-OCT were categorized as the non-VMT group. Vitreomacular traction (VMT) was defined as an attachment of vitreous cortex within a 3-mm radius centred on the fovea with intraretinal structural changes, according to the classification of the International Vitreomacular Traction Study (IVTS) group (Fig. 1) (Duker et al. 2013).

Surgical procedures
All patients underwent 25-gauge sutureless pars plana vitrectomy combined with cataract surgery because of loss of VA or metamorphopsia. A single vitreoretinal surgeon (HJK) operated on all the patients using a Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA). Cataract surgery was performed by phacoemulsification and insertion of a one-piece intraocular lens (AcrySof IQ; Alcon Surgical, Inc., Fort Worth, TX, USA) before vitrectomy. When complete PVD was not present, PVD was induced during surgery and extended to the major retinal vascular arcade. After core vitrectomy, the surgeon used microforceps to peel the ERM and ILM over the macular area after staining the ILM with 0.5% indocyanine green. No intraocular tamponade including gas or silicone oil was used. Postoperative antibiotic and steroid (Vigamox and PredForte) were used in all patients for 2 months.
Outcome measurements
At the baseline, all patients underwent complete ophthalmic examinations including best-corrected visual acuity (BCVA), intraocular pressure (IOP), mean spherical equivalent (SE) and axial length (AL) measurements; slit-lamp biomicroscopy; indirect ophthalmoscopic examination; colour fundus photography; and SD-OCT (Spectralis HRA+OCT; Heidelberg Engineering GmbH, Dossenheim, Germany) with enhanced depth imaging (EDI). Best-corrected visual acuity (BCVA) was measured using the Snellen chart and converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analysis. Intraocular pressure (IOP) was measured using Goldmann applanation tonometry, and the AL was measured using IOLMaster (Carl Zeiss Meditec, Jena, Germany). The SFCT defined as the vertical distance from the outer edge of the hyper-reflective RPE to the hyper-reflective line of the choroid–sclera junction was measured manually using the caliper tool in the OCT Heidelberg Eye Explorer software (Heidelberg Engineering). The CSFT within an inner 1-mm diameter circle corresponding to the Early Treatment Diabetic Retinopathy Study (ETDRS) grid was measured using a thickness map after adjusting the fovea centre. The measurements for SFCT and CSFT were calculated by two independent retinal specialists (ECK and KHL) who were masked to the clinical information, and the mean values were used in statistical analysis. Postoperatively, BCVA and IOP were obtained at 1 week and 1, 3, and 6 months after surgery and SD-OCT with EDI was performed at 3 and 6 months after surgery to measure SFCT and CSFT. Postoperative complications such as severe hypotony (<6 mmHg), severe IOP elevation (>30 mmHg), recurrent ERM, endophthalmitis, and retinal detachment were evaluated.
Statistical analysis
All statistical values were analysed using spss software (version 18.0; SPSS Inc., Chicago, IL, USA) for Windows. Comparison of the qualitative data including sex and the presence of hypertension was performed using a chi-square test. Fisher's exact test was performed to compare the incidence of surgical complications including severe hypotony and hypertony. The preoperative quantitative data including age and the AL, mean SE, BCVA, IOP, CSFT and SFCT were compared using independent Student's t-tests. To compare the continuous variables including BCVA, CSFT and SFCT, repeated measures analysis of variance (repeated measures anova) and post hoc analysis with Bonferroni correction was performed. Interobserver agreement on CSFT and SFCT measurements was evaluated using the intraclass correlation coefficient (ICC). An excellent agreement in measuring CSFT and SFCT between the two independent observers was noted (ICC = 0.892 and 0.824, respectively). A p-value less than 0.05 was considered statistically significant in this study.
Results
Baseline characteristics
Two hundred and twenty-eight eyes that had undergone vitrectomy and cataract surgery for ERM and cataract were enrolled in this study. There were 21 eyes in the VMT group and 207 eyes in the non-VMT group, and baseline characteristics of the two groups are shown in Table 1. The age, gender, BCVA, IOP, AL, mean SE, mean CSFT and the presence of hypertension were not significantly different between the two groups (p > 0.05). On the other hand, mean SFCT at the baseline was significantly higher in the VMT group compared with the non-VMT group (270.3 ± 93.4 vs. 223.7 ± 82.1 μm; p = 0.015).
| Characteristic | Group | p-value | |
|---|---|---|---|
| VMT | non-VMT | ||
| Number of eyes (%) | 21 (9.6) | 207 (90.4) | |
| Gender (M/F) | 7/14 | 70/137 | 1.000 |
| Mean age ± SD (years) | 63.0 ± 8.2 | 65.8 ± 8.1 | 0.125 |
| BCVA ± SD (logMAR) | 0.453 ± 0.286 | 0.382 ± 0.267 | 0.288 |
| IOP ± SD (mmHg) | 13.2 ± 3.6 | 13.5 ± 3.0 | 0.674 |
| Anxial lenghth ± SD (mm) | 23.41 ± 0.81 | 23.62 ± 1.40 | 0.500 |
| Mean spherical equivalent ± SD (Diopter) | 0.61 ± 1.03 | −0.30 ± 2.75 | 0.151 |
| Mean SFCT ± SD (μm) | 270.3 ± 93.4 | 223.7 ± 82.1 | 0.015 |
| Mean CSFT ± SD (μm) | 493.2 ± 131.6 | 453.8 ± 91.4 | 0.073 |
| Hypertension (%) | 6 (28.6) | 99 (47.8) | 0.110 |
- BCVA = best-corrected visual acuity; CSFT = central subfield thickness; IOP = intraocular pressure; logMAR = logarithm of the Mminimum Aangle of Rresolution; SD = standard deviation; SFCT = subfoveal choroidal thickness.
Visual acuity
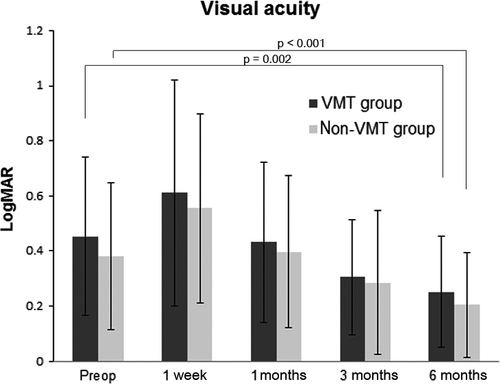
In the VMT group, the mean logMAR VA was 0.453 ± 0.286 preoperatively, 0.611 ± 0.411 at 1 week, 0.433 ± 0.291 at 1 month, 0.305 ± 0.212 at 3 months and 0.252 ± 0.202 at 6 months after the surgery. In the non-VMT group, the mean logMAR VA was 0.382 ± 0.267 preoperatively and 0.555 ± 0.344 at 1 week, 0.396 ± 0.276 at 1 month, 0.285 ± 0.261 at 3 months and 0.205 ± 0.189 at 6 months after the surgery. There was no significant difference between the two groups at all visits (p > 0.05). The logMAR VA was improved compared with that at baseline in both the VMT and the non-VMT groups at 6 months after the surgery (p = 0.002 and p < 0.001, respectively; Fig. 2).
Subfoveal choroidal thickness
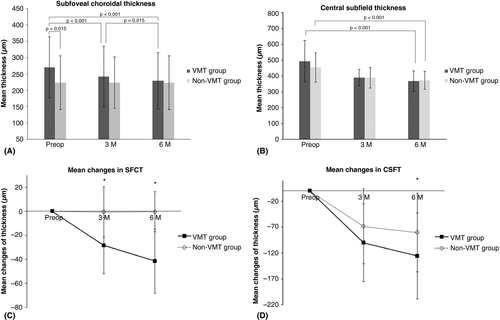
Although the mean SFCT was higher in the VMT group at baseline, it showed no difference between the two groups at 3 months and 6 months after the surgery (p = 0.339 and p = 0.772, respectively). In the VMT group, the mean SFCT decreased from 270.3 ± 93.4 μm at the baseline to 241.7 ± 92.3 μm at 3 months and 228.8 ± 86.4 μm at 6 months after the surgery (p < 0.001; Fig. 3A). In the non-VMT group, the mean SFCT showed no temporal changes (223.7 ± 82.1 μm at the baseline, 223.6 ± 78.9 μm at 3 months and 223.3 ± 82.6 μm at 6 months after the surgery; p = 0.696). The mean changes in SFCT from the baseline were significantly greater in the VMT group compared with the non-VMT group [−28.50 ± 23.68 vs. −0.70 ± 20.97 μm at 3 months (p < 0.001); −41.57 ± 26.62 vs. −0.48 ± 16.62 μm at 6 months (p < 0.001)] (Fig. 3C).
Central subfield thickness
There was no difference between the mean CSFT between the two groups at 3 months and 6 months after the surgery (p = 0.946 and p = 0.648, respectively). In the VMT group, the mean CSFT reduced from 493.2 ± 131.6 μm before surgery to 390.1 ± 51.1 μm at 3 months and 367.3 ± 64.4 μm at 6 months after the surgery (p < 0.001). In the non-VMT group, the mean CSFT continuously decreased from 453.8 ± 91.4 μm at the baseline to 389.0 ± 64.6 μm at 3 months and 373.4 ± 56.9 μm at 6 months after the surgery (p < 0.001; Fig. 3B). The mean change in CSFT from the baseline was not different between the VMT and non-VMT groups at 3 months (−100.35 ± 75.06 vs. −68.53 ± 72.57 μm; p = 0.086), but significantly greater in the VMT group at 6 months after the surgery (−125.90 ± 82.99 vs. −80.45 ± 75.85 μm; p = 0.010; Fig. 3D).
Postoperative complications
One eye with severe hypotony (IOP <6 mmHg) at 1 week after surgery and one eye with severe hypertony (IOP >30 mmHg) at 3 months after the surgery were noted in the non-VMT group, but there were no such findings in the VMT group. The incidence of severe hypertony or hypotony was not different between the two groups (p = 1.000). Endophthalmitis, intraoperative iatrogenic retinal break, postoperative wound leakage, retinal detachment and recurred ERM were not observed in either group during the 6-month follow-up period.
Discussion
Our retrospective comparative case series investigated changes in choroidal thickness after vitrectomy in eyes with ERM with and without VMT. Our study showed that the mean SFCT was greater at the baseline in the VMT group than in the non-VMT group. In the VMT group, the mean SFCT decreased gradually from the baseline to 6 months after vitrectomy with ERM removal and ILM peeling (Fig. 4). However, in the non-VMT group, it remained unchanged from the baseline to 6 months after the surgery. The mean changes in SFCT were much greater in the VMT group compared with the non-VMT group at 3 months and 6 months after the surgery. Moreover, reduction in CSFT, compared with baseline, was significantly greater in the VMT group than non-VMT group at 6 months after surgery.
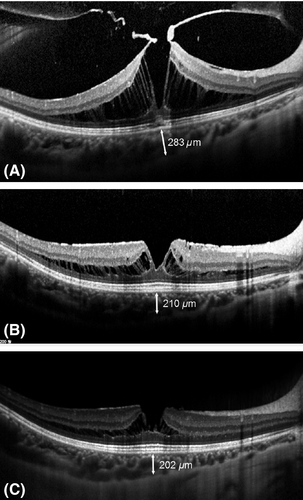
The age, AL and mean SE, which could be associated with the choroidal thickness, were not statistically different between the two groups (Ding et al. 2011; Gupta et al. 2015). However, the mean SFCT was greater in the VMT group than in the non-VMT group before surgery, and this led us to speculate that VMT could induce thickening of the choroid. To the best of our knowledge, there is no previous report that the presence of VMT might be associated with increased choroid thickness. Vitreomacular traction (VMT) might lead to increased thickness of the choroid through various possible mechanisms. First, mechanical forces in VMT may pull the choroid apart. Vitreomacular traction (VMT) is typically in an anteroposterior direction, whereas ERM is generally associated with tangential traction that distorts configuration of the inner retinal surface (Sebag 2009; Jackson et al. 2013). Moreover, one study revealed that α-SMA (smooth muscle actin)-positive cells were more frequently observed in specimens with VMT compared with those with ERM with complete PVD (Zhao et al. 2013). The α-SMA in myofibroblast-like cells is known as one of the most important elements involved in contraction of the ERM, and greater numbers of these cells in eyes with VMT might have led to increased AP traction in the VMT group (Bu et al. 2014). The AP traction on the retina can affect the RPE and choroid according to Newton's third law (every action has an equal and opposite reaction) when the RPE is attached to the retina with sufficient force (Stefansson 2009). On the contrary, subretinal fluid between the RPE and retina might disrupt the AP traction on the choroid. In fact, there was no patient with subretinal fluid in the VMT group in our study. Additionally, the AP forces in VMT might have been exacerbated when saccadic eye movement occurred. Another possible mechanism is that VMT can cause mechanical stretching of the RPE, and this stress may increase the level of VEGF in RPE cells (Reese et al. 1967; Kinoshita et al. 2014). Elevation of VEGF induces choroidal vascular hyperpermeability and subsequent leaking of oncotic proteins into the interstitial space of the choroid (Nickla & Wallman 2010). Third, chronic and low-grade inflammation may be present at the choroidal level in eyes with VMT. Elimination of these effects of VMT by vitrectomy might have resulted in the marked decrease in SFCT in the VMT group at 6 months after the surgery in our study.
On the contrary, there was no change in mean SFCT from the baseline to 6 months after the surgery in the non-VMT group. In other words, ERM alone, which is associated with tangential traction without AP traction, may not affect choroidal thickness. A recent study showed decreased choroidal thickness at 3 months after vitrectomy with ILM peeling (Kang & Koh 2015). However, the sample size in the study was small (21 eyes in 21 patients), and the presence or absence of VMT was not described. Therefore, the reduced choroidal thickness after vitrectomy might have been caused by the inclusion in the study of patients who had ERM with VMT.
The VMT group tended to have greater CSFT compared with the non-VMT group, even though there was no statistical significance (493.2 ± 131.6 vs. 453.8 ± 91.4 μm; p = 0.073). The AP traction in VMT might explain the increased mean CSFT in the VMT group. Vitreomacular traction (VMT) is associated with many vitreoretinal diseases including diabetic macular oedema (DME) (Akbar Khan et al. 2015), macular hole (Steel & Lotery 2013) and neovascular AMD (Green-Simms & Bakri 2011; Michalewska et al. 2015). Vitreomacular traction (VMT) could pull the retina anteriorly and reduce the pressure in the interstitial space of the retina. As a result, an influx of the fluid from the capillary networks can occur, according to Starling's law of hydrostatic pressure (Simpson et al. 2012). Previous studies showed that surgical removal of VMT might help to reduce macular thickness in eyes with DME and neovascular AMD (Shah & Haller 2012; Golan & Loewenstein 2014). Likewise, greater mean changes in CSFT from the baseline to 6 months after the surgery in the VMT group might have been related to alleviation of VMT by vitrectomy. It is possible that greater reductions in CSFT may be expected after vitrectomy for ERM if VMT is present.
Visual acuity (VA) improved after vitrectomy for ERM in both groups, and this result was consistent with the findings of previous studies (Dawson et al. 2014). Although cataract surgery might have had an effect on VA, a previous study found no clinically significant difference in improvement of VA between vitrectomy alone and vitrectomy combined with cataract surgery (Aung et al. 2013). Our study demonstrated no different surgical outcome in VA between the two groups.
Vitreomacular traction (VMT) has previously been reported in patients with ERM; however, the prevalence of ERM combined with VMT was not reported in previous studies (Jackson et al. 2013). Our results showed that 9.6% (21/228 eyes) of the eyes with ERM had VMT at baseline, and a further study is needed to evaluate the association between VMT and ERM.
The present study has several limitations. The cataract surgery combined with vitrectomy could have affected the surgical outcomes including VA, SFCT and CSFT. However, only patients who had undergone vitrectomy with cataract surgery were enrolled in this study; therefore, the comparison of surgical outcomes between the two groups would not have been affected by the type of surgical procedure. Furthermore, we did not include other examinations such as fluorescein angiography or indocyanine green angiography, which can help us to understand the status of choroidal circulation. Another limitation is that there is no definite evidence that SD-OCT can detect all cases of VMT, and this might have affected the classification of the patients into the two groups. This study is also limited by the small number of eyes with VMT (n = 21) compared with those without VMT (n = 207). Additionally, we evaluated surgical outcomes including VA, CSFT and SFCT during a relatively short follow-up period (6 months) after the surgery, and postoperative complications such as recurrence of ERM could not be reliably evaluated in this time period.
In conclusion, the mean SFCT was higher in ERM with VMT, compared with ERM without VMT, and surgical resolution of VMT with the use of vitrectomy resulted in normalization of the thickened choroid. However, eyes with ERM but without VMT did not have a thickened choroid preoperatively, and vitrectomy for ERM without VMT did not change the choroidal thickness up to 6 months after surgery. Furthermore, increased mean CSFT and loss of VA were improved by vitrectomy from the baseline to 6 months after surgery independent of the presence of VMT.




