Comparative analysis of corneal measurements obtained from a Scheimpflug camera and an integrated Placido-optical coherence tomography device in normal and keratoconic eyes
The authors would like to acknowledge the statistical expertise kindly provided by Associate Prof. Peter Petocz of the Department of Biostatistics, Macquarie University, Sydney. No author has a financial or proprietary interest in any material or method mentioned.
Abstract
Purpose
To assess the agreement between a Scheimpflug camera (Pentacam) and a combined Placido-optical coherence tomography device (Visante OMNI) in measuring corneal curvature, thickness and elevation values in normal and keratoconic eyes.
Methods
Corneal measurements of 110 normal eyes (one eye per subject) and 70 keratoconic eyes were obtained from both devices and compared. Agreement was determined using the Bland–Altman analysis 95% limits of agreement (LoA).
Results
The Pentacam measured significantly greater keratometry readings in the flattest (K1) and steepest meridians (K2) in normal and keratoconic eyes. The 95% LoA in normal eyes were −0.32 to 0.59 dioptres (D) (K1) and −0.41 to 0.74 D (K2). In keratoconic eyes, the 95% LoA were −1.35 to 1.92 D (K1) and −1.38 to 1.99 D (K2). The Pentacam recorded significantly higher central corneal thickness (CCT) values in both groups of eyes. The 95% LoA were −4.31 to 39.89 microns (μ) and −12.92 to 41.35 μ in normal and keratoconic eyes, respectively. Pentacam anterior and posterior corneal elevations were significantly greater in both groups of eyes. The devices demonstrated excellent repeatability and reproducibility for corneal curvature and thickness but not elevation measurements.
Conclusions
The Pentacam measured significantly greater corneal curvature, thickness and elevation values compared to the Visante OMNI in normal and keratoconic eyes. The devices agree moderately for anterior corneal elevations in normal eyes and do not appear to be interchangeable for corneal measurements in clinical practice.
Introduction
Refractive surgery is an increasingly popular option for correction of ametropia. Screening patients to ensure their candidature for surgery and to exclude keratoconus or other corneal anomalies is of paramount importance. This involves obtaining precise corneal curvature, thickness and elevation measurements. Furthermore, highly repeatable and reproducible measurements are mandatory to observe time-related corneal changes.
Keratoconus is an asymmetric corneal disorder characterized by progressive corneal thinning and protrusion (Rabinowitz 1998). There is great emphasis on early detection and subsequent management of these cases using newer refractive surgical options (Park & Gritz 2013; Raiskup & Spoerl 2013). Accurate corneal imaging is therefore essential to diagnose keratoconus, facilitate patient selection and study the efficacy of these procedures.
Conventional corneal topography systems have been based on the Placido principle that measures the anterior corneal curvature (Mejía-Barbosa & Malacara-Hernández 2001). Clinical studies have demonstrated that examination of the posterior corneal surface can often reveal pathology in eyes that have completely normal anterior curvature (Schlegel et al. 2008). Elevation-based Scheimpflug imaging has the ability to analyse both anterior and posterior corneal surfaces and thus offers an advantage over isolated placido topography (Chen & Lam 2007; Schlegel et al. 2008; Miyata et al. 2013).
Ultrasound pachymetry is currently considered the gold standard for corneal thickness measurement. Being a contact technique, it is associated with certain inherent disadvantages (Marsich & Bullimore 2000; Miglior et al. 2004). The Pentacam (Oculus Inc, Wetzlar, Germany) is a rotating Scheimpflug-based camera that provides a reliable assessment of the anterior and posterior corneal surface and corneal thickness (Kawamorita et al. 2009; McAlinden et al. 2011a). Optical coherence tomography (OCT) is another established technology for imaging the anterior segment and providing non-contact corneal thickness measurements (Li et al. 2006)
The Visante OMNI (Carl Zeiss Meditec, Jena, Germany) is a newer corneal imaging device. This is a hybrid created by linking the Placido-based topographer (Atlas) to an optical coherence tomography device (Visante OCT). The OMNI integrates Atlas corneal topography data with the OCT corneal thickness data and provides, in addition, posterior corneal elevation data (Srivannaboon et al. 2012). The purpose of this study is to assess and compare corneal measurements in normal and keratoconic eyes generated by these imaging systems using different technologies.
Patients and Methods
Study participants
This study was conducted at the Macquarie University Ophthalmology Clinic, Australian School of Advanced Medicine, Sydney. The study protocol was approved by the medical ethics committee and adhered to the principles proposed by the declaration of Helsinki. One hundred and ten normal eyes (110 subjects) and 70 keratoconic eyes (40 subjects) were included after obtaining a written informed consent.
Normal eyes were associated with no ocular disease, previous ocular surgery or trauma, no contact lens use and had refractive errors ≤1.00 dioptres (D) (spherical equivalent). Keratoconic eyes were diagnosed based on the presence of typical corneal steepening and one or more of the following signs: corneal thinning, Fleischer's ring, Vogt striae and apical scarring (Maeda et al. 1994; Rabinowitz 1998). Eyes with previous corneal hydrops or corneal surgery were excluded from the study. Contact lens wearers were instructed to avoid wearing lenses for 72 hr prior to measurements.
Measurements
Each individual was measured with the Pentacam and the Visante OMNI in a random sequence by a single experienced operator. A subset of 20 normal subjects (20 eyes) underwent repeat measurements, once by the same operator and thereafter by another experienced operator to assess intra-operator repeatability and interoperator reproducibility. For repeat measurements, the participants were repositioned and measurements were taken approximately 3–5 min apart.
The following corneal parameters were measured from both Pentacam and the Visante OMNI: corneal dioptric powers (simulated K readings) in the flattest (K1) and steepest (K2) meridians, central corneal thickness (CCT) and minimum corneal thickness (MCT). Corneal elevations were read in relation to a best-fit toric ellipsoid reference (BFTE 8 mm) at the thinnest point of the cornea.
Pentacam
The Pentacam device uses a measurement wavelength of 475 nm (blue light-emitting diode), and there are 25 000 measurement points. A Scheimpflug high-resolution camera takes multiple slit images of the anterior segment in <2 seconds while rotating 180° around the eye. Measurements were obtained with subjects in a sitting position looking at the fixation target according to the manufacturer's instructions. For this study, the 25-images-per-scan mode and the auto-measurement mode were chosen. Only scans that the Pentacam's ‘quality specification’(QS) function determined as ‘OK’ were included for analysis.
Visante OMNI
Initially, corneal measurements were performed on each eye of the subject using the Atlas corneal topographer (version 3.0, Carl Zeiss Meditec, Jena, Germany). The subject's information and anterior corneal surface data (7960 data points) are automatically transferred to the Visante OCT via the network link. Then, the subject was positioned at the Visante OCT station to measure corneal thickness (global pachymetry: 2048 data points) using the auto corneal vertex alignment. Posterior corneal elevation and curvature data were derived by the system software by integrating the anterior corneal surface data derived from the Atlas with the corneal thickness data from the Visante OCT through precise corneal vertex alignment (Srivannaboon et al. 2012). The anterior and posterior corneal elevations were obtained by placing the cursor at the thinnest point.
Statistics
For each normal subject, the data from one randomly selected eye (Microsoft excel randomization function) were included for analysis. Keratoconus being an asymmetric disorder, data from both eyes were used. Statistical analysis was performed using SPSS software (version 19.0; SPSS Inc, Chicago, IL, USA). The measurements obtained from both devices were described as mean ± standard deviation (SD), and the difference between these values was examined using a paired t-test.
Bland–Altman analysis was performed to assess the 95% limits of agreement (mean of the difference ± 1.96 times standard deviation) between both systems (Bland & Altman 1986). The level of agreement between two devices is determined by the magnitude of these limits with lower values indicating better agreement and vice versa. This judgement regarding the limit at which it is acceptable to use the two devices interchangeably is a clinical decision (McAlinden et al. 2011b).
Repeatability and reproducibility were determined using the intraclass correlation coefficients (ICCs). The ICCs range from 0 to 1 and are interpreted as follows: <0.75 (poor agreement), 0.75 to 0.90 (moderate agreement) and >0.90 (good agreement) (McGraw & Wong 1996). For all analysis, a p value of <0.05 was considered statistically significant.
Results
One hundred and ten eyes of 110 normal subjects (49 males, 61 females) and 70 eyes of 40 keratoconic subjects (22 males, 18 females) were included. The mean age of normal subjects was 32.49 ± 13.22 years (range 15–72 years). The mean age of keratoconic subjects was 33.74 ± 12.35 years (range 17–63 years).
Corneal curvature
Compared to Visante OMNI, the Pentacam measured significantly greater simulated keratometry readings in normal and keratoconic eyes (Table 1). In normal eyes, the 95% LoA were from −0.32 to 0.59 dioptres (D) (K1) and from −0.41 to 0.74 D (K2). In keratoconic eyes, wider 95% LoA were noted from −1.35 to 1.92 D (K1) and from −1.38 to 1.99 D (K2). Bland–Altman plots for differences in corneal curvature values are shown in Fig. 1.
| Parameter | Normal eyes | Keratoconic eyes |
|---|---|---|
| Pentacam K1 (D) | 42.82 ± 1.42 | 44.72 ± 3.27 |
| Visante OMNI K1(D) | 42.69 ± 1.45 | 44.44 ± 2.89 |
| p Value | <0.0001 | 0.007 |
| Pentacam K2 (D) | 43.75 ± 1.50 | 47.97 ± 4.11 |
| Visante OMNI K2(D) | 43.59 ± 1.51 | 47.66 ± 3.85 |
| p Value | <0.0001 | 0.005 |
- K1 = simulated K reading in the flattest meridian, D = dioptres; K2 = simulated K reading in the steepest meridian.

Corneal thickness
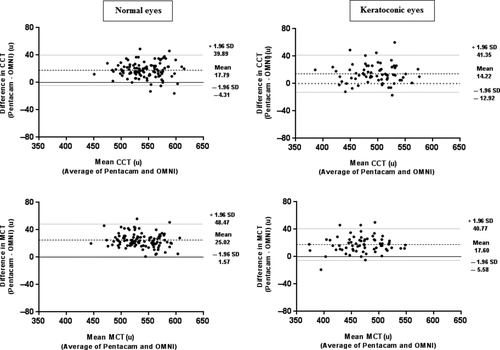
Corneal thickness values obtained from the Visante OMNI were significantly thinner in both groups of eyes (Table 2). In normal eyes, the Visante OMNI recorded lower CCT by 17.79 ± 11.27 μ and MCT by 25.02 ± 11.96 μ. The 95% LoA were from −4.31 to 39.89 μ for CCT and from 1.57 to 48.47 μ for MCT.
| Parameter | Normal eyes | Keratoconic eyes |
|---|---|---|
| Pentacam CCT (μ) | 552.11 ± 32.61 | 501.04 ± 37.61 |
| Visante OMNI CCT (μ) | 534.32 ± 32.78 | 486.83 ± 37.42 |
| p Value | <0.0001 | <0.0001 |
| Pentacam MCT (μ) | 549.15 ± 32.59 | 477.63 ± 40.00 |
| Visante OMNI MCT (μ) | 524.13 ± 34.17 | 460.03 ± 38.74 |
| p Value | <0.0001 | <0.0001 |
- CCT = central corneal thickness, μ = microns, MCT = minimum corneal thickness.
In keratoconic eyes, the Visante OMNI underestimated CCT by 14.22 ± 14.03 μ and MCT by 17.60 ± 11.82 μ. The 95% LoA were from −12.92 to 41.35 μ for CCT and from −5.58 to 40.77 μ for MCT. Bland–Altman plots for differences in corneal thickness values are shown in Fig. 2.
Corneal elevations
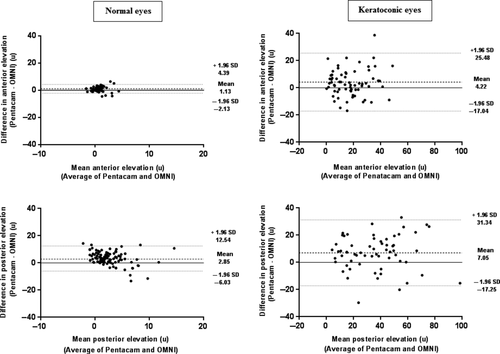
Pentacam anterior and posterior corneal elevations at the thinnest point were significantly higher in normal and keratoconic eyes (Table 3). The 95% LoA were from −2.13 to 4.39 μ for anterior elevations and from −6.03 to 12.54 μ for posterior elevations in normal eyes. Similar to corneal curvatures, wider 95% LoA were noted from −17.04 to 25.48 μ (anterior elevations) and from −17.25 to 31.34 μ (posterior elevations) in keratoconic eyes.
| Parameter | Normal eyes | Keratoconic eyes |
|---|---|---|
| Pentacam AE (μ) | 1.17 ± 1.28 | 20.51 ± 14.70 |
| Visante OMNI AE (μ) | 0.04 ± 1.14 | 16.29 ± 12.60 |
| p Value | <0.0001 | 0.002 |
| Pentacam PE (μ) | 4.24 ± 3.05 | 38.94 ± 21.77 |
| Visante OMNI PE (μ) | 0.98 ± 4.16 | 31.89 ± 21.24 |
| p Value | <0.0001 | <0.0001 |
- AE = anterior elevation at the thinnest point, PE = posterior elevation at the thinnest point,
- μ = microns.
Figure 3 shows the Bland–Altman plots for differences between both devices in corneal elevations. For posterior corneal elevations in normal eyes, the plot shows the pattern in which variation of at least 1 device depends strongly on the magnitude of measurements.
Repeatability and reproducibility
Based on ICC values, both Pentacam and Visante OMNI demonstrated excellent intra-operator repeatability and interoperator reproducibility for corneal curvature and thickness measurements. In contrast, moderate to poor repeatability and reproducibility were observed for elevations, particularly posterior elevation values (Table 4). The coefficient of repeatability values was as follows: K1(Pentacam 0.18, Visante 0.30), K2 (Pentacam 0.18, Visante 0.54), CCT (Pentacam 10.44, Visante 10.84), MCT (Pentacam 10.42, Visante 10.96), anterior elevations (Pentacam 0.89, Visante 1.01) and posterior elevations (Pentacam 2.69, Visante 3.06).
| Parameter | Repeatability ICC | Reproducibility ICC |
|---|---|---|
| Pentacam K1 (D) | 0.995 (0.984–0.998) | 0.997 (0.990–0.909) |
| Visante OMNI K1(D) | 0.985 (0.952–0.995) | 0.995 (0.983–0.998) |
| Pentacam K2 (D) | 0.993 (0.977–0.998) | 0.995 (0.985–0.999) |
| Visante OMNI K2(D) | 0.958 (0.874–0.987) | 0.994 (0.979–0.998) |
| Pentacam CCT (μ) | 0.984 (0.948–0.995) | 0.988 (0.961–0.996) |
| Visante OMNI CCT (μ) | 0.960 (0.878–0.988) | 0.984 (0.947–0.995) |
| Pentacam MCT (μ) | 0.984 (0.948–0.995) | 0.994 (0.981–0.998) |
| Visante OMNI MCT (μ) | 0.968 (0.901–0.990) | 0.990 (0.966–0.997) |
| Pentacam AE (μ) | 0.852 (0.593–0.952) | 0.892 (0.641–0.967) |
| Visante OMNI AE (μ) | 0.828 (0.500–0.942) | 0.854 (0.519–0.955) |
| Pentacam PE (μ) | 0.824 (0.465–0.941) | 0.768 (0.393–0.923) |
| Visante OMNI PE (μ) | 0.741 (0.192–0.920) | 0.606 (0.178–0.845) |
- ICC = intraclass correlation coefficient, K1 = simulated K reading in the flattest meridian, D = dioptres, K2 = simulated K reading in the steepest meridian, CCT = central corneal thickness, μ = microns, MCT = minimum corneal thickness, AE = anterior elevation at thinnest point, PE = posterior elevation at thinnest point.
Discussion
This study aimed to compare corneal parameters in normal and keratoconic eyes obtained from two commercially available imaging systems: the routinely used Pentacam and the recently introduced Visante OMNI. Corneal curvature and thickness measurements are important not only for screening patients for refractive surgery but also for diagnosing corneal disorders and for assessing glaucomatous eyes. To the best of our knowledge, this is the first study in the literature to compare these two devices.
The Atlas corneal topographer incorporated in the Visante OMNI works on the Placido principle whereby the anterior corneal surface is illuminated by concentric rings creating a reflected image (Mejía-Barbosa & Malacara-Hernández 2001). A corneal curvature colour map is then generated from computer analysis of this image. In comparison, the Pentacam derives keratometry data from cross-sectional Scheimpflug images.
There is a paucity of studies comparing corneal curvature values between the Pentacam and the Atlas topographer. We observed that the Pentacam measured significantly greater keratometry readings in normal and keratoconic eyes. Mean differences in keratometry values were 0.13 D (K1) and 0.16 D (K2) in normal eyes and 0.28 D (K1) and 0.31 D (K2) in keratoconic eyes. The 95% LoA were from −0.32 to 0.59 D (K1) and from −0.41 to 0.74 D (K2) in normal eyes. In keratoconic eyes, wider limits were noted from −1.35 to 1.92 D (K1) and from −1.38 to 1.99 D (K2). In contrast, Doménech et al. (2009) noted wide limits of agreement from to 0.25 to −1.48D (K1) and from 0.38 to −1.54 D (K2) with the Pentacam recording lower keratometry values than the Atlas in normal eyes. This discrepancy is clinically significant as the corneal curvature is one of the principal parameters widely used to detect keratoconus and monitor disease progression (McMahon et al. 2006; Romero-Jime′nez et al. 2010). Wider limits of agreement essentially mean that the devices vary significantly in the measured values and therefore are not potentially interchangeable.
Recently, non-contact methods of measuring corneal thickness are preferred over conventional ultrasonic pachymetry. This is due to associated patient discomfort, risk of corneal epithelial defects, potential for transmission of infections and variability of results with this technique (Marsich & Bullimore 2000; Miglior et al. 2004). Majority of earlier studies comparing the Pentacam to Visante OCT reported significantly lower CCT values with the Visante OCT in normal eyes (Doors et al. 2009; Yazici et al. 2010; Gorgun et al. 2011). Interestingly, O'Donnell et al. (2012) noted greater CCT measurements with Visante OCT as compared to Pentacam whereas a study by Ponce et al. showed no significant discrepancy(Prospero Ponce et al. 2009). Regarding keratoconic eyes, a recent study did not show any significant difference in corneal thickness measurements between both devices (Mencucci et al. 2012). In the present study, the Visante OMNI underestimated CCT by 17.79 ± 11.27 μ and MCT values by 25.02 ± 11.96 μ in normal eyes. Similar findings were noted in keratoconic eyes.
These differences in corneal thickness values may be significant in a clinical setting. An overestimation of CCT may falsely offer reassurance to surgeons that the available corneal thickness is appropriate for refractive surgery. An underestimation may unnecessarily exclude otherwise eligible normal patients from having surgery or alter their surgical options from LASIK to Surface Ablation or to consider intra-ocular refractive surgery. Similarly, in keratoconic eyes, an underestimation could potentially prevent patients from undergoing collagen cross-linking and an overestimation would make the procedure potentially unsafe. Our results indicate that the Pentacam and the Visante OMNI are not interchangeable in clinical practice for corneal thickness values.
With respect to anterior and posterior corneal elevations at the thinnest point, the Pentacam measured significantly higher values in both normal and keratoconic eyes. Moderate agreement was observed only for anterior corneal elevations in normal eyes. While Bland–Altman analysis demonstrated an inappropriate pattern of agreement for posterior elevations in normal eyes, wide limits were noted between both devices for anterior and posterior elevations in keratoconic eyes. The 95% LoA for posterior elevations were from −6.03 to 12.54 μ in normal eyes and from −17.25 to 31.34 μ in keratoconic eyes. We chose to study posterior corneal elevations at the thinnest point as these values are considered to be particularly sensitive in detecting keratoconus (Kovács et al. 2011). In our literature search, we did not come across similar studies comparing thinnest point corneal elevations between the Pentacam and the Visante OMNI.
One aspect to be considered is that both devices use different techniques to measure the corneal curvature, thickness and elevation values. While the Pentacam uses a Scheimpflug technique to take cross-sectional scans, the OMNI combines two technologies: the Placido technique and the OCT scanning slit technology. The Pentacam measures corneal thickness between the air–tear film interface and the posterior corneal surface and calculates corneal elevations using a software system that constructs a three-dimensional image of the anterior segment from elevation points captured by the rotating camera (Amano et al. 2006). The Visante OMNI measures corneal thickness using OCT and then subtracts this data from the anterior corneal surface elevations (measured by ATLAS topographer) to obtain the posterior corneal elevations (Srivannaboon et al. 2012).
Studies on the individual devices have demonstrated high repeatability and reproducibility for corneal curvature and thickness measurements (Chen & Lam 2007; Piñero et al. 2008). In this study, both Pentacam and Visante OMNI demonstrated excellent intra-operator repeatability and interoperator reproducibility for corneal curvature and thickness values. However, lower repeatability and reproducibility were observed for thinnest point elevations, particularly posterior elevation values. Amongst the devices, the Pentacam ICCs were generally noted to be better. This could be because Visante OMNI measurements require the subject to shift between two devices (Atlas corneal topographer and Visante OCT), thus prolonging the scan acquisition time.
Our results compare well with Guilbert et al. (2012) who compared corneal thickness, curvature and elevation values between a combined Placido-Scheimpflug system and a combined Placido-scanning-slit system and noted poor repeatability only for elevation measurements from both devices. Similarly, Núñez et al. observed poor repeatability for Pentacam posterior elevation measurements (Núñez & Blanco 2009). Recently, Srivannaboon et al. (2012) demonstrated that the Visante OMNI provides good repeatable and reproducible posterior corneal elevation values.
However, these studies measured corneal elevations with respect to a best-fit sphere (BFS) reference. In this study, we chose a BFTE reference as this closely resembles the actual corneal surface and therefore is more sensitive to local changes than a reference sphere (Kovács et al. 2011). The reason for obtaining poorly repeatable elevation measurements has not been elucidated. From our results, it appears that posterior elevations are not reliable for monitoring keratoconus progression.
To summarize, our results indicate that the Pentacam measured significantly greater corneal curvature, thickness and elevation values compared to Visante OMNI in normal and keratoconic eyes. The scatter and variability in measurements is higher for keratoconic eyes than normal eyes probably due to an aberrated corneal surface associated with the condition. The Pentacam and the Visante OMNI agree well only for anterior corneal elevation measurements in normal eyes. Therefore, these two devices are not interchangeable in a clinical setting. One of the strengths of this study is the inclusion of keratoconic eyes in addition to normal eyes. Further studies with larger sample sizes of both normal and abnormal corneas including keratoconus suspect and postrefractive surgery are justified.